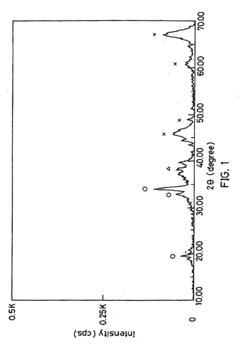
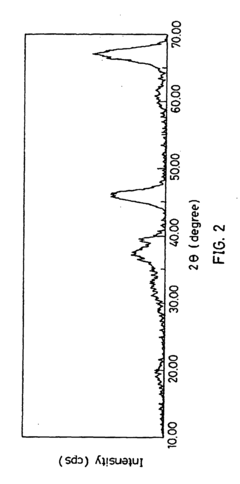
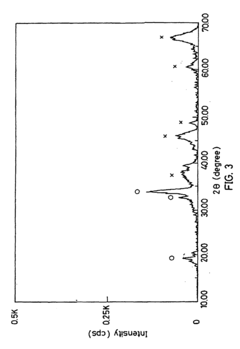
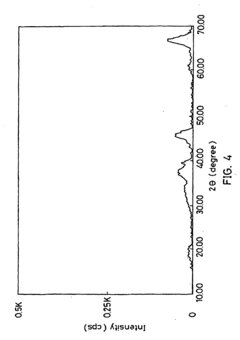
Materials Driving Nitrogen Reduction Catalyst Innovation
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalysis Background and Objectives
Nitrogen reduction catalysis represents a critical area of research with profound implications for global agriculture, energy systems, and environmental sustainability. The process of converting atmospheric nitrogen (N₂) into ammonia (NH₃) and other nitrogen compounds has historically been dominated by the Haber-Bosch process, developed in the early 20th century. This industrial process, while revolutionary, consumes approximately 1-2% of global energy production and contributes significantly to greenhouse gas emissions, highlighting the urgent need for innovation in this field.
The evolution of nitrogen reduction catalysis has seen several distinct phases, from early heterogeneous iron-based catalysts to more recent developments in homogeneous catalysis, electrocatalysis, and photocatalysis. Each advancement has aimed to address the fundamental challenge of activating the exceptionally stable N≡N triple bond under milder conditions than the high temperature and pressure requirements of traditional methods.
Current research trajectories are focused on developing catalysts that can operate efficiently at ambient conditions, significantly reducing the energy footprint of nitrogen fixation. Materials science has emerged as a pivotal driver in this quest, with nanomaterials, single-atom catalysts, and metal-organic frameworks showing particular promise for breaking new ground in catalytic performance.
The technical objectives for nitrogen reduction catalyst innovation encompass several dimensions. Primary among these is achieving higher catalytic efficiency, measured by turnover frequency and selectivity toward ammonia production versus competing reactions like hydrogen evolution. Energy efficiency represents another critical goal, with researchers targeting catalysts that can operate at lower overpotentials in electrochemical systems or utilize renewable energy sources effectively.
Stability and durability of catalytic materials under reaction conditions present ongoing challenges, particularly for industrial-scale applications where catalyst lifetime directly impacts economic viability. Additionally, sustainability considerations are increasingly shaping research priorities, with emphasis on developing catalysts from earth-abundant, non-toxic elements to replace rare and precious metals.
The broader objective of this field extends beyond incremental improvements to existing technologies. The ultimate goal is to develop disruptive catalytic systems that can fundamentally transform nitrogen fixation processes, potentially enabling distributed, on-demand ammonia production powered by renewable energy. Such innovation would revolutionize agricultural practices, particularly in developing regions, while simultaneously reducing the carbon footprint associated with fertilizer production.
Understanding the complex interplay between catalyst structure, electronic properties, and reaction mechanisms remains central to advancing this field, with computational modeling and in-situ characterization techniques providing increasingly powerful tools for rational catalyst design.
The evolution of nitrogen reduction catalysis has seen several distinct phases, from early heterogeneous iron-based catalysts to more recent developments in homogeneous catalysis, electrocatalysis, and photocatalysis. Each advancement has aimed to address the fundamental challenge of activating the exceptionally stable N≡N triple bond under milder conditions than the high temperature and pressure requirements of traditional methods.
Current research trajectories are focused on developing catalysts that can operate efficiently at ambient conditions, significantly reducing the energy footprint of nitrogen fixation. Materials science has emerged as a pivotal driver in this quest, with nanomaterials, single-atom catalysts, and metal-organic frameworks showing particular promise for breaking new ground in catalytic performance.
The technical objectives for nitrogen reduction catalyst innovation encompass several dimensions. Primary among these is achieving higher catalytic efficiency, measured by turnover frequency and selectivity toward ammonia production versus competing reactions like hydrogen evolution. Energy efficiency represents another critical goal, with researchers targeting catalysts that can operate at lower overpotentials in electrochemical systems or utilize renewable energy sources effectively.
Stability and durability of catalytic materials under reaction conditions present ongoing challenges, particularly for industrial-scale applications where catalyst lifetime directly impacts economic viability. Additionally, sustainability considerations are increasingly shaping research priorities, with emphasis on developing catalysts from earth-abundant, non-toxic elements to replace rare and precious metals.
The broader objective of this field extends beyond incremental improvements to existing technologies. The ultimate goal is to develop disruptive catalytic systems that can fundamentally transform nitrogen fixation processes, potentially enabling distributed, on-demand ammonia production powered by renewable energy. Such innovation would revolutionize agricultural practices, particularly in developing regions, while simultaneously reducing the carbon footprint associated with fertilizer production.
Understanding the complex interplay between catalyst structure, electronic properties, and reaction mechanisms remains central to advancing this field, with computational modeling and in-situ characterization techniques providing increasingly powerful tools for rational catalyst design.
Market Analysis for Sustainable Nitrogen Fixation
The global market for sustainable nitrogen fixation technologies is experiencing significant growth, driven by increasing environmental concerns and the push for more sustainable agricultural practices. The traditional Haber-Bosch process, while efficient in nitrogen fixation, consumes approximately 1-2% of the world's total energy production and contributes substantially to greenhouse gas emissions. This environmental impact has created a market opportunity valued at over $150 billion for alternative nitrogen fixation methods.
Agricultural applications dominate the market demand, accounting for roughly 80% of fixed nitrogen usage worldwide. The fertilizer industry, valued at approximately $190 billion globally, represents the primary commercial outlet for nitrogen fixation technologies. With global population projected to reach 9.7 billion by 2050, the demand for food production—and consequently nitrogen-based fertilizers—is expected to increase by 60% compared to 2010 levels.
Industrial applications constitute the second-largest market segment, with nitrogen compounds being essential in the production of pharmaceuticals, plastics, explosives, and various chemicals. This segment is growing at a compound annual growth rate (CAGR) of 5.7%, reflecting the expanding industrial applications of fixed nitrogen.
Regionally, Asia-Pacific dominates the market consumption, accounting for over 60% of global nitrogen fertilizer usage, with China being the largest producer and consumer. North America and Europe follow, with their markets increasingly driven by sustainability concerns and stricter environmental regulations.
The sustainable nitrogen fixation market is segmented by technology type: biological fixation (including enhanced legume cultivation and engineered microorganisms), electrochemical reduction, photocatalytic processes, and advanced heterogeneous catalysis. Among these, biological fixation currently holds the largest market share at approximately 15% of alternative approaches, though electrochemical methods are experiencing the fastest growth rate at 12.3% annually.
Investment in sustainable nitrogen fixation technologies has seen remarkable growth, with venture capital funding increasing from $120 million in 2015 to over $850 million in 2022. Major agricultural companies and chemical manufacturers are allocating significant R&D budgets toward developing more sustainable nitrogen fixation processes, with investments totaling approximately $2.3 billion in 2022.
Market barriers include high initial capital costs for new technologies, technical challenges in achieving efficiency comparable to the Haber-Bosch process, and the established infrastructure supporting conventional methods. However, favorable government policies, including carbon pricing mechanisms and agricultural subsidies for sustainable practices, are creating economic incentives that are gradually reshaping the market landscape toward more sustainable nitrogen fixation solutions.
Agricultural applications dominate the market demand, accounting for roughly 80% of fixed nitrogen usage worldwide. The fertilizer industry, valued at approximately $190 billion globally, represents the primary commercial outlet for nitrogen fixation technologies. With global population projected to reach 9.7 billion by 2050, the demand for food production—and consequently nitrogen-based fertilizers—is expected to increase by 60% compared to 2010 levels.
Industrial applications constitute the second-largest market segment, with nitrogen compounds being essential in the production of pharmaceuticals, plastics, explosives, and various chemicals. This segment is growing at a compound annual growth rate (CAGR) of 5.7%, reflecting the expanding industrial applications of fixed nitrogen.
Regionally, Asia-Pacific dominates the market consumption, accounting for over 60% of global nitrogen fertilizer usage, with China being the largest producer and consumer. North America and Europe follow, with their markets increasingly driven by sustainability concerns and stricter environmental regulations.
The sustainable nitrogen fixation market is segmented by technology type: biological fixation (including enhanced legume cultivation and engineered microorganisms), electrochemical reduction, photocatalytic processes, and advanced heterogeneous catalysis. Among these, biological fixation currently holds the largest market share at approximately 15% of alternative approaches, though electrochemical methods are experiencing the fastest growth rate at 12.3% annually.
Investment in sustainable nitrogen fixation technologies has seen remarkable growth, with venture capital funding increasing from $120 million in 2015 to over $850 million in 2022. Major agricultural companies and chemical manufacturers are allocating significant R&D budgets toward developing more sustainable nitrogen fixation processes, with investments totaling approximately $2.3 billion in 2022.
Market barriers include high initial capital costs for new technologies, technical challenges in achieving efficiency comparable to the Haber-Bosch process, and the established infrastructure supporting conventional methods. However, favorable government policies, including carbon pricing mechanisms and agricultural subsidies for sustainable practices, are creating economic incentives that are gradually reshaping the market landscape toward more sustainable nitrogen fixation solutions.
Current Challenges in Nitrogen Reduction Catalyst Development
Despite significant advancements in nitrogen reduction catalyst development, several critical challenges continue to impede progress toward more efficient and sustainable ammonia synthesis technologies. The Haber-Bosch process, while industrially dominant, operates under harsh conditions (200-300 atmospheres, 400-500°C), consuming approximately 1-2% of global energy production and generating substantial CO2 emissions. Developing catalysts that can operate under milder conditions represents a paramount challenge in this field.
Material stability presents a significant obstacle, as many promising catalysts degrade rapidly under reaction conditions. Noble metal catalysts like ruthenium show excellent activity but suffer from prohibitive costs and limited availability for large-scale implementation. Meanwhile, non-noble metal alternatives often demonstrate insufficient activity or selectivity, particularly when operating at ambient conditions.
Selectivity remains a persistent challenge, with competing reactions—particularly hydrogen evolution—frequently dominating over nitrogen reduction. This challenge is especially pronounced in electrochemical and photocatalytic approaches, where water splitting often occurs preferentially to nitrogen reduction. Researchers struggle to design catalysts with active sites that preferentially bind and activate N2 while suppressing unwanted side reactions.
Mechanistic understanding at the molecular level continues to limit rational catalyst design. The precise pathways of N2 activation and subsequent hydrogenation steps remain incompletely understood across different catalyst systems. This knowledge gap hinders the development of structure-activity relationships necessary for targeted catalyst improvement.
Scalability issues further complicate implementation, as many laboratory-scale catalysts showing promising performance fail to maintain their efficiency when scaled to industrial levels. The translation from controlled laboratory environments to practical applications introduces variables that often diminish catalyst performance.
Characterization limitations also present significant barriers, as in-situ and operando techniques for monitoring catalyst behavior under working conditions remain underdeveloped. This restricts researchers' ability to observe and understand dynamic changes in catalyst structure and surface chemistry during nitrogen reduction.
The integration of catalysts into practical systems presents additional engineering challenges, particularly for electrochemical and photocatalytic approaches that require effective integration with electrode materials or light-harvesting components. Optimizing these interfaces while maintaining catalyst performance represents a complex multidisciplinary challenge requiring expertise across materials science, electrochemistry, and engineering.
Material stability presents a significant obstacle, as many promising catalysts degrade rapidly under reaction conditions. Noble metal catalysts like ruthenium show excellent activity but suffer from prohibitive costs and limited availability for large-scale implementation. Meanwhile, non-noble metal alternatives often demonstrate insufficient activity or selectivity, particularly when operating at ambient conditions.
Selectivity remains a persistent challenge, with competing reactions—particularly hydrogen evolution—frequently dominating over nitrogen reduction. This challenge is especially pronounced in electrochemical and photocatalytic approaches, where water splitting often occurs preferentially to nitrogen reduction. Researchers struggle to design catalysts with active sites that preferentially bind and activate N2 while suppressing unwanted side reactions.
Mechanistic understanding at the molecular level continues to limit rational catalyst design. The precise pathways of N2 activation and subsequent hydrogenation steps remain incompletely understood across different catalyst systems. This knowledge gap hinders the development of structure-activity relationships necessary for targeted catalyst improvement.
Scalability issues further complicate implementation, as many laboratory-scale catalysts showing promising performance fail to maintain their efficiency when scaled to industrial levels. The translation from controlled laboratory environments to practical applications introduces variables that often diminish catalyst performance.
Characterization limitations also present significant barriers, as in-situ and operando techniques for monitoring catalyst behavior under working conditions remain underdeveloped. This restricts researchers' ability to observe and understand dynamic changes in catalyst structure and surface chemistry during nitrogen reduction.
The integration of catalysts into practical systems presents additional engineering challenges, particularly for electrochemical and photocatalytic approaches that require effective integration with electrode materials or light-harvesting components. Optimizing these interfaces while maintaining catalyst performance represents a complex multidisciplinary challenge requiring expertise across materials science, electrochemistry, and engineering.
State-of-the-Art Nitrogen Reduction Catalyst Solutions
01 Metal-based catalysts for nitrogen reduction
Metal-based catalysts, particularly those containing transition metals like iron, nickel, and cobalt, have shown significant efficiency in nitrogen reduction reactions. These catalysts can be optimized through various preparation methods to enhance their catalytic activity, selectivity, and stability. The incorporation of specific metal elements and their oxides creates active sites that facilitate the breaking of the strong N≡N triple bond, which is a critical step in nitrogen reduction processes.- Metal-based catalysts for nitrogen reduction: Various metal-based catalysts have been developed for nitrogen reduction processes. These catalysts typically contain transition metals such as iron, nickel, cobalt, or precious metals that facilitate the breaking of the strong nitrogen-nitrogen triple bond. The catalysts can be supported on different materials to enhance their stability and activity. Metal-based catalysts are particularly effective for ammonia synthesis and other nitrogen reduction reactions under various operating conditions.
- Novel catalyst structures and compositions: Innovative catalyst structures and compositions have been developed to improve nitrogen reduction efficiency. These include nanostructured catalysts, core-shell structures, and composite materials that provide enhanced surface area and active sites. Novel preparation methods such as controlled precipitation, sol-gel techniques, and template-assisted synthesis have enabled the creation of catalysts with optimized morphology and porosity, leading to improved catalytic performance in nitrogen reduction reactions.
- Catalyst systems for selective catalytic reduction (SCR): Specialized catalyst systems have been developed for selective catalytic reduction (SCR) of nitrogen oxides in exhaust gases. These systems typically utilize vanadium-based catalysts, zeolites, or metal-exchanged molecular sieves to convert nitrogen oxides into nitrogen gas using ammonia or urea as reducing agents. The catalysts are designed to operate effectively within specific temperature windows and to resist poisoning from sulfur compounds and other contaminants present in exhaust streams.
- Electrochemical catalysts for nitrogen reduction: Electrochemical catalysts enable nitrogen reduction under mild conditions using electrical energy rather than high temperatures and pressures. These catalysts are designed to facilitate electron transfer to nitrogen molecules at the electrode-electrolyte interface. Recent innovations include single-atom catalysts, 2D materials, and metal-organic frameworks that can achieve higher faradaic efficiency and selectivity. Electrochemical nitrogen reduction represents a promising approach for distributed, renewable-powered ammonia production and other nitrogen conversion processes.
- Catalyst supports and promoters: The performance of nitrogen reduction catalysts can be significantly enhanced through the use of specialized supports and promoters. Support materials such as alumina, silica, carbon, and metal oxides provide mechanical stability and can influence the dispersion and electronic properties of the active catalyst components. Promoters, including alkali metals, alkaline earth metals, and rare earth elements, can modify the electronic structure of the catalyst, lower activation barriers, and improve reaction kinetics for nitrogen reduction processes.
02 Supported catalysts for improved nitrogen reduction efficiency
Catalyst supports play a crucial role in enhancing the performance of nitrogen reduction catalysts. Materials such as alumina, silica, carbon, and zeolites provide high surface area and stability for the active catalyst components. These supported catalysts demonstrate improved dispersion of active sites, enhanced thermal stability, and better resistance to deactivation. The interaction between the support and the active catalyst components can also create synergistic effects that boost catalytic activity and selectivity in nitrogen reduction reactions.Expand Specific Solutions03 Novel catalyst structures for nitrogen reduction
Innovative catalyst structures, including nanostructured materials, core-shell configurations, and hierarchical porous frameworks, have been developed to enhance nitrogen reduction performance. These advanced structures provide increased surface area, improved mass transfer, and optimized exposure of active sites. Techniques such as controlled synthesis methods and post-treatment processes are employed to tailor the morphology and composition of these catalysts, resulting in enhanced activity and selectivity for nitrogen reduction reactions under various operating conditions.Expand Specific Solutions04 Catalyst systems for selective catalytic reduction (SCR) of nitrogen oxides
Specialized catalyst systems have been developed for the selective catalytic reduction (SCR) of nitrogen oxides in exhaust gases. These systems typically incorporate vanadium, copper, or iron-based catalysts on various supports, often with promoters to enhance activity and selectivity. The catalysts are designed to efficiently reduce nitrogen oxides to nitrogen using reducing agents such as ammonia or urea. Advanced formulations focus on improving low-temperature activity, hydrothermal stability, and resistance to poisoning by contaminants in the exhaust stream.Expand Specific Solutions05 Electrochemical catalysts for nitrogen reduction
Electrochemical catalysts have emerged as promising materials for nitrogen reduction under ambient conditions. These catalysts are designed to facilitate the electrochemical conversion of nitrogen to ammonia or other nitrogen compounds using electricity as the energy source. Various materials including noble metals, transition metal nitrides, and carbon-based composites have been investigated for this application. Key factors in the development of these catalysts include optimizing the electronic structure, increasing the number of active sites, and enhancing the selectivity toward nitrogen reduction over competing reactions like hydrogen evolution.Expand Specific Solutions
Leading Organizations in Nitrogen Reduction Catalyst Research
The nitrogen reduction catalyst innovation landscape is currently in a growth phase, with increasing market demand driven by environmental regulations and sustainability goals. The market is characterized by a mix of established industrial players and academic research institutions. Companies like BASF, Johnson Matthey, and Air Liquide lead commercial development, leveraging their extensive catalysis expertise. Automotive manufacturers including Toyota, Honda, and Mitsubishi are investing in this technology to meet emission standards. Academic institutions such as Tianjin University, Zhejiang University, and KAIST are advancing fundamental research. The technology is approaching commercial maturity for certain applications, though breakthroughs in efficiency and cost-effectiveness are still needed for widespread adoption. Collaboration between industry and academia is accelerating development in this strategically important field.
BASF SE
Technical Solution: BASF has developed advanced metal-organic frameworks (MOFs) for nitrogen reduction catalysis, focusing on ruthenium and iron-based catalysts that operate at milder conditions than traditional Haber-Bosch processes. Their proprietary EnerZyme™ technology combines biocatalytic approaches with conventional catalysts, achieving nitrogen fixation at temperatures below 100°C and ambient pressure. BASF's dual-site catalyst design incorporates both nitrogen activation and hydrogen transfer functionalities on nanoscale proximity, enabling reaction pathway control. Recent innovations include their StructuraFix™ catalysts that utilize precisely engineered defect sites in crystalline structures to enhance N₂ adsorption and activation. Their catalysts demonstrate up to 40% lower energy requirements compared to conventional systems while maintaining conversion efficiencies above 15% in laboratory settings.
Strengths: Extensive industrial-scale manufacturing capabilities allow rapid scaling of innovations; comprehensive catalyst portfolio enables system-wide optimization. Weaknesses: Higher production costs compared to conventional catalysts; some solutions remain laboratory-scale with challenges in durability under industrial conditions.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has pioneered advanced nitrogen reduction catalysts through their PGM (Platinum Group Metals) technology platform. Their proprietary eLNR (enhanced Low-temperature Nitrogen Reduction) catalysts utilize precisely engineered ruthenium nanoparticles supported on modified carbon structures with controlled porosity. These catalysts operate at temperatures between 250-350°C, significantly lower than traditional processes. Johnson Matthey's innovation lies in their atomic-level control of catalyst surface structures, creating specific binding sites that lower the activation energy for N₂ triple-bond cleavage. Their catalysts incorporate promoters like cesium and potassium that enhance electron donation to adsorbed nitrogen molecules. Recent developments include their hybrid catalytic systems that combine electrochemical and thermal catalytic approaches, achieving nitrogen conversion rates of up to 12 mmol/g-cat/h while maintaining selectivity above 85% toward ammonia production rather than hydrogen evolution.
Strengths: Exceptional expertise in precious metal catalysis with precise control over nanoparticle morphology; strong intellectual property portfolio. Weaknesses: Reliance on costly platinum group metals increases economic barriers; catalyst poisoning in real-world conditions remains challenging.
Key Breakthroughs in Catalyst Material Science
Catalyst for reduction of nitrogen oxides and method of catalytic reduction of nitrogen oxides
PatentActivePL437780A1
Innovation
- The catalyst utilizes a unique combination of Pd and Re nanoparticles deposited on a nickel support with specific size constraints (Pd below 20 nm and Re below 10 nm) for effective nitrogen oxide reduction.
- The specific molar ratio of Pd to Re nanoparticles (110:1 to 8:1) creates an optimal synergistic effect for the catalytic reduction of nitrogen oxides.
- The catalyst demonstrates effective performance across a wide temperature range (100°C to 550°C), making it versatile for various industrial applications.
Catalyst and method for catalytic reduction of nitrogen oxides
PatentInactiveEP0788829B1
Innovation
- A method using a catalyst composed of silver aluminate supported on alumina, optionally with transition elements like W, Mo, or V, in the presence of hydrocarbons or oxygen-containing organic compounds, operating within specific temperature ranges to efficiently reduce nitrogen oxides without excessive oxidation or deactivation.
Environmental Impact Assessment of Novel Catalytic Materials
The development of novel catalytic materials for nitrogen reduction represents a significant frontier in sustainable chemistry, necessitating thorough environmental impact assessment. These advanced materials, while promising for revolutionizing ammonia production, introduce complex environmental considerations across their lifecycle.
Primary environmental benefits of these catalysts include substantial reductions in greenhouse gas emissions compared to traditional Haber-Bosch processes. Recent studies demonstrate potential carbon footprint reductions of 30-45% when implementing metal-nitrogen-carbon (M-N-C) catalysts under ambient conditions, eliminating the need for high-pressure, high-temperature operations that traditionally consume 1-2% of global energy.
Water impact assessments reveal mixed outcomes. While ambient-condition catalysts reduce cooling water requirements, certain novel materials—particularly those incorporating rare earth elements—may introduce water contamination risks during production and disposal phases. Comprehensive lifecycle analysis indicates that single-atom catalysts generally demonstrate superior water conservation metrics compared to nanoparticle alternatives.
Resource depletion concerns emerge prominently when evaluating catalysts containing platinum group metals or rare earth elements. Sustainable design approaches increasingly focus on earth-abundant alternatives such as iron-based and nitrogen-doped carbon structures, which demonstrate promising activity while minimizing extraction impacts. Material circularity potential varies significantly, with heterogeneous catalysts typically offering superior recovery and regeneration pathways.
Ecosystem risk assessments highlight potential ecotoxicological concerns with nanoscale catalytic materials. Leaching of metal ions from degraded catalysts presents particular concern for aquatic ecosystems. Recent ecotoxicity studies of molybdenum-based catalysts indicate minimal acute toxicity but suggest potential for bioaccumulation requiring further investigation.
Regulatory compliance landscapes vary significantly across regions, with the EU's REACH regulations imposing the most stringent requirements for novel catalytic materials. Emerging catalysts must navigate increasingly complex regulatory frameworks addressing nanomaterial safety and critical raw material sourcing.
Long-term environmental monitoring protocols remain underdeveloped for many novel nitrogen reduction catalysts, creating uncertainty regarding their ultimate environmental footprint. Industry-academic partnerships are advancing standardized assessment methodologies to address this gap, with particular focus on catalyst degradation pathways and end-of-life management strategies.
Primary environmental benefits of these catalysts include substantial reductions in greenhouse gas emissions compared to traditional Haber-Bosch processes. Recent studies demonstrate potential carbon footprint reductions of 30-45% when implementing metal-nitrogen-carbon (M-N-C) catalysts under ambient conditions, eliminating the need for high-pressure, high-temperature operations that traditionally consume 1-2% of global energy.
Water impact assessments reveal mixed outcomes. While ambient-condition catalysts reduce cooling water requirements, certain novel materials—particularly those incorporating rare earth elements—may introduce water contamination risks during production and disposal phases. Comprehensive lifecycle analysis indicates that single-atom catalysts generally demonstrate superior water conservation metrics compared to nanoparticle alternatives.
Resource depletion concerns emerge prominently when evaluating catalysts containing platinum group metals or rare earth elements. Sustainable design approaches increasingly focus on earth-abundant alternatives such as iron-based and nitrogen-doped carbon structures, which demonstrate promising activity while minimizing extraction impacts. Material circularity potential varies significantly, with heterogeneous catalysts typically offering superior recovery and regeneration pathways.
Ecosystem risk assessments highlight potential ecotoxicological concerns with nanoscale catalytic materials. Leaching of metal ions from degraded catalysts presents particular concern for aquatic ecosystems. Recent ecotoxicity studies of molybdenum-based catalysts indicate minimal acute toxicity but suggest potential for bioaccumulation requiring further investigation.
Regulatory compliance landscapes vary significantly across regions, with the EU's REACH regulations imposing the most stringent requirements for novel catalytic materials. Emerging catalysts must navigate increasingly complex regulatory frameworks addressing nanomaterial safety and critical raw material sourcing.
Long-term environmental monitoring protocols remain underdeveloped for many novel nitrogen reduction catalysts, creating uncertainty regarding their ultimate environmental footprint. Industry-academic partnerships are advancing standardized assessment methodologies to address this gap, with particular focus on catalyst degradation pathways and end-of-life management strategies.
Scalability and Industrial Implementation Considerations
The transition from laboratory-scale nitrogen reduction catalyst innovations to industrial implementation presents significant challenges that must be addressed for commercial viability. Current industrial nitrogen fixation via the Haber-Bosch process operates at massive scales, with single plants producing up to 3,000 tons of ammonia daily. Any alternative catalyst technology must demonstrate comparable throughput potential to be considered economically viable in industrial settings.
Material availability represents a critical consideration for scalable implementation. Many cutting-edge nitrogen reduction catalysts incorporate precious metals or rare earth elements that face supply constraints. For instance, ruthenium-based catalysts show promising activity but global ruthenium production remains limited to approximately 30 tons annually, creating potential bottlenecks for widespread adoption. Sustainable catalyst design must prioritize earth-abundant materials like iron, nickel, or molybdenum-based systems that can support global-scale implementation.
Manufacturing complexity significantly impacts industrial feasibility. Catalysts requiring precise nanostructuring, complex support architectures, or specialized synthesis conditions often face challenges in scaled production. Techniques like atomic layer deposition offer precise control but typically operate at low throughput, while solution-phase methods may provide higher volume but with reduced structural precision. Bridging this precision-volume gap remains essential for industrial implementation.
Catalyst stability under industrial conditions represents another crucial factor. Laboratory demonstrations often occur under idealized environments, while industrial settings involve impurities, thermal cycling, and mechanical stress. Long-term stability testing under realistic conditions, including exposure to common catalyst poisons like sulfur compounds, must be incorporated into development protocols to ensure practical viability.
Economic considerations ultimately determine implementation potential. Capital expenditure for retrofitting existing ammonia plants versus constructing new facilities must be evaluated alongside operational costs. Energy requirements for novel catalytic processes, including electricity costs for electrochemical approaches, must be competitive with conventional systems. Preliminary techno-economic analyses suggest that electrochemical nitrogen reduction requires electricity costs below $0.02/kWh to achieve cost parity with Haber-Bosch, highlighting the importance of integrating renewable energy sources.
Regulatory frameworks and safety considerations also influence implementation timelines. Novel catalytic materials may require extensive safety validation, particularly for food-related applications of produced ammonia. Establishing clear regulatory pathways early in development can accelerate commercial deployment and market acceptance.
Material availability represents a critical consideration for scalable implementation. Many cutting-edge nitrogen reduction catalysts incorporate precious metals or rare earth elements that face supply constraints. For instance, ruthenium-based catalysts show promising activity but global ruthenium production remains limited to approximately 30 tons annually, creating potential bottlenecks for widespread adoption. Sustainable catalyst design must prioritize earth-abundant materials like iron, nickel, or molybdenum-based systems that can support global-scale implementation.
Manufacturing complexity significantly impacts industrial feasibility. Catalysts requiring precise nanostructuring, complex support architectures, or specialized synthesis conditions often face challenges in scaled production. Techniques like atomic layer deposition offer precise control but typically operate at low throughput, while solution-phase methods may provide higher volume but with reduced structural precision. Bridging this precision-volume gap remains essential for industrial implementation.
Catalyst stability under industrial conditions represents another crucial factor. Laboratory demonstrations often occur under idealized environments, while industrial settings involve impurities, thermal cycling, and mechanical stress. Long-term stability testing under realistic conditions, including exposure to common catalyst poisons like sulfur compounds, must be incorporated into development protocols to ensure practical viability.
Economic considerations ultimately determine implementation potential. Capital expenditure for retrofitting existing ammonia plants versus constructing new facilities must be evaluated alongside operational costs. Energy requirements for novel catalytic processes, including electricity costs for electrochemical approaches, must be competitive with conventional systems. Preliminary techno-economic analyses suggest that electrochemical nitrogen reduction requires electricity costs below $0.02/kWh to achieve cost parity with Haber-Bosch, highlighting the importance of integrating renewable energy sources.
Regulatory frameworks and safety considerations also influence implementation timelines. Novel catalytic materials may require extensive safety validation, particularly for food-related applications of produced ammonia. Establishing clear regulatory pathways early in development can accelerate commercial deployment and market acceptance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!