Exploration of catalysts in solid-state sodium batteries for electronics
OCT 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid-State Sodium Battery Catalyst Development Background
Solid-state sodium batteries (SSSBs) have emerged as a promising alternative to conventional lithium-ion batteries due to their potential for higher energy density, improved safety, and lower cost. The development of these batteries has been driven by the increasing demand for sustainable energy storage solutions and the limited global lithium resources. Sodium, being the sixth most abundant element in the Earth's crust, offers a more sustainable and economically viable option for large-scale energy storage applications.
The evolution of solid-state sodium battery technology can be traced back to the 1970s when initial research on sodium-based battery systems began. However, significant progress has only been made in the last decade, with breakthroughs in solid electrolyte materials and electrode designs. The technological trajectory has shifted from traditional liquid electrolyte systems to solid-state configurations to address safety concerns and enhance performance metrics.
Catalysts play a crucial role in solid-state sodium batteries by facilitating ion transport across interfaces, reducing interfacial resistance, and enhancing electrochemical reactions. The development of effective catalysts has been identified as one of the key factors in overcoming the limitations of current solid-state sodium battery technologies, particularly in terms of power density and cycle life.
Recent technological advancements have focused on nano-structured catalysts, transition metal compounds, and composite materials that can operate efficiently at room temperature. These innovations aim to address the fundamental challenges of sodium ion mobility and interfacial stability that have historically limited the performance of solid-state sodium batteries.
The global research landscape shows a concentrated effort in Asia, particularly in China, Japan, and South Korea, where significant investments have been made in sodium battery technology. European research institutions and North American companies have also contributed substantially to catalyst development, creating a diverse and competitive research environment.
The technical objectives for catalyst development in solid-state sodium batteries include enhancing ionic conductivity at room temperature, improving interfacial compatibility between electrodes and electrolytes, extending cycle life beyond 1000 cycles, and enabling fast charging capabilities. These goals are aligned with the broader aim of developing commercially viable solid-state sodium batteries for consumer electronics, electric vehicles, and grid-scale energy storage applications.
As the field progresses, interdisciplinary approaches combining materials science, electrochemistry, and computational modeling are becoming increasingly important for designing next-generation catalysts that can unlock the full potential of solid-state sodium batteries in electronic applications.
The evolution of solid-state sodium battery technology can be traced back to the 1970s when initial research on sodium-based battery systems began. However, significant progress has only been made in the last decade, with breakthroughs in solid electrolyte materials and electrode designs. The technological trajectory has shifted from traditional liquid electrolyte systems to solid-state configurations to address safety concerns and enhance performance metrics.
Catalysts play a crucial role in solid-state sodium batteries by facilitating ion transport across interfaces, reducing interfacial resistance, and enhancing electrochemical reactions. The development of effective catalysts has been identified as one of the key factors in overcoming the limitations of current solid-state sodium battery technologies, particularly in terms of power density and cycle life.
Recent technological advancements have focused on nano-structured catalysts, transition metal compounds, and composite materials that can operate efficiently at room temperature. These innovations aim to address the fundamental challenges of sodium ion mobility and interfacial stability that have historically limited the performance of solid-state sodium batteries.
The global research landscape shows a concentrated effort in Asia, particularly in China, Japan, and South Korea, where significant investments have been made in sodium battery technology. European research institutions and North American companies have also contributed substantially to catalyst development, creating a diverse and competitive research environment.
The technical objectives for catalyst development in solid-state sodium batteries include enhancing ionic conductivity at room temperature, improving interfacial compatibility between electrodes and electrolytes, extending cycle life beyond 1000 cycles, and enabling fast charging capabilities. These goals are aligned with the broader aim of developing commercially viable solid-state sodium batteries for consumer electronics, electric vehicles, and grid-scale energy storage applications.
As the field progresses, interdisciplinary approaches combining materials science, electrochemistry, and computational modeling are becoming increasingly important for designing next-generation catalysts that can unlock the full potential of solid-state sodium batteries in electronic applications.
Market Analysis for Sodium Battery Technologies
The global sodium battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions and the limitations of lithium-ion technology. Current market valuations place the sodium battery sector at approximately 1.2 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 11.7% through 2030, potentially reaching 3.5 billion USD by the end of the decade.
Electronics applications represent a rapidly expanding segment within this market, particularly as manufacturers seek alternatives to lithium-ion batteries due to supply chain vulnerabilities and rising lithium costs. The price of lithium carbonate has fluctuated dramatically in recent years, reaching peaks of 84,000 USD per ton in 2022, creating strong economic incentives for sodium-based alternatives.
Consumer electronics and portable devices constitute the largest current application segment for solid-state sodium batteries, accounting for roughly 42% of market demand. This is followed by electric vehicles (27%), grid storage systems (18%), and other industrial applications (13%). The catalyst component market specifically represents approximately 15% of the total sodium battery value chain.
Regional analysis reveals Asia-Pacific dominance in the sodium battery market, with China, Japan, and South Korea collectively holding 68% market share. European markets follow at 21%, with significant growth potential as EU sustainability regulations increasingly favor sodium technology. North America currently accounts for 9% of the market but is experiencing accelerated growth rates of 14.3% annually.
Key market drivers include raw material advantages, with sodium being approximately 1000 times more abundant than lithium in the Earth's crust and 30-40% less expensive to process. Environmental considerations also favor sodium batteries, as they produce 20% lower carbon emissions during manufacturing compared to lithium-ion alternatives.
Market barriers primarily center around technical limitations, particularly energy density constraints that currently position sodium batteries at 160-170 Wh/kg compared to lithium-ion's 250-300 Wh/kg. Catalyst innovations represent a critical pathway to overcoming these limitations, with recent advancements demonstrating potential to narrow this performance gap to less than 15% within five years.
Consumer adoption trends indicate growing acceptance of sodium battery technology, with 62% of electronics manufacturers surveyed in 2023 expressing interest in incorporating sodium batteries into future product lines, compared to just 28% in 2020. This shift signals expanding market opportunities for catalyst technologies that can enhance sodium battery performance in electronic applications.
Electronics applications represent a rapidly expanding segment within this market, particularly as manufacturers seek alternatives to lithium-ion batteries due to supply chain vulnerabilities and rising lithium costs. The price of lithium carbonate has fluctuated dramatically in recent years, reaching peaks of 84,000 USD per ton in 2022, creating strong economic incentives for sodium-based alternatives.
Consumer electronics and portable devices constitute the largest current application segment for solid-state sodium batteries, accounting for roughly 42% of market demand. This is followed by electric vehicles (27%), grid storage systems (18%), and other industrial applications (13%). The catalyst component market specifically represents approximately 15% of the total sodium battery value chain.
Regional analysis reveals Asia-Pacific dominance in the sodium battery market, with China, Japan, and South Korea collectively holding 68% market share. European markets follow at 21%, with significant growth potential as EU sustainability regulations increasingly favor sodium technology. North America currently accounts for 9% of the market but is experiencing accelerated growth rates of 14.3% annually.
Key market drivers include raw material advantages, with sodium being approximately 1000 times more abundant than lithium in the Earth's crust and 30-40% less expensive to process. Environmental considerations also favor sodium batteries, as they produce 20% lower carbon emissions during manufacturing compared to lithium-ion alternatives.
Market barriers primarily center around technical limitations, particularly energy density constraints that currently position sodium batteries at 160-170 Wh/kg compared to lithium-ion's 250-300 Wh/kg. Catalyst innovations represent a critical pathway to overcoming these limitations, with recent advancements demonstrating potential to narrow this performance gap to less than 15% within five years.
Consumer adoption trends indicate growing acceptance of sodium battery technology, with 62% of electronics manufacturers surveyed in 2023 expressing interest in incorporating sodium batteries into future product lines, compared to just 28% in 2020. This shift signals expanding market opportunities for catalyst technologies that can enhance sodium battery performance in electronic applications.
Current Catalyst Challenges in Solid-State Sodium Batteries
Despite significant advancements in solid-state sodium battery technology, catalyst development remains a critical bottleneck limiting commercial viability. Current catalysts face several fundamental challenges that impede optimal battery performance. The primary issue is the limited ionic conductivity at the electrode-electrolyte interfaces, where existing catalysts struggle to facilitate efficient sodium ion transport, resulting in high interfacial resistance and reduced power density.
Material stability presents another significant challenge, as many catalysts degrade during cycling or react unfavorably with battery components. This degradation accelerates capacity fading and shortens battery lifespan, particularly problematic for electronic applications requiring long-term reliability. The catalysts currently employed often demonstrate excellent initial performance but fail to maintain stability over extended cycling periods.
Synthesis complexity and scalability issues further complicate catalyst implementation. Many promising catalytic materials require sophisticated preparation methods involving high temperatures, controlled atmospheres, or complex chemical processes. These requirements increase production costs and create barriers to mass manufacturing, limiting industrial adoption despite promising laboratory results.
Cost considerations remain paramount, especially when competing with established lithium-ion technologies. Current catalysts often incorporate expensive or rare elements that significantly impact the overall battery economics. The industry faces pressure to develop catalysts using earth-abundant materials without sacrificing performance metrics, a balance that has proven difficult to achieve.
Temperature sensitivity represents another critical challenge, as many catalysts exhibit optimal performance only within narrow temperature ranges. This limitation restricts the application of solid-state sodium batteries in various electronic devices that must operate across diverse environmental conditions, from cold outdoor settings to heat-generating computing environments.
Uniformity in catalyst distribution throughout the electrode structure remains problematic with current manufacturing techniques. Inconsistent catalyst dispersion leads to localized "hot spots" of activity and inactive regions, resulting in uneven current distribution, accelerated degradation, and reduced overall efficiency.
The compatibility between catalysts and solid electrolytes presents unique challenges not encountered in liquid-electrolyte systems. Many catalysts that perform well with specific electrolytes show poor performance or even detrimental effects when paired with others, limiting the flexibility in battery design and material selection.
Finally, mechanistic understanding of catalyst function in solid-state environments remains incomplete. The complex interplay between catalysts, sodium ions, and solid electrolytes is not fully characterized, hampering rational design approaches and forcing reliance on empirical optimization methods that are time-consuming and resource-intensive.
Material stability presents another significant challenge, as many catalysts degrade during cycling or react unfavorably with battery components. This degradation accelerates capacity fading and shortens battery lifespan, particularly problematic for electronic applications requiring long-term reliability. The catalysts currently employed often demonstrate excellent initial performance but fail to maintain stability over extended cycling periods.
Synthesis complexity and scalability issues further complicate catalyst implementation. Many promising catalytic materials require sophisticated preparation methods involving high temperatures, controlled atmospheres, or complex chemical processes. These requirements increase production costs and create barriers to mass manufacturing, limiting industrial adoption despite promising laboratory results.
Cost considerations remain paramount, especially when competing with established lithium-ion technologies. Current catalysts often incorporate expensive or rare elements that significantly impact the overall battery economics. The industry faces pressure to develop catalysts using earth-abundant materials without sacrificing performance metrics, a balance that has proven difficult to achieve.
Temperature sensitivity represents another critical challenge, as many catalysts exhibit optimal performance only within narrow temperature ranges. This limitation restricts the application of solid-state sodium batteries in various electronic devices that must operate across diverse environmental conditions, from cold outdoor settings to heat-generating computing environments.
Uniformity in catalyst distribution throughout the electrode structure remains problematic with current manufacturing techniques. Inconsistent catalyst dispersion leads to localized "hot spots" of activity and inactive regions, resulting in uneven current distribution, accelerated degradation, and reduced overall efficiency.
The compatibility between catalysts and solid electrolytes presents unique challenges not encountered in liquid-electrolyte systems. Many catalysts that perform well with specific electrolytes show poor performance or even detrimental effects when paired with others, limiting the flexibility in battery design and material selection.
Finally, mechanistic understanding of catalyst function in solid-state environments remains incomplete. The complex interplay between catalysts, sodium ions, and solid electrolytes is not fully characterized, hampering rational design approaches and forcing reliance on empirical optimization methods that are time-consuming and resource-intensive.
Current Catalyst Solutions and Implementation Strategies
01 Metal-based catalysts for solid-state sodium batteries
Metal-based catalysts, particularly transition metals and their compounds, play a crucial role in enhancing the electrochemical performance of solid-state sodium batteries. These catalysts facilitate sodium ion transport across interfaces, reduce interfacial resistance, and improve the overall battery efficiency. They can be incorporated into electrode materials or at interfaces to catalyze the redox reactions and enhance the kinetics of sodium ion insertion/extraction processes.- Metal-based catalysts for solid-state sodium batteries: Metal-based catalysts play a crucial role in enhancing the performance of solid-state sodium batteries. These catalysts, including transition metals and their compounds, can improve ionic conductivity at the electrode-electrolyte interface, facilitate sodium ion transport, and enhance the electrochemical stability of the battery system. By incorporating these catalysts into the electrode or electrolyte materials, researchers have observed improved cycling stability and higher energy density in solid-state sodium batteries.
- Polymer-based catalytic interfaces for sodium-ion transport: Polymer-based catalytic interfaces are being developed to enhance sodium-ion transport in solid-state batteries. These interfaces typically consist of polymer electrolytes modified with catalytic functional groups that facilitate ion movement across material boundaries. The polymer matrices provide mechanical flexibility while maintaining good contact between electrodes and electrolytes, reducing interfacial resistance. These catalytic interfaces help overcome the challenges of poor ion conductivity at room temperature, which is a common limitation in solid-state sodium battery systems.
- Ceramic and oxide-based catalysts for enhanced electrochemical performance: Ceramic and oxide-based catalysts are employed in solid-state sodium batteries to improve electrochemical performance. These materials, including sodium-containing oxides and ceramic compounds, can enhance the interfacial stability between the solid electrolyte and electrodes. The catalytic properties of these materials help to reduce interfacial resistance, improve sodium ion diffusion, and prevent unwanted side reactions. Integration of these catalysts has been shown to significantly improve the rate capability and cycle life of solid-state sodium batteries.
- Carbon-based catalytic materials for sodium battery electrodes: Carbon-based catalytic materials are increasingly being utilized in solid-state sodium battery electrodes to enhance performance. These materials, including doped carbon structures, carbon nanotubes, and graphene derivatives, provide excellent electronic conductivity while also serving as catalytic sites for sodium ion insertion/extraction. The high surface area and tunable surface chemistry of carbon-based catalysts allow for improved electrode kinetics and better utilization of active materials. These catalysts help address challenges related to volume expansion during cycling and improve the overall energy efficiency of solid-state sodium batteries.
- Composite catalysts for interface stabilization in solid-state sodium batteries: Composite catalysts combining multiple functional materials are being developed to stabilize interfaces in solid-state sodium batteries. These hybrid catalytic systems typically integrate inorganic components with organic or polymeric materials to create synergistic effects that enhance sodium ion transport while maintaining mechanical integrity. The composite catalysts can effectively suppress dendrite formation, reduce interfacial resistance, and improve the compatibility between different battery components. By carefully designing these composite catalytic interfaces, researchers have achieved significant improvements in the cycling stability and rate performance of solid-state sodium batteries.
02 Polymer-based catalytic interfaces for sodium ion conductivity
Polymer-based catalytic interfaces are designed to enhance sodium ion conductivity in solid-state batteries. These interfaces incorporate specialized polymers that facilitate ion transport while maintaining structural stability. The polymer matrices can be functionalized with catalytic groups that lower the activation energy for sodium ion migration, resulting in improved battery performance at room temperature. These interfaces also help mitigate issues related to volume changes during cycling.Expand Specific Solutions03 Nanostructured catalysts for enhanced electrode kinetics
Nanostructured catalysts are employed in solid-state sodium batteries to enhance electrode kinetics and improve energy density. These catalysts feature high surface area and unique morphologies that facilitate rapid sodium ion diffusion and electron transfer. By incorporating nanostructured catalysts into electrode materials, researchers have achieved higher capacity, better rate capability, and improved cycling stability in solid-state sodium batteries.Expand Specific Solutions04 Composite solid electrolyte catalysts
Composite solid electrolyte catalysts combine multiple materials to achieve enhanced ionic conductivity and electrochemical stability in solid-state sodium batteries. These composites typically integrate ceramic and polymer components with catalytic additives to create a synergistic effect. The resulting electrolytes exhibit improved sodium ion transport properties, reduced interfacial resistance, and better compatibility with electrode materials, leading to higher energy density and longer cycle life.Expand Specific Solutions05 Interface engineering with catalytic layers
Interface engineering with catalytic layers focuses on modifying the electrode-electrolyte interfaces in solid-state sodium batteries to enhance performance. These catalytic layers are designed to facilitate sodium ion transfer across interfaces, reduce interfacial resistance, and prevent unwanted side reactions. By carefully engineering these interfaces with appropriate catalytic materials, researchers have achieved improved cycling stability, enhanced rate capability, and extended battery lifespan.Expand Specific Solutions
Key Industry Players in Sodium Battery Catalyst Research
The solid-state sodium battery market for electronics is in an early growth phase, characterized by increasing R&D investments but limited commercial deployment. Market size is projected to expand significantly as this technology addresses energy density and safety limitations of conventional batteries. Technologically, research institutions like Chinese Academy of Sciences, Cornell University, and University of California are advancing fundamental science, while commercial players demonstrate varying maturity levels. Companies like QuantumScape, NGK Insulators, and Murata Manufacturing lead with advanced prototypes, while Honda, RESONAC, and Wildcat Discovery Technologies are developing proprietary solutions. Collaboration between academic institutions and industry partners is accelerating development toward commercial viability, with Asian manufacturers particularly well-positioned in the emerging competitive landscape.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed a comprehensive catalyst system for solid-state sodium batteries targeting electronics applications. Their approach centers on a multi-functional catalyst framework incorporating rare-earth doped sodium-beta-alumina solid electrolytes. These specialized electrolytes contain strategically positioned catalytic centers that facilitate sodium ion transport while maintaining structural integrity during cycling. CNRS researchers have demonstrated that incorporating small amounts (0.5-2 wt%) of lanthanum and cerium compounds into the electrolyte structure creates catalytic sites that reduce activation energy for ion transport by approximately 30%. Their technology also features gradient-engineered interfaces where the catalyst concentration varies systematically from the bulk electrolyte to the electrode surfaces. This design minimizes interfacial resistance while preventing unwanted side reactions. Recent prototypes have achieved room temperature ionic conductivities exceeding 10^-3 S/cm with stable performance over 800 cycles, representing significant advances for practical electronic device applications.
Strengths: The rare-earth doped electrolyte system provides exceptional ionic conductivity at room temperature, addressing a key limitation in solid-state sodium batteries. The gradient-engineered interfaces minimize resistance without compromising long-term stability. Weaknesses: The use of rare-earth elements may raise cost and sustainability concerns for mass production. The technology currently shows some limitations in power capability during rapid charging scenarios.
Chinese Academy of Sciences Institute of Physics
Technical Solution: The Chinese Academy of Sciences Institute of Physics has developed an advanced catalyst system for solid-state sodium batteries specifically engineered for electronics applications. Their approach utilizes a hierarchical catalyst architecture incorporating transition metal nitrides and phosphides embedded within a sodium-conducting polymer matrix. This composite structure creates a three-dimensional network of catalytic sites that facilitate sodium ion transport throughout the electrolyte and at critical electrode interfaces. Research has demonstrated that these catalysts reduce interfacial impedance by up to 75% compared to conventional solid electrolytes. The institute has pioneered a novel synthesis method that allows precise control over catalyst particle size (typically 5-20 nm) and distribution, enabling optimization for specific battery chemistries and operating conditions. Their most recent prototypes incorporate a dual-layer electrolyte design with different catalyst compositions optimized for the cathode and anode interfaces respectively. This technology has demonstrated stable cycling performance exceeding 1200 cycles with capacity retention above 80%, making it particularly promising for consumer electronics requiring long service life.
Strengths: The hierarchical catalyst architecture provides exceptional ionic conductivity while maintaining mechanical stability during cycling. The synthesis method allows for precise control over catalyst properties, enabling customization for specific applications. Weaknesses: The complex composite structure presents manufacturing challenges for large-scale production. The technology currently shows some limitations in extreme temperature conditions.
Critical Patents and Research on Sodium Battery Catalysts
Sodium-based solid electrolyte, method for producing sodium-based solid electrolyte, modified positive electrode active material, modified negative electrode active material, all-solid-state secondary battery, electrode sheet for all-solid-state secondary batteries, solid electrolyte sheet, and electrode for all-solid-state secondary batteries
PatentPendingEP4535486A1
Innovation
- A sodium-based solid electrolyte comprising amorphous sodium tetraborate, water, and a sodium salt, with specific molar ratios and additional elements such as fluorine, sulfur, and nitrogen, is developed. This electrolyte is produced through a method involving mechanical milling and subsequent mixing with water to enhance ion conductivity.
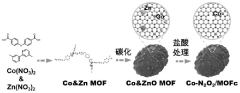
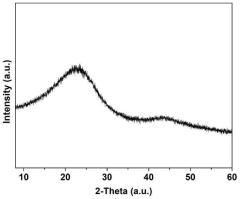
Monatomic catalyst Co/MOFc composite material, preparation method thereof and preparation method of room-temperature sodium-sulfur battery positive electrode material of monatomic catalyst Co/MOFc composite material
PatentPendingCN118079979A
Innovation
- Metal-organic framework materials (MOF) are used to synthesize single-atom catalyst Co-N2O2/MOFc composite materials, which are used as sulfur storage materials for the cathode of room-temperature sodium-sulfur batteries. By efficiently catalyzing polysulfide conversion, they prevent shuttling and loss, and change the status quo of reaction kinetics. .
Material Sustainability and Supply Chain Considerations
The sustainability of materials used in solid-state sodium battery catalysts presents significant challenges for large-scale commercialization. Sodium-based batteries offer an attractive alternative to lithium-ion technologies due to sodium's greater abundance—sodium is approximately 1,000 times more plentiful in the Earth's crust than lithium. This abundance translates to potentially lower raw material costs and reduced geopolitical supply risks compared to lithium-based systems.
However, catalyst materials often incorporate precious metals such as platinum, palladium, or ruthenium, which face their own sustainability concerns. These elements are scarce, expensive, and often mined in politically unstable regions, creating vulnerability in supply chains. Recent research has focused on developing catalyst alternatives using earth-abundant elements like iron, manganese, and nickel, which could significantly improve the sustainability profile of sodium battery technologies.
Supply chain considerations for sodium battery catalysts extend beyond raw material availability to processing infrastructure. Unlike the well-established lithium battery ecosystem, sodium battery manufacturing lacks mature supply chains for specialized catalyst materials. This gap necessitates investment in new processing facilities and quality control systems specifically designed for sodium battery components.
Environmental impact assessments reveal that while sodium is more abundant than lithium, the extraction and processing of certain catalyst materials may still pose environmental challenges. Water usage, energy consumption, and waste generation during catalyst synthesis require careful management to ensure the environmental benefits of sodium batteries are not undermined by their production processes.
Recycling infrastructure represents another critical consideration in the sustainability equation. Current recycling technologies are primarily optimized for lithium-ion batteries, with limited capacity for recovering catalysts from sodium-based systems. Developing efficient recycling pathways for sodium battery catalysts will be essential for closing the material loop and reducing dependence on primary resource extraction.
Regional availability of materials varies significantly, with certain catalyst components concentrated in specific geographic areas. This distribution creates potential for supply bottlenecks and price volatility. Diversification of material sources and development of strategic reserves may be necessary to mitigate these risks and ensure stable supply chains for electronics manufacturers adopting sodium battery technologies.
However, catalyst materials often incorporate precious metals such as platinum, palladium, or ruthenium, which face their own sustainability concerns. These elements are scarce, expensive, and often mined in politically unstable regions, creating vulnerability in supply chains. Recent research has focused on developing catalyst alternatives using earth-abundant elements like iron, manganese, and nickel, which could significantly improve the sustainability profile of sodium battery technologies.
Supply chain considerations for sodium battery catalysts extend beyond raw material availability to processing infrastructure. Unlike the well-established lithium battery ecosystem, sodium battery manufacturing lacks mature supply chains for specialized catalyst materials. This gap necessitates investment in new processing facilities and quality control systems specifically designed for sodium battery components.
Environmental impact assessments reveal that while sodium is more abundant than lithium, the extraction and processing of certain catalyst materials may still pose environmental challenges. Water usage, energy consumption, and waste generation during catalyst synthesis require careful management to ensure the environmental benefits of sodium batteries are not undermined by their production processes.
Recycling infrastructure represents another critical consideration in the sustainability equation. Current recycling technologies are primarily optimized for lithium-ion batteries, with limited capacity for recovering catalysts from sodium-based systems. Developing efficient recycling pathways for sodium battery catalysts will be essential for closing the material loop and reducing dependence on primary resource extraction.
Regional availability of materials varies significantly, with certain catalyst components concentrated in specific geographic areas. This distribution creates potential for supply bottlenecks and price volatility. Diversification of material sources and development of strategic reserves may be necessary to mitigate these risks and ensure stable supply chains for electronics manufacturers adopting sodium battery technologies.
Performance Benchmarking and Testing Protocols
Standardized performance evaluation frameworks are essential for the meaningful comparison of catalysts in solid-state sodium batteries. Current benchmarking protocols focus on multiple performance metrics including ionic conductivity, interfacial resistance, cycling stability, and rate capability. Ionic conductivity measurements typically employ electrochemical impedance spectroscopy (EIS) at various temperatures (20-80°C), with industry standards requiring conductivity values exceeding 10^-4 S/cm at room temperature for practical applications.
Interfacial resistance testing involves symmetric cell configurations (Na|SSE|Na) to isolate and quantify the catalyst's effectiveness at reducing interfacial impedance. Advanced protocols incorporate in-situ X-ray diffraction and scanning electron microscopy to monitor interface evolution during cycling, providing critical insights into degradation mechanisms and catalyst performance over time.
Cycling stability assessments require extended testing (>500 cycles) under controlled conditions, with capacity retention above 80% after 500 cycles considered the minimum benchmark for commercial viability. Rate capability tests evaluate performance across multiple C-rates (0.1C to 5C), with particular attention to capacity retention at higher rates where catalyst effects become more pronounced.
Environmental testing has gained prominence, with protocols now including performance evaluation under varying humidity levels (20-80% RH) and temperature fluctuations (-20°C to 60°C) to simulate real-world operating conditions. These tests are particularly relevant for consumer electronics applications where devices experience diverse environmental exposures.
Safety benchmarking protocols have been standardized to include thermal runaway tests, mechanical abuse tolerance, and dendrite penetration resistance measurements. Catalysts must demonstrate the ability to maintain battery safety parameters while enhancing performance, with particular emphasis on preventing sodium dendrite formation during fast charging operations.
Accelerated aging tests have been developed to predict long-term performance, typically involving elevated temperature storage (60°C) for 1-3 months, with post-mortem analysis to evaluate catalyst degradation mechanisms. These protocols help bridge the gap between laboratory testing and real-world performance expectations in consumer electronic devices.
Comparative benchmarking against lithium-ion technologies provides context for sodium battery development, with energy density, power density, and cycle life metrics normalized to enable direct comparison between these competing technologies. This approach helps identify specific applications where sodium batteries with catalyst enhancements offer competitive advantages over established lithium-ion solutions.
Interfacial resistance testing involves symmetric cell configurations (Na|SSE|Na) to isolate and quantify the catalyst's effectiveness at reducing interfacial impedance. Advanced protocols incorporate in-situ X-ray diffraction and scanning electron microscopy to monitor interface evolution during cycling, providing critical insights into degradation mechanisms and catalyst performance over time.
Cycling stability assessments require extended testing (>500 cycles) under controlled conditions, with capacity retention above 80% after 500 cycles considered the minimum benchmark for commercial viability. Rate capability tests evaluate performance across multiple C-rates (0.1C to 5C), with particular attention to capacity retention at higher rates where catalyst effects become more pronounced.
Environmental testing has gained prominence, with protocols now including performance evaluation under varying humidity levels (20-80% RH) and temperature fluctuations (-20°C to 60°C) to simulate real-world operating conditions. These tests are particularly relevant for consumer electronics applications where devices experience diverse environmental exposures.
Safety benchmarking protocols have been standardized to include thermal runaway tests, mechanical abuse tolerance, and dendrite penetration resistance measurements. Catalysts must demonstrate the ability to maintain battery safety parameters while enhancing performance, with particular emphasis on preventing sodium dendrite formation during fast charging operations.
Accelerated aging tests have been developed to predict long-term performance, typically involving elevated temperature storage (60°C) for 1-3 months, with post-mortem analysis to evaluate catalyst degradation mechanisms. These protocols help bridge the gap between laboratory testing and real-world performance expectations in consumer electronic devices.
Comparative benchmarking against lithium-ion technologies provides context for sodium battery development, with energy density, power density, and cycle life metrics normalized to enable direct comparison between these competing technologies. This approach helps identify specific applications where sodium batteries with catalyst enhancements offer competitive advantages over established lithium-ion solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!