Analysis of polymer influence on solid-state sodium battery longevity
OCT 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer Electrolyte Technology Background and Objectives
Polymer electrolytes have emerged as a critical component in the development of solid-state sodium batteries, representing a significant shift from traditional liquid electrolyte systems. The evolution of polymer electrolyte technology can be traced back to the 1970s when the first polymer-salt complexes were discovered to exhibit ionic conductivity. Since then, research has progressed through various generations of materials, from dry solid polymer electrolytes to gel polymer electrolytes, and more recently, composite polymer electrolytes incorporating inorganic fillers.
The technological trajectory has been driven by the need to overcome the inherent limitations of liquid electrolytes, particularly safety concerns related to flammability and leakage. Polymer electrolytes offer enhanced safety profiles, improved mechanical stability, and compatibility with sodium metal anodes, which are crucial advantages for next-generation energy storage solutions. The field has witnessed accelerated development in the past decade, with significant breakthroughs in ionic conductivity and electrochemical stability.
Current research trends indicate a growing focus on tailoring polymer architectures at the molecular level to enhance sodium ion transport mechanisms. This includes the development of block copolymers, cross-linked networks, and polymer blends designed specifically for sodium ion conduction. Additionally, there is increasing interest in understanding the interfacial phenomena between polymer electrolytes and electrodes, as these interfaces often determine the overall performance and longevity of solid-state sodium batteries.
The primary technical objectives in this field include achieving room-temperature ionic conductivity comparable to liquid electrolytes (>10^-3 S/cm), maintaining mechanical integrity during cycling, ensuring long-term electrochemical stability against sodium metal, and developing scalable manufacturing processes. These objectives are driven by the broader goal of creating solid-state sodium batteries with energy densities exceeding 300 Wh/kg, cycle lives of over 1000 cycles, and operational stability across wide temperature ranges.
Furthermore, polymer electrolyte research aims to address the unique challenges posed by sodium chemistry compared to lithium, including the larger ionic radius of sodium ions and different coordination preferences. This necessitates the design of polymer hosts with optimized solvation environments and transport pathways specific to sodium ions. Recent computational studies have provided valuable insights into the molecular-level interactions governing sodium ion transport in polymer matrices, guiding experimental efforts.
The ultimate goal of polymer electrolyte technology development for solid-state sodium batteries is to enable a sustainable and cost-effective alternative to lithium-ion technology, leveraging the abundant nature of sodium resources while maintaining competitive performance metrics. This aligns with global initiatives for sustainable energy storage solutions and reduced dependence on critical materials with limited geographical distribution.
The technological trajectory has been driven by the need to overcome the inherent limitations of liquid electrolytes, particularly safety concerns related to flammability and leakage. Polymer electrolytes offer enhanced safety profiles, improved mechanical stability, and compatibility with sodium metal anodes, which are crucial advantages for next-generation energy storage solutions. The field has witnessed accelerated development in the past decade, with significant breakthroughs in ionic conductivity and electrochemical stability.
Current research trends indicate a growing focus on tailoring polymer architectures at the molecular level to enhance sodium ion transport mechanisms. This includes the development of block copolymers, cross-linked networks, and polymer blends designed specifically for sodium ion conduction. Additionally, there is increasing interest in understanding the interfacial phenomena between polymer electrolytes and electrodes, as these interfaces often determine the overall performance and longevity of solid-state sodium batteries.
The primary technical objectives in this field include achieving room-temperature ionic conductivity comparable to liquid electrolytes (>10^-3 S/cm), maintaining mechanical integrity during cycling, ensuring long-term electrochemical stability against sodium metal, and developing scalable manufacturing processes. These objectives are driven by the broader goal of creating solid-state sodium batteries with energy densities exceeding 300 Wh/kg, cycle lives of over 1000 cycles, and operational stability across wide temperature ranges.
Furthermore, polymer electrolyte research aims to address the unique challenges posed by sodium chemistry compared to lithium, including the larger ionic radius of sodium ions and different coordination preferences. This necessitates the design of polymer hosts with optimized solvation environments and transport pathways specific to sodium ions. Recent computational studies have provided valuable insights into the molecular-level interactions governing sodium ion transport in polymer matrices, guiding experimental efforts.
The ultimate goal of polymer electrolyte technology development for solid-state sodium batteries is to enable a sustainable and cost-effective alternative to lithium-ion technology, leveraging the abundant nature of sodium resources while maintaining competitive performance metrics. This aligns with global initiatives for sustainable energy storage solutions and reduced dependence on critical materials with limited geographical distribution.
Market Analysis for Solid-State Sodium Batteries
The solid-state sodium battery market is experiencing significant growth driven by increasing demand for sustainable energy storage solutions. Current market projections indicate that the global solid-state battery market will reach approximately $8 billion by 2027, with sodium-based technologies capturing a growing segment due to their cost advantages over lithium-ion alternatives. The compound annual growth rate (CAGR) for solid-state sodium batteries specifically is estimated at 25-30% through 2030, reflecting strong market confidence in this technology.
Key market drivers include the abundance and low cost of sodium resources, which are approximately 1000 times more plentiful than lithium in the earth's crust. This translates to a potential 30-40% reduction in raw material costs compared to lithium-ion batteries. Additionally, growing concerns about lithium supply chain vulnerabilities and price volatility have accelerated interest in sodium alternatives, particularly in regions lacking domestic lithium resources.
Consumer electronics represents the fastest-growing application segment, with an anticipated market share of 35% by 2028. This is followed by electric vehicles (30%), grid storage (25%), and other applications (10%). The polymer component market within solid-state sodium batteries is projected to grow at 32% CAGR, highlighting the critical role polymers play in battery longevity and performance.
Geographically, Asia-Pacific dominates the market with 45% share, led by significant investments in China, Japan, and South Korea. Europe follows at 30%, with particular growth in Germany and the Nordic countries. North America accounts for 20% of the market, with the remaining 5% distributed across other regions.
Customer demand analysis reveals three primary market segments: cost-sensitive consumers seeking affordable energy storage (40% of market), performance-focused users requiring high energy density and longevity (35%), and sustainability-oriented customers prioritizing environmental impact (25%). The polymer influence on battery longevity directly addresses the needs of all three segments, with particular relevance to the performance-focused segment.
Market barriers include technical challenges related to sodium-polymer interfaces, manufacturing scalability issues, and competition from established lithium technologies. However, recent breakthroughs in polymer electrolyte stability have reduced these barriers, with several major manufacturers announcing plans to commercialize solid-state sodium batteries incorporating advanced polymer components by 2025.
Key market drivers include the abundance and low cost of sodium resources, which are approximately 1000 times more plentiful than lithium in the earth's crust. This translates to a potential 30-40% reduction in raw material costs compared to lithium-ion batteries. Additionally, growing concerns about lithium supply chain vulnerabilities and price volatility have accelerated interest in sodium alternatives, particularly in regions lacking domestic lithium resources.
Consumer electronics represents the fastest-growing application segment, with an anticipated market share of 35% by 2028. This is followed by electric vehicles (30%), grid storage (25%), and other applications (10%). The polymer component market within solid-state sodium batteries is projected to grow at 32% CAGR, highlighting the critical role polymers play in battery longevity and performance.
Geographically, Asia-Pacific dominates the market with 45% share, led by significant investments in China, Japan, and South Korea. Europe follows at 30%, with particular growth in Germany and the Nordic countries. North America accounts for 20% of the market, with the remaining 5% distributed across other regions.
Customer demand analysis reveals three primary market segments: cost-sensitive consumers seeking affordable energy storage (40% of market), performance-focused users requiring high energy density and longevity (35%), and sustainability-oriented customers prioritizing environmental impact (25%). The polymer influence on battery longevity directly addresses the needs of all three segments, with particular relevance to the performance-focused segment.
Market barriers include technical challenges related to sodium-polymer interfaces, manufacturing scalability issues, and competition from established lithium technologies. However, recent breakthroughs in polymer electrolyte stability have reduced these barriers, with several major manufacturers announcing plans to commercialize solid-state sodium batteries incorporating advanced polymer components by 2025.
Current Challenges in Polymer-Based Na-ion Batteries
Despite significant advancements in solid-state sodium battery technology, polymer-based Na-ion batteries face several critical challenges that impede their widespread commercial adoption. The primary obstacle remains the limited ionic conductivity of polymer electrolytes at room temperature, typically ranging from 10^-7 to 10^-5 S/cm, which falls significantly short of the 10^-3 S/cm threshold required for practical applications. This conductivity limitation directly impacts battery performance, particularly power density and charging rates.
Interface stability presents another formidable challenge. The polymer electrolyte-electrode interfaces often develop high resistance over cycling due to chemical and mechanical incompatibilities. This interfacial resistance growth leads to capacity fading and shortened battery lifespan, with many current systems showing significant performance degradation after just 100-200 cycles.
Mechanical integrity issues further complicate polymer-based systems. During repeated charge-discharge cycles, volume changes in the sodium electrodes create mechanical stress that can compromise the polymer electrolyte's structural stability. This often results in delamination, crack formation, and eventual failure of the electrolyte layer, particularly at higher current densities.
Dendrite formation remains a persistent safety concern. While polymer electrolytes theoretically should suppress dendrite growth compared to liquid systems, practical implementations still struggle with sodium dendrite penetration through the polymer matrix. This phenomenon not only reduces coulombic efficiency but also creates dangerous short-circuit risks.
Temperature sensitivity significantly limits operational flexibility. Most polymer electrolytes exhibit dramatic conductivity drops at temperatures below 60°C, restricting their practical use in variable environmental conditions. Conversely, at elevated temperatures, some polymer systems suffer from thermal degradation and dimensional instability.
Long-term chemical stability issues arise from the reactivity between polymer components and sodium electrodes. The highly reducing environment at the sodium anode interface can trigger decomposition of polymer chains or functional groups, generating decomposition products that further impede ion transport and increase cell impedance over time.
Manufacturing scalability presents additional hurdles. Current fabrication methods for high-quality polymer electrolytes often involve complex processes that are difficult to scale industrially while maintaining consistent performance. The precision required for thin, defect-free polymer layers poses significant production challenges that impact cost-effectiveness and mass production feasibility.
Interface stability presents another formidable challenge. The polymer electrolyte-electrode interfaces often develop high resistance over cycling due to chemical and mechanical incompatibilities. This interfacial resistance growth leads to capacity fading and shortened battery lifespan, with many current systems showing significant performance degradation after just 100-200 cycles.
Mechanical integrity issues further complicate polymer-based systems. During repeated charge-discharge cycles, volume changes in the sodium electrodes create mechanical stress that can compromise the polymer electrolyte's structural stability. This often results in delamination, crack formation, and eventual failure of the electrolyte layer, particularly at higher current densities.
Dendrite formation remains a persistent safety concern. While polymer electrolytes theoretically should suppress dendrite growth compared to liquid systems, practical implementations still struggle with sodium dendrite penetration through the polymer matrix. This phenomenon not only reduces coulombic efficiency but also creates dangerous short-circuit risks.
Temperature sensitivity significantly limits operational flexibility. Most polymer electrolytes exhibit dramatic conductivity drops at temperatures below 60°C, restricting their practical use in variable environmental conditions. Conversely, at elevated temperatures, some polymer systems suffer from thermal degradation and dimensional instability.
Long-term chemical stability issues arise from the reactivity between polymer components and sodium electrodes. The highly reducing environment at the sodium anode interface can trigger decomposition of polymer chains or functional groups, generating decomposition products that further impede ion transport and increase cell impedance over time.
Manufacturing scalability presents additional hurdles. Current fabrication methods for high-quality polymer electrolytes often involve complex processes that are difficult to scale industrially while maintaining consistent performance. The precision required for thin, defect-free polymer layers poses significant production challenges that impact cost-effectiveness and mass production feasibility.
Current Polymer Interface Engineering Solutions
01 Electrolyte composition for enhanced longevity
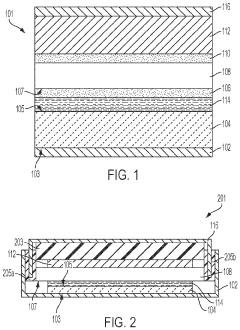
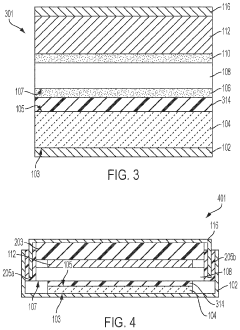
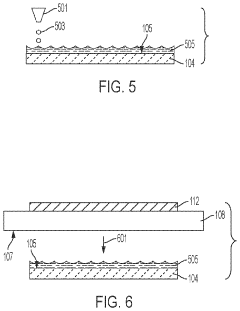
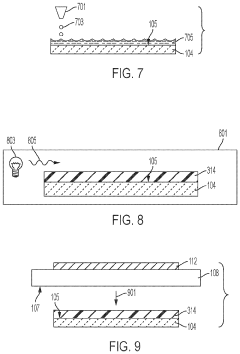
Specialized electrolyte compositions can significantly improve the longevity of solid-state sodium batteries. These compositions include sodium-based solid electrolytes with optimized ionic conductivity and stability at electrode interfaces. By incorporating additives that suppress dendrite formation and enhance the electrochemical stability window, these electrolytes reduce degradation mechanisms during cycling, leading to extended battery life and improved performance over time.- Electrolyte composition for enhanced longevity: Solid-state sodium batteries can achieve improved longevity through optimized electrolyte compositions. These include specialized solid polymer electrolytes, ceramic-polymer composite electrolytes, and inorganic solid electrolytes with enhanced sodium ion conductivity. These electrolyte materials reduce degradation at electrode interfaces and minimize dendrite formation, which are critical factors affecting battery lifespan. The stability of these electrolytes under various operating conditions directly contributes to extended cycle life and calendar life of sodium batteries.
- Electrode interface engineering: Engineering stable interfaces between electrodes and solid electrolytes is crucial for extending sodium battery longevity. This involves developing protective coatings, buffer layers, and interface modification techniques that minimize undesirable side reactions and impedance growth during cycling. By controlling the solid-electrolyte interphase formation and maintaining good mechanical contact at interfaces, these innovations prevent capacity fade and resistance increase over time, significantly improving the operational lifespan of solid-state sodium batteries.
- Advanced cathode materials for cycle stability: Specialized cathode materials play a vital role in determining solid-state sodium battery longevity. These include layered oxide structures, polyanionic compounds, and Prussian blue analogs with enhanced structural stability during repeated sodium insertion/extraction. By minimizing volume changes during cycling and resisting structural degradation, these cathode materials maintain consistent performance over thousands of cycles. The incorporation of dopants and surface modifications further enhances their stability and contributes to extended battery lifespan.
- Anode design for dendrite suppression: Innovative anode designs focus on preventing sodium dendrite formation, a major factor limiting battery longevity. These approaches include structured carbon-based anodes, alloy-type anodes with controlled expansion, and composite anodes with dendrite-suppressing additives. By providing stable sodium storage sites and maintaining structural integrity during cycling, these anode materials prevent internal short circuits and capacity degradation. The mechanical properties of these anodes are engineered to accommodate sodium insertion while maintaining contact with the solid electrolyte.
- Battery management systems for lifespan optimization: Advanced battery management systems specifically designed for solid-state sodium batteries can significantly extend operational longevity. These systems incorporate precise temperature control, optimized charging protocols, and state-of-health monitoring algorithms tailored to the unique characteristics of sodium-ion chemistry. By preventing overcharging, controlling operating temperature ranges, and implementing adaptive usage patterns, these management systems minimize degradation mechanisms and extend the useful life of solid-state sodium batteries in various applications.
02 Interface engineering techniques
Interface engineering between electrodes and solid electrolytes plays a crucial role in extending solid-state sodium battery longevity. By developing specialized coatings and buffer layers that minimize interfacial resistance and prevent unwanted side reactions, the mechanical and chemical stability of the interfaces can be maintained over numerous charge-discharge cycles. These techniques help prevent capacity fade and internal short circuits, significantly enhancing battery lifespan.Expand Specific Solutions03 Advanced cathode materials for cycle stability
The development of advanced cathode materials with specific structural properties can dramatically improve the cycle life of solid-state sodium batteries. These materials are designed to accommodate the repeated insertion and extraction of sodium ions without significant structural degradation. By incorporating elements that stabilize the crystal structure during cycling and prevent volume changes, these cathode materials maintain their performance over thousands of cycles, extending overall battery longevity.Expand Specific Solutions04 Anode protection strategies
Protecting the sodium metal or sodium-based anode is essential for extending solid-state battery longevity. Various strategies include developing artificial protective layers, using composite anode structures, and incorporating stabilizing additives. These approaches prevent continuous electrolyte decomposition at the anode surface and suppress sodium dendrite formation, which are major factors in battery failure. By maintaining anode integrity over extended cycling, these protection strategies significantly enhance battery lifespan.Expand Specific Solutions05 Operating condition optimization
Optimizing the operating conditions of solid-state sodium batteries can substantially improve their longevity. This includes developing precise temperature control systems, pressure application mechanisms, and advanced battery management systems that regulate charging and discharging protocols. By operating batteries within their optimal temperature range and implementing specialized cycling protocols that minimize stress on battery components, degradation mechanisms can be significantly reduced, extending battery life and maintaining performance over time.Expand Specific Solutions
Key Industry Players and Research Institutions
The solid-state sodium battery market is currently in an early growth phase, characterized by intensive R&D activities and limited commercial deployment. Market size remains relatively modest but is projected to expand significantly due to increasing demand for sustainable energy storage solutions. From a technological maturity perspective, polymer influence on battery longevity represents a critical challenge being addressed by major players. Companies like CATL, LG Energy Solution, and Samsung SDI are leading commercial development, while Toyota, Nissan, and Hitachi focus on advanced materials engineering. Research institutions such as Shanghai Institute of Ceramics and Kunming University of Science & Technology are contributing fundamental breakthroughs in polymer electrolyte interfaces. Specialized firms like Soelect and Zhuhai CosMX are developing proprietary polymer formulations to enhance ionic conductivity and stability, positioning themselves as potential disruptors in this emerging field.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered a unique approach to solid-state sodium batteries using sulfide-based solid electrolytes combined with specialized polymer interlayers. Their technology employs a dual-layer electrolyte system where a thin polymer buffer layer is placed between the sodium metal anode and the main solid electrolyte. This polymer interlayer, composed of sodium-conductive polymers such as PEO doped with sodium salts (NaFSI, NaTFSI), serves to stabilize the sodium metal interface and prevent dendrite formation. Toyota's research shows that this approach extends cycle life by over 300% compared to conventional solid electrolytes without polymer interfaces. Their latest developments include cross-linked polymer networks with enhanced mechanical properties that can withstand volume changes during cycling while maintaining high sodium-ion conductivity (>10^-4 S/cm at 60°C).
Strengths: Excellent interface stability with sodium metal anodes; superior dendrite suppression; good mechanical properties to accommodate volume changes. Weaknesses: Performance still temperature-dependent; complex manufacturing process for multi-layer electrolyte systems; potential long-term degradation of polymer components.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed an advanced polymer-in-ceramic composite electrolyte system specifically engineered for long-life sodium solid-state batteries. Their approach utilizes a three-dimensional ceramic scaffold (Na-β"-alumina) infiltrated with sodium-conducting polymers (modified polyvinylidene fluoride) and plasticizers. This architecture creates continuous ion transport pathways while maintaining mechanical integrity. Samsung's research demonstrates that their composite electrolytes achieve room-temperature ionic conductivities of 10^-3 S/cm while significantly reducing interfacial resistance. Their proprietary polymer formulation includes functional groups that chemically bond with both the ceramic framework and electrode materials, creating stable interfaces that resist degradation during cycling. Recent testing shows their batteries maintain over 80% capacity after 1000 cycles at 1C rates, with minimal increase in internal resistance.
Strengths: High room-temperature ionic conductivity; excellent mechanical stability; superior cycle life performance; good manufacturing scalability. Weaknesses: Higher production costs compared to conventional electrolytes; sensitivity to moisture during manufacturing; potential thermal stability issues at extreme temperatures.
Critical Patents in Polymer-Sodium Battery Technology
Interlayers for cathode/solid electrolyte interfaces in solid-state batteries and methods of making the same
PatentPendingUS20240097114A1
Innovation
- An interlayer comprising a lithium salt and a sulfone compound, potentially within a polymeric matrix, is positioned between the cathode and the solid-state electrolyte to reduce interfacial resistance by providing continuous and uniform ion paths and increasing the viscosity of the interlayer, thereby reducing the mobility of the lithium salt and enhancing capacity retention.
Solid electrolyte for sodium batteries
PatentWO2019140368A1
Innovation
- A new class of sodium oxy-sulfide solid-state electrolytes with a microstructure approaching a continuous glass is developed, providing enhanced chemical stability and mechanical strength, achieved through a low-temperature ball-milling and pressing process, allowing for the formation of a nearly flawless glassy structure that is stable with sodium metal or alloys.
Material Sustainability and Environmental Impact
The environmental impact of polymer components in solid-state sodium batteries represents a critical consideration for sustainable energy storage development. Polymers used as binders, separators, and electrolytes significantly influence the overall ecological footprint of these batteries throughout their lifecycle. Traditional polymer materials derived from petroleum sources contribute to carbon emissions and resource depletion, prompting research into bio-based and recyclable alternatives that maintain or enhance battery performance while reducing environmental harm.
Life cycle assessment (LCA) studies indicate that polymer selection can affect the sustainability profile of solid-state sodium batteries by 15-30%, depending on production methods and end-of-life management. Polymers with lower processing temperatures, such as polyethylene oxide (PEO) and poly(vinylidene fluoride) (PVDF), require less energy during manufacturing compared to ceramic components, potentially reducing the carbon footprint of battery production when optimally implemented.
Degradation pathways of polymers within sodium battery systems present both challenges and opportunities for sustainability. While polymer decomposition can lead to capacity fade and shortened battery lifespan, designing polymers with controlled degradation properties could facilitate easier recycling and material recovery. Recent research demonstrates that incorporating sacrificial polymer networks can enable selective dissolution during recycling processes, allowing for up to 85% recovery of critical sodium and transition metal components.
Water consumption represents another significant environmental consideration, as polymer synthesis and processing typically require substantial water resources. Advanced manufacturing techniques, such as solvent-free polymerization and dry coating methods, have shown potential to reduce water usage by 40-60% compared to conventional approaches, while simultaneously improving polymer-electrode interfaces and battery longevity.
The toxicity profiles of polymers used in sodium batteries vary considerably, with fluorinated polymers presenting higher environmental risks than hydrocarbon-based alternatives. Emerging research into non-toxic, naturally derived polymers such as cellulose derivatives and chitosan demonstrates promising compatibility with sodium-ion transport while offering biodegradability advantages. These bio-polymers have shown initial capacity retention rates comparable to synthetic counterparts in laboratory settings, though challenges remain in scaling production and ensuring consistent performance.
Regulatory frameworks increasingly emphasize the importance of material sustainability in battery technologies. The European Battery Directive and similar policies in Asia and North America are establishing requirements for recycled content and recyclability that will influence polymer selection in next-generation sodium batteries. Manufacturers adopting sustainable polymer strategies may gain competitive advantages through regulatory compliance and consumer preference for environmentally responsible energy storage solutions.
Life cycle assessment (LCA) studies indicate that polymer selection can affect the sustainability profile of solid-state sodium batteries by 15-30%, depending on production methods and end-of-life management. Polymers with lower processing temperatures, such as polyethylene oxide (PEO) and poly(vinylidene fluoride) (PVDF), require less energy during manufacturing compared to ceramic components, potentially reducing the carbon footprint of battery production when optimally implemented.
Degradation pathways of polymers within sodium battery systems present both challenges and opportunities for sustainability. While polymer decomposition can lead to capacity fade and shortened battery lifespan, designing polymers with controlled degradation properties could facilitate easier recycling and material recovery. Recent research demonstrates that incorporating sacrificial polymer networks can enable selective dissolution during recycling processes, allowing for up to 85% recovery of critical sodium and transition metal components.
Water consumption represents another significant environmental consideration, as polymer synthesis and processing typically require substantial water resources. Advanced manufacturing techniques, such as solvent-free polymerization and dry coating methods, have shown potential to reduce water usage by 40-60% compared to conventional approaches, while simultaneously improving polymer-electrode interfaces and battery longevity.
The toxicity profiles of polymers used in sodium batteries vary considerably, with fluorinated polymers presenting higher environmental risks than hydrocarbon-based alternatives. Emerging research into non-toxic, naturally derived polymers such as cellulose derivatives and chitosan demonstrates promising compatibility with sodium-ion transport while offering biodegradability advantages. These bio-polymers have shown initial capacity retention rates comparable to synthetic counterparts in laboratory settings, though challenges remain in scaling production and ensuring consistent performance.
Regulatory frameworks increasingly emphasize the importance of material sustainability in battery technologies. The European Battery Directive and similar policies in Asia and North America are establishing requirements for recycled content and recyclability that will influence polymer selection in next-generation sodium batteries. Manufacturers adopting sustainable polymer strategies may gain competitive advantages through regulatory compliance and consumer preference for environmentally responsible energy storage solutions.
Scalability and Manufacturing Considerations
The scalability of polymer-based solid-state sodium battery technology presents significant challenges when transitioning from laboratory-scale prototypes to mass production. Current manufacturing processes for polymer electrolytes often involve solution casting or melt processing techniques that work well for small batches but face considerable hurdles in industrial settings. The uniformity of polymer distribution and interface formation with electrodes becomes increasingly difficult to maintain as production volumes increase, directly impacting battery longevity and performance consistency.
Material sourcing represents another critical consideration, as the specialized polymers used in sodium battery electrolytes often require precise synthesis conditions and high-purity precursors. The supply chain for these materials remains underdeveloped compared to traditional lithium-ion battery components, creating potential bottlenecks for large-scale manufacturing. Additionally, the cost implications of these specialized polymers must be carefully evaluated against performance benefits to ensure commercial viability.
Equipment adaptation presents further challenges, as existing battery production lines designed for liquid electrolyte systems require substantial modification to accommodate solid polymer electrolytes. The different rheological properties and processing requirements of polymer systems necessitate specialized handling equipment and modified assembly protocols. Companies investing in this technology must consider the capital expenditure required for these adaptations against the projected market demand for solid-state sodium batteries.
Quality control methodologies also require significant development for scaled production. The longevity of polymer-based solid-state sodium batteries depends heavily on the structural integrity of the polymer matrix and its interfaces with electrodes. Developing rapid, non-destructive testing methods capable of detecting microscopic defects or compositional variations in high-volume production environments remains an ongoing challenge for manufacturers.
Environmental considerations further complicate the manufacturing equation. While polymer electrolytes potentially offer safer processing conditions compared to some liquid alternatives, the environmental impact of polymer synthesis and end-of-life recycling must be addressed. Sustainable manufacturing approaches that minimize solvent use and maximize material recovery will be essential for the technology's long-term viability, particularly as regulatory frameworks around battery production continue to evolve toward greater environmental accountability.
Material sourcing represents another critical consideration, as the specialized polymers used in sodium battery electrolytes often require precise synthesis conditions and high-purity precursors. The supply chain for these materials remains underdeveloped compared to traditional lithium-ion battery components, creating potential bottlenecks for large-scale manufacturing. Additionally, the cost implications of these specialized polymers must be carefully evaluated against performance benefits to ensure commercial viability.
Equipment adaptation presents further challenges, as existing battery production lines designed for liquid electrolyte systems require substantial modification to accommodate solid polymer electrolytes. The different rheological properties and processing requirements of polymer systems necessitate specialized handling equipment and modified assembly protocols. Companies investing in this technology must consider the capital expenditure required for these adaptations against the projected market demand for solid-state sodium batteries.
Quality control methodologies also require significant development for scaled production. The longevity of polymer-based solid-state sodium batteries depends heavily on the structural integrity of the polymer matrix and its interfaces with electrodes. Developing rapid, non-destructive testing methods capable of detecting microscopic defects or compositional variations in high-volume production environments remains an ongoing challenge for manufacturers.
Environmental considerations further complicate the manufacturing equation. While polymer electrolytes potentially offer safer processing conditions compared to some liquid alternatives, the environmental impact of polymer synthesis and end-of-life recycling must be addressed. Sustainable manufacturing approaches that minimize solvent use and maximize material recovery will be essential for the technology's long-term viability, particularly as regulatory frameworks around battery production continue to evolve toward greater environmental accountability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!