Investigating the Stability of Nitrogen Reduction Catalyst at High Temperatures
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalyst Evolution and Research Objectives
Nitrogen reduction catalysts have undergone significant evolution since their initial development in the early 20th century. The Haber-Bosch process, developed in 1909, represented the first major breakthrough in nitrogen fixation, utilizing iron-based catalysts at high temperatures and pressures. This process revolutionized agriculture by enabling industrial-scale ammonia production, though with considerable energy requirements and environmental impact.
The 1970s and 1980s witnessed the emergence of ruthenium-based catalysts, offering improved activity at lower operating pressures compared to traditional iron catalysts. This period marked a shift toward more efficient nitrogen reduction approaches, though temperature stability remained a persistent challenge for widespread industrial adoption.
Recent decades have seen accelerated development in catalyst technology, with significant advancements in nanomaterial-based catalysts. Metal nitrides, particularly those incorporating molybdenum and vanadium, have demonstrated promising nitrogen reduction capabilities. Simultaneously, research into single-atom catalysts has opened new possibilities for atomic-level precision in catalyst design, potentially addressing long-standing stability issues at elevated temperatures.
The current technological landscape is characterized by increasing focus on sustainable nitrogen reduction methods. Electrocatalytic and photocatalytic approaches have gained prominence as potential alternatives to traditional thermochemical processes, offering pathways to operate under milder conditions while potentially mitigating stability concerns associated with high-temperature operations.
Our research objectives center on investigating the fundamental mechanisms of catalyst degradation at temperatures exceeding 500°C, with particular emphasis on structural transformations, surface reconstruction phenomena, and the role of support materials in enhancing thermal stability. We aim to develop novel catalyst formulations that maintain activity and selectivity under prolonged high-temperature exposure, potentially enabling more energy-efficient nitrogen reduction processes.
Additionally, we seek to establish comprehensive stability metrics and accelerated testing protocols that can accurately predict catalyst longevity in industrial settings. This includes developing in-situ characterization techniques capable of monitoring catalyst evolution under reaction conditions, providing real-time insights into degradation pathways.
The ultimate goal is to design next-generation nitrogen reduction catalysts with exceptional thermal stability, capable of maintaining performance for extended periods at elevated temperatures. Such advancements would significantly impact industrial ammonia synthesis, NOx reduction technologies, and emerging applications in sustainable energy systems, potentially reducing energy consumption and environmental impact across multiple sectors.
The 1970s and 1980s witnessed the emergence of ruthenium-based catalysts, offering improved activity at lower operating pressures compared to traditional iron catalysts. This period marked a shift toward more efficient nitrogen reduction approaches, though temperature stability remained a persistent challenge for widespread industrial adoption.
Recent decades have seen accelerated development in catalyst technology, with significant advancements in nanomaterial-based catalysts. Metal nitrides, particularly those incorporating molybdenum and vanadium, have demonstrated promising nitrogen reduction capabilities. Simultaneously, research into single-atom catalysts has opened new possibilities for atomic-level precision in catalyst design, potentially addressing long-standing stability issues at elevated temperatures.
The current technological landscape is characterized by increasing focus on sustainable nitrogen reduction methods. Electrocatalytic and photocatalytic approaches have gained prominence as potential alternatives to traditional thermochemical processes, offering pathways to operate under milder conditions while potentially mitigating stability concerns associated with high-temperature operations.
Our research objectives center on investigating the fundamental mechanisms of catalyst degradation at temperatures exceeding 500°C, with particular emphasis on structural transformations, surface reconstruction phenomena, and the role of support materials in enhancing thermal stability. We aim to develop novel catalyst formulations that maintain activity and selectivity under prolonged high-temperature exposure, potentially enabling more energy-efficient nitrogen reduction processes.
Additionally, we seek to establish comprehensive stability metrics and accelerated testing protocols that can accurately predict catalyst longevity in industrial settings. This includes developing in-situ characterization techniques capable of monitoring catalyst evolution under reaction conditions, providing real-time insights into degradation pathways.
The ultimate goal is to design next-generation nitrogen reduction catalysts with exceptional thermal stability, capable of maintaining performance for extended periods at elevated temperatures. Such advancements would significantly impact industrial ammonia synthesis, NOx reduction technologies, and emerging applications in sustainable energy systems, potentially reducing energy consumption and environmental impact across multiple sectors.
Market Analysis for High-Temperature Stable Catalysts
The high-temperature stable catalyst market for nitrogen reduction is experiencing robust growth, driven primarily by increasing demand in ammonia production, which consumes approximately 2% of global energy and accounts for over 500 million tons of annual production. This market segment is projected to expand at a compound annual growth rate of 4.7% through 2030, significantly outpacing the broader catalyst market's 3.2% growth rate.
Industrial sectors represent the dominant application area, particularly fertilizer manufacturing which constitutes nearly 80% of ammonia usage globally. The energy sector is emerging as a secondary growth driver, with ammonia increasingly viewed as a potential hydrogen carrier and carbon-free fuel. This application could represent a market opportunity exceeding $30 billion by 2035 if technological barriers regarding catalyst stability are overcome.
Geographically, Asia-Pacific commands the largest market share at approximately 45%, with China alone accounting for 30% of global high-temperature catalyst consumption. North America and Europe follow with 25% and 20% market shares respectively, though Europe demonstrates the fastest growth trajectory due to aggressive green hydrogen initiatives and stringent emission regulations.
Price sensitivity varies significantly by application segment. Traditional industrial applications remain highly cost-conscious, with catalyst price premiums typically limited to 15-20% over conventional alternatives. Conversely, emerging applications in green ammonia production demonstrate considerably higher willingness to pay for performance, with premiums of 50-100% acceptable if substantial efficiency improvements or extended catalyst lifespans can be demonstrated.
Market barriers include high capital investment requirements, technical complexity of integration, and regulatory hurdles related to safety standards. The average implementation cycle for new catalyst technologies in industrial settings spans 3-5 years, creating significant commercialization challenges for emerging solutions.
Customer requirements increasingly emphasize not only thermal stability but also resistance to common catalyst poisons, particularly sulfur compounds and carbon deposition. Performance metrics prioritized by end-users include conversion efficiency maintenance above 90% after 5,000 operating hours at temperatures exceeding 450°C, pressure tolerance up to 300 bar, and minimal precious metal content to control costs.
The competitive landscape features both established chemical companies and specialized catalyst manufacturers, with recent market consolidation through strategic acquisitions indicating growing recognition of this segment's value. Intellectual property protection remains strong, with approximately 350 active patents specifically addressing high-temperature stability of nitrogen reduction catalysts filed in the past decade.
Industrial sectors represent the dominant application area, particularly fertilizer manufacturing which constitutes nearly 80% of ammonia usage globally. The energy sector is emerging as a secondary growth driver, with ammonia increasingly viewed as a potential hydrogen carrier and carbon-free fuel. This application could represent a market opportunity exceeding $30 billion by 2035 if technological barriers regarding catalyst stability are overcome.
Geographically, Asia-Pacific commands the largest market share at approximately 45%, with China alone accounting for 30% of global high-temperature catalyst consumption. North America and Europe follow with 25% and 20% market shares respectively, though Europe demonstrates the fastest growth trajectory due to aggressive green hydrogen initiatives and stringent emission regulations.
Price sensitivity varies significantly by application segment. Traditional industrial applications remain highly cost-conscious, with catalyst price premiums typically limited to 15-20% over conventional alternatives. Conversely, emerging applications in green ammonia production demonstrate considerably higher willingness to pay for performance, with premiums of 50-100% acceptable if substantial efficiency improvements or extended catalyst lifespans can be demonstrated.
Market barriers include high capital investment requirements, technical complexity of integration, and regulatory hurdles related to safety standards. The average implementation cycle for new catalyst technologies in industrial settings spans 3-5 years, creating significant commercialization challenges for emerging solutions.
Customer requirements increasingly emphasize not only thermal stability but also resistance to common catalyst poisons, particularly sulfur compounds and carbon deposition. Performance metrics prioritized by end-users include conversion efficiency maintenance above 90% after 5,000 operating hours at temperatures exceeding 450°C, pressure tolerance up to 300 bar, and minimal precious metal content to control costs.
The competitive landscape features both established chemical companies and specialized catalyst manufacturers, with recent market consolidation through strategic acquisitions indicating growing recognition of this segment's value. Intellectual property protection remains strong, with approximately 350 active patents specifically addressing high-temperature stability of nitrogen reduction catalysts filed in the past decade.
Current Challenges in High-Temperature Catalyst Stability
The stability of nitrogen reduction catalysts at high temperatures represents one of the most significant challenges in the field of catalytic chemistry. Current catalysts, particularly those used in ammonia synthesis, face severe degradation when exposed to temperatures exceeding 500°C for extended periods. This degradation manifests primarily through three mechanisms: sintering, poisoning, and phase transformation, all of which contribute to diminished catalytic performance and shortened operational lifespans.
Sintering, the agglomeration of catalyst particles at elevated temperatures, remains particularly problematic for nitrogen reduction catalysts. Recent studies have demonstrated that traditional iron-based catalysts can lose up to 40% of their active surface area after just 100 hours of operation at temperatures above 550°C. This reduction in surface area directly correlates with decreased catalytic efficiency and nitrogen conversion rates.
Catalyst poisoning presents another significant challenge, especially in industrial settings where feed gas impurities are unavoidable. Sulfur compounds, even at concentrations as low as 1 ppm, can irreversibly bind to active sites on ruthenium and iron-based catalysts, rendering them permanently deactivated. The poisoning effect intensifies at higher temperatures, with research indicating a 2.5-fold increase in poisoning rates for every 50°C increase above 450°C.
Phase transformation under high-temperature conditions further complicates catalyst stability. Many promising nitrogen reduction catalysts undergo crystalline structure changes when subjected to thermal cycling, particularly those based on transition metal nitrides. For instance, molybdenum nitride catalysts have been observed to transform from the catalytically active γ-Mo2N phase to less active phases when exposed to temperatures above 600°C, resulting in up to 70% activity loss.
The development of support materials that maintain structural integrity at high temperatures represents another unresolved challenge. Conventional supports like alumina and silica exhibit significant surface area reduction and pore collapse at elevated temperatures, compromising their ability to disperse and stabilize active catalyst components. Advanced ceramic supports show promise but introduce new challenges related to catalyst-support interactions and synthesis complexity.
Economic considerations further constrain solutions to these stability challenges. While precious metal catalysts (particularly ruthenium-based systems) demonstrate superior thermal stability, their high cost prohibits widespread industrial adoption. The trade-off between catalyst cost, performance, and longevity remains a central dilemma for industrial applications, particularly in regions with limited access to precious metals or specialized manufacturing capabilities.
Recent attempts to address these challenges through core-shell architectures and atomic layer deposition techniques have shown promise in laboratory settings but face significant hurdles in scaling to industrial production volumes. The gap between laboratory demonstrations and commercially viable solutions continues to widen as temperature requirements for energy-efficient nitrogen reduction processes increase.
Sintering, the agglomeration of catalyst particles at elevated temperatures, remains particularly problematic for nitrogen reduction catalysts. Recent studies have demonstrated that traditional iron-based catalysts can lose up to 40% of their active surface area after just 100 hours of operation at temperatures above 550°C. This reduction in surface area directly correlates with decreased catalytic efficiency and nitrogen conversion rates.
Catalyst poisoning presents another significant challenge, especially in industrial settings where feed gas impurities are unavoidable. Sulfur compounds, even at concentrations as low as 1 ppm, can irreversibly bind to active sites on ruthenium and iron-based catalysts, rendering them permanently deactivated. The poisoning effect intensifies at higher temperatures, with research indicating a 2.5-fold increase in poisoning rates for every 50°C increase above 450°C.
Phase transformation under high-temperature conditions further complicates catalyst stability. Many promising nitrogen reduction catalysts undergo crystalline structure changes when subjected to thermal cycling, particularly those based on transition metal nitrides. For instance, molybdenum nitride catalysts have been observed to transform from the catalytically active γ-Mo2N phase to less active phases when exposed to temperatures above 600°C, resulting in up to 70% activity loss.
The development of support materials that maintain structural integrity at high temperatures represents another unresolved challenge. Conventional supports like alumina and silica exhibit significant surface area reduction and pore collapse at elevated temperatures, compromising their ability to disperse and stabilize active catalyst components. Advanced ceramic supports show promise but introduce new challenges related to catalyst-support interactions and synthesis complexity.
Economic considerations further constrain solutions to these stability challenges. While precious metal catalysts (particularly ruthenium-based systems) demonstrate superior thermal stability, their high cost prohibits widespread industrial adoption. The trade-off between catalyst cost, performance, and longevity remains a central dilemma for industrial applications, particularly in regions with limited access to precious metals or specialized manufacturing capabilities.
Recent attempts to address these challenges through core-shell architectures and atomic layer deposition techniques have shown promise in laboratory settings but face significant hurdles in scaling to industrial production volumes. The gap between laboratory demonstrations and commercially viable solutions continues to widen as temperature requirements for energy-efficient nitrogen reduction processes increase.
Existing High-Temperature Catalyst Stabilization Approaches
01 Metal-based catalysts for nitrogen reduction stability
Various metal-based catalysts have been developed to enhance stability during nitrogen reduction processes. These catalysts typically incorporate noble metals, transition metals, or their combinations to resist deactivation under reaction conditions. The catalyst formulations often include specific metal ratios and preparation methods that contribute to their long-term performance and resistance to poisoning during nitrogen reduction reactions.- Metal-based catalysts for nitrogen reduction stability: Various metal-based catalysts have been developed to enhance stability during nitrogen reduction processes. These catalysts typically incorporate noble metals, transition metals, or their combinations to resist deactivation under reaction conditions. The stability is achieved through specific structural configurations, support materials, and preparation methods that prevent sintering and poisoning during prolonged operation.
- Support materials for enhancing catalyst longevity: The choice of support materials significantly impacts nitrogen reduction catalyst stability. Materials such as alumina, silica, zeolites, and carbon-based supports provide thermal resistance and prevent catalyst agglomeration. These supports maintain high surface area and dispersion of active sites, while some specialized supports offer additional benefits like improved resistance to sulfur poisoning and water tolerance during extended operation periods.
- Promoters and additives for stability enhancement: Various promoters and additives are incorporated into nitrogen reduction catalysts to improve their stability. These compounds, including alkali metals, alkaline earth metals, and rare earth elements, modify the electronic properties of the catalyst surface and create protective layers that prevent deactivation. Some additives specifically target resistance to common poisons like sulfur compounds and carbon deposition, extending catalyst lifetime under industrial conditions.
- Regeneration techniques for deactivated catalysts: Methods for regenerating deactivated nitrogen reduction catalysts have been developed to extend their operational lifetime. These techniques include controlled oxidation treatments, reduction processes, washing with specific solvents, and thermal treatments under controlled atmospheres. Some advanced regeneration approaches incorporate plasma or microwave-assisted treatments that can restore catalyst activity while preserving the structural integrity of the catalyst system.
- Novel catalyst structures for enhanced stability: Innovative structural designs have been developed to enhance nitrogen reduction catalyst stability. These include core-shell structures, hierarchical porous frameworks, encapsulated active sites, and atomically dispersed catalysts. Such architectural approaches protect active sites from sintering and poisoning while maintaining accessibility to reactants. Some designs incorporate self-healing mechanisms or sacrificial components that preferentially react with catalyst poisons, significantly extending operational lifetime under harsh reaction conditions.
02 Support materials for improving catalyst stability
The choice of support material significantly impacts nitrogen reduction catalyst stability. Various supports such as alumina, silica, zeolites, and carbon-based materials can enhance catalyst durability by providing thermal stability, preventing sintering, and improving dispersion of active components. Modified support structures with specific surface characteristics and porosity help maintain catalyst activity over extended operational periods by minimizing degradation mechanisms.Expand Specific Solutions03 Promoters and additives for enhanced catalyst stability
Incorporating specific promoters and additives into nitrogen reduction catalysts can significantly improve their stability. These components, including alkali metals, alkaline earth metals, and rare earth elements, help prevent catalyst deactivation by neutralizing poisons, inhibiting sintering, and maintaining active site accessibility. The strategic addition of these stabilizing agents in precise concentrations extends catalyst lifetime under harsh reaction conditions.Expand Specific Solutions04 Thermal and hydrothermal stability enhancement techniques
Various techniques have been developed to enhance the thermal and hydrothermal stability of nitrogen reduction catalysts. These include controlled calcination procedures, specific synthesis methods that create robust structures, and incorporation of stabilizing elements that prevent structural collapse at high temperatures. Advanced preparation methods such as core-shell structures and encapsulation techniques protect active components from sintering and phase transformation during operation.Expand Specific Solutions05 Regeneration methods for deactivated nitrogen reduction catalysts
Effective regeneration methods have been developed to restore activity to deactivated nitrogen reduction catalysts. These processes include controlled oxidation treatments, washing with specific solvents, thermal treatments under controlled atmospheres, and chemical treatments to remove deposited contaminants. Regeneration protocols are designed to remove poisons and restore the original catalyst structure without causing additional damage to the active components.Expand Specific Solutions
Leading Research Institutions and Industrial Manufacturers
The nitrogen reduction catalyst stability market at high temperatures is in a growth phase, with increasing demand driven by industrial applications and environmental regulations. The competitive landscape is characterized by established chemical companies like Umicore SA, BASF, and Tosoh Corp. leading innovation in catalyst technology, while automotive manufacturers such as Hyundai, Kia, and Nissan are significant end-users driving application-specific developments. Research institutions including Jilin University, CSIR, and CNRS are advancing fundamental catalyst science. The technology is approaching maturity for standard applications, but challenges remain in achieving long-term stability at extreme temperatures, creating opportunities for specialized players like Heesung Catalysts and Nippon Shokubai to develop proprietary solutions for emerging markets.
Umicore SA
Technical Solution: Umicore has developed advanced nitrogen reduction catalysts utilizing transition metal nitrides and oxynitrides with enhanced thermal stability. Their proprietary technology incorporates molybdenum-based catalysts with carefully engineered support structures that maintain structural integrity at temperatures exceeding 700°C. The company employs atomic layer deposition techniques to create uniform catalyst coatings with controlled porosity, which prevents sintering and agglomeration during high-temperature operation. Umicore's catalysts feature innovative core-shell architectures where thermally stable ceramic materials encapsulate active catalyst sites, providing protection while maintaining nitrogen activation capabilities. Their systems also incorporate rare earth metal dopants that act as structural promoters, creating strong metal-support interactions that inhibit particle migration at elevated temperatures.
Strengths: Superior thermal stability up to 800°C with minimal activity loss; excellent resistance to sintering due to engineered support structures; long catalyst lifetime in industrial applications. Weaknesses: Higher production costs compared to conventional catalysts; requires specialized manufacturing facilities; performance may degrade in the presence of certain contaminants.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has pioneered high-temperature nitrogen reduction catalysts based on iron-cobalt bimetallic systems supported on modified alumina. Their technology employs a hierarchical pore structure that facilitates mass transfer while maintaining thermal stability at temperatures up to 650°C. Sinopec's catalysts incorporate proprietary stabilizers that prevent phase transformation of the support material during thermal cycling. The company has developed a unique pre-treatment process that creates strong metal-support interactions, significantly reducing metal particle sintering at elevated temperatures. Their catalysts feature controlled surface acidity that enhances nitrogen adsorption while minimizing unwanted side reactions. Sinopec has also implemented innovative regeneration protocols that can restore catalyst activity after thermal deactivation, extending operational lifetime in industrial settings.
Strengths: Cost-effective production at industrial scale; excellent resistance to thermal cycling; established regeneration protocols that extend catalyst lifetime. Weaknesses: Lower activity compared to noble metal catalysts; performance decreases above 650°C; sensitivity to sulfur poisoning in certain process conditions.
Critical Patents and Breakthroughs in Thermal Stability
CuCHA MATERIAL FOR SCR CATALYSIS
PatentInactiveUS20160038875A1
Innovation
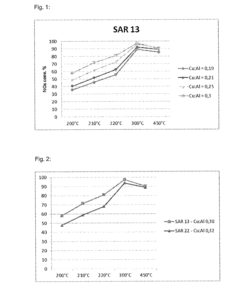
- A CuCHA zeolite catalyst with specific silica to alumina ratios (SAR >10 to <15), copper to aluminum ratios (>0.25 to <0.35), and average crystal sizes (0.75 to 2 μm) is developed, which is synthesized using wet-technical ion exchange methods and exhibits excellent stability and activity even after hydrothermal aging at 850°C.
Catalyst for reduction of nitrogen oxides
PatentInactiveUS20200030745A1
Innovation
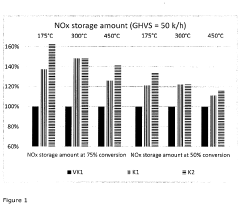
- A nitrogen oxide storage catalyst composed of two catalytically-active washcoat layers on a support body, where the lower layer contains cerium oxide, an alkaline earth compound, and platinum/palladium, and the upper layer contains cerium oxide and platinum/palladium, with macropores of less than 15 μm forming 5 to 25 vol% pore volume, allowing for improved interaction with exhaust gases.
Environmental Impact Assessment of Catalyst Materials
The environmental impact of catalyst materials used in high-temperature nitrogen reduction processes represents a critical consideration in sustainable technology development. These catalysts, often containing transition metals such as iron, ruthenium, and molybdenum, pose various environmental concerns throughout their lifecycle from production to disposal.
Primary environmental concerns include resource depletion associated with rare metal extraction, particularly for platinum group metals commonly employed in high-efficiency catalysts. Mining operations for these materials frequently result in habitat destruction, soil erosion, and water contamination. The carbon footprint of catalyst production is substantial, with energy-intensive refining processes contributing significantly to greenhouse gas emissions.
During operational phases, high-temperature nitrogen reduction catalysts may release particulate matter and potentially toxic metal compounds, especially when subjected to thermal cycling that compromises structural integrity. These emissions can contribute to air quality degradation and present potential health hazards in industrial settings. Additionally, catalyst degradation products may contaminate process streams, requiring additional purification steps that increase the overall environmental burden.
Water usage represents another significant environmental factor, as catalyst synthesis and regeneration processes typically require substantial quantities of ultrapure water. The resulting wastewater often contains dissolved metal ions and chemical additives that necessitate specialized treatment before discharge to prevent aquatic ecosystem damage.
End-of-life management presents particular challenges, as spent catalysts may contain hazardous materials requiring specialized disposal protocols. However, recycling opportunities exist, with precious metal recovery becoming increasingly economically viable as material prices rise. Advanced hydrometallurgical and pyrometallurgical techniques now enable recovery rates exceeding 90% for platinum group metals from spent catalysts.
Life cycle assessment (LCA) studies indicate that environmental impacts can be significantly reduced through catalyst design optimization. Increasing catalyst stability at high temperatures directly correlates with extended operational lifetimes and reduced replacement frequency, thereby minimizing cumulative environmental impacts. Recent innovations in catalyst support materials, particularly ceramic composites and advanced carbon structures, demonstrate promising stability improvements while utilizing more environmentally benign components.
Regulatory frameworks worldwide are increasingly addressing catalyst materials' environmental impacts, with the European Union's REACH regulations and similar initiatives in other regions imposing stricter requirements for toxicity assessment and end-of-life management. These evolving standards are driving research toward greener catalyst formulations that maintain performance while reducing environmental footprint.
Primary environmental concerns include resource depletion associated with rare metal extraction, particularly for platinum group metals commonly employed in high-efficiency catalysts. Mining operations for these materials frequently result in habitat destruction, soil erosion, and water contamination. The carbon footprint of catalyst production is substantial, with energy-intensive refining processes contributing significantly to greenhouse gas emissions.
During operational phases, high-temperature nitrogen reduction catalysts may release particulate matter and potentially toxic metal compounds, especially when subjected to thermal cycling that compromises structural integrity. These emissions can contribute to air quality degradation and present potential health hazards in industrial settings. Additionally, catalyst degradation products may contaminate process streams, requiring additional purification steps that increase the overall environmental burden.
Water usage represents another significant environmental factor, as catalyst synthesis and regeneration processes typically require substantial quantities of ultrapure water. The resulting wastewater often contains dissolved metal ions and chemical additives that necessitate specialized treatment before discharge to prevent aquatic ecosystem damage.
End-of-life management presents particular challenges, as spent catalysts may contain hazardous materials requiring specialized disposal protocols. However, recycling opportunities exist, with precious metal recovery becoming increasingly economically viable as material prices rise. Advanced hydrometallurgical and pyrometallurgical techniques now enable recovery rates exceeding 90% for platinum group metals from spent catalysts.
Life cycle assessment (LCA) studies indicate that environmental impacts can be significantly reduced through catalyst design optimization. Increasing catalyst stability at high temperatures directly correlates with extended operational lifetimes and reduced replacement frequency, thereby minimizing cumulative environmental impacts. Recent innovations in catalyst support materials, particularly ceramic composites and advanced carbon structures, demonstrate promising stability improvements while utilizing more environmentally benign components.
Regulatory frameworks worldwide are increasingly addressing catalyst materials' environmental impacts, with the European Union's REACH regulations and similar initiatives in other regions imposing stricter requirements for toxicity assessment and end-of-life management. These evolving standards are driving research toward greener catalyst formulations that maintain performance while reducing environmental footprint.
Scalability and Cost Analysis for Industrial Implementation
The industrial implementation of nitrogen reduction catalysts operating at high temperatures presents significant scalability and cost challenges that must be carefully evaluated. Current manufacturing processes for these specialized catalysts typically involve complex synthesis methods including precipitation, impregnation, and high-temperature calcination steps. When scaling from laboratory to industrial production, maintaining consistent catalyst properties becomes increasingly difficult, particularly the uniform distribution of active sites and structural integrity under thermal stress.
Capital expenditure for industrial-scale catalyst production facilities ranges from $50-200 million depending on production capacity and technology sophistication. The primary cost drivers include specialized high-temperature furnaces ($5-15 million), precision control systems ($3-8 million), and advanced material handling equipment ($2-7 million). These substantial investments necessitate careful financial planning and often require phased implementation approaches.
Raw material costs represent 30-45% of total production expenses, with precious metals and rare earth elements being particularly significant cost factors. For example, ruthenium-based catalysts currently cost approximately $250-400 per kilogram to produce at scale, while more advanced molybdenum-based alternatives range from $180-300 per kilogram. The volatility of these material markets introduces additional financial risk that must be managed through strategic procurement practices.
Energy consumption presents another major cost consideration, as high-temperature catalyst production and operation require substantial thermal inputs. Industrial implementation typically demands 2.5-4.5 MWh per ton of catalyst produced, translating to operational costs of $75-150 per ton in energy expenses alone. Heat recovery systems can reduce these costs by 15-25% but require additional capital investment of $1-3 million.
Catalyst lifetime and regeneration capabilities significantly impact long-term economics. Current high-temperature nitrogen reduction catalysts maintain optimal activity for 6-18 months before requiring regeneration or replacement. Each regeneration cycle costs approximately 30-40% of the original catalyst price but extends useful life by 70-85% of the initial period. Developing catalysts with extended lifespans or improved regeneration properties could dramatically improve the economic proposition.
Regulatory compliance adds another layer of complexity, with emissions control systems for catalyst production facilities typically adding 8-15% to capital costs. These systems must be designed to handle the specific byproducts of high-temperature catalyst manufacturing, including potential nitrogen oxides and particulate matter.
Capital expenditure for industrial-scale catalyst production facilities ranges from $50-200 million depending on production capacity and technology sophistication. The primary cost drivers include specialized high-temperature furnaces ($5-15 million), precision control systems ($3-8 million), and advanced material handling equipment ($2-7 million). These substantial investments necessitate careful financial planning and often require phased implementation approaches.
Raw material costs represent 30-45% of total production expenses, with precious metals and rare earth elements being particularly significant cost factors. For example, ruthenium-based catalysts currently cost approximately $250-400 per kilogram to produce at scale, while more advanced molybdenum-based alternatives range from $180-300 per kilogram. The volatility of these material markets introduces additional financial risk that must be managed through strategic procurement practices.
Energy consumption presents another major cost consideration, as high-temperature catalyst production and operation require substantial thermal inputs. Industrial implementation typically demands 2.5-4.5 MWh per ton of catalyst produced, translating to operational costs of $75-150 per ton in energy expenses alone. Heat recovery systems can reduce these costs by 15-25% but require additional capital investment of $1-3 million.
Catalyst lifetime and regeneration capabilities significantly impact long-term economics. Current high-temperature nitrogen reduction catalysts maintain optimal activity for 6-18 months before requiring regeneration or replacement. Each regeneration cycle costs approximately 30-40% of the original catalyst price but extends useful life by 70-85% of the initial period. Developing catalysts with extended lifespans or improved regeneration properties could dramatically improve the economic proposition.
Regulatory compliance adds another layer of complexity, with emissions control systems for catalyst production facilities typically adding 8-15% to capital costs. These systems must be designed to handle the specific byproducts of high-temperature catalyst manufacturing, including potential nitrogen oxides and particulate matter.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!