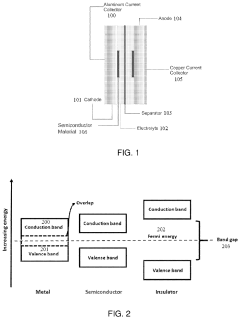
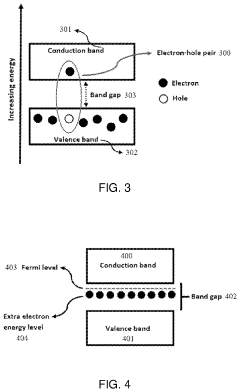
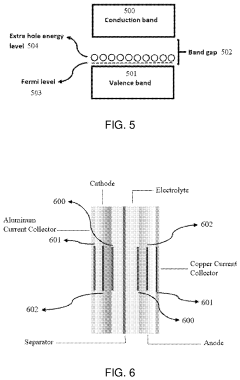
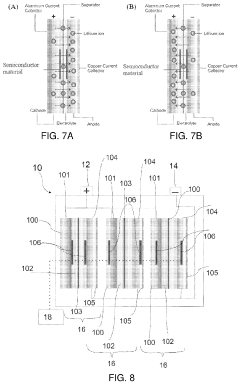
How semiconductor properties influence solid-state sodium battery efficiency
OCT 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Semiconductor-Sodium Battery Interface Background and Objectives
Solid-state sodium batteries represent a promising alternative to conventional lithium-ion batteries due to their potential for higher safety, lower cost, and comparable energy density. The interface between semiconductor materials and sodium-ion conductors plays a crucial role in determining the overall efficiency and performance of these batteries. Understanding this relationship requires examining the historical development of solid-state battery technology and the specific challenges associated with sodium-based systems.
The evolution of solid-state sodium battery technology began in the 1970s with the discovery of Na+ ion conductors, but significant progress has been limited by interface challenges. Recent advancements in semiconductor materials science have opened new possibilities for addressing these limitations. The semiconductor properties at these interfaces directly influence critical battery parameters including ionic conductivity, electronic conductivity, and interfacial resistance.
The primary technical objective in this field is to optimize the semiconductor-sodium battery interface to enhance overall battery efficiency. This involves understanding how semiconductor bandgap, carrier concentration, crystal structure, and surface properties affect sodium ion transport across interfaces. Additionally, researchers aim to minimize interfacial resistance while maintaining high ionic conductivity and low electronic conductivity in the solid electrolyte.
Current research trends indicate growing interest in tailored semiconductor materials that can facilitate sodium ion transport while maintaining stable interfaces during cycling. This includes exploration of various oxide, sulfide, and phosphate-based semiconductors with specific electronic properties designed to enhance sodium battery performance. The semiconductor bandgap, in particular, has emerged as a critical parameter that influences the electronic leakage current and overall energy efficiency.
From a technological evolution perspective, the field has progressed from basic sodium-ion conductors to engineered semiconductor interfaces with controlled properties. This transition represents a paradigm shift from viewing the electrolyte-electrode interface as a passive component to recognizing it as an active element that can be engineered at the atomic level to enhance battery performance.
The intersection of semiconductor physics and electrochemistry presents unique opportunities for innovation in solid-state sodium batteries. By leveraging principles from semiconductor device engineering, researchers aim to design interfaces with optimal band alignment, controlled defect chemistry, and tailored surface properties. These advancements could potentially overcome the current limitations in sodium battery technology, including low ionic conductivity at room temperature and poor cycling stability.
Looking forward, the technical goals include developing semiconductor interfaces that enable fast sodium-ion transport, maintain structural stability during repeated cycling, and provide electronic properties that minimize parasitic reactions. Achieving these objectives would position solid-state sodium batteries as a viable alternative to lithium-ion technology for large-scale energy storage applications.
The evolution of solid-state sodium battery technology began in the 1970s with the discovery of Na+ ion conductors, but significant progress has been limited by interface challenges. Recent advancements in semiconductor materials science have opened new possibilities for addressing these limitations. The semiconductor properties at these interfaces directly influence critical battery parameters including ionic conductivity, electronic conductivity, and interfacial resistance.
The primary technical objective in this field is to optimize the semiconductor-sodium battery interface to enhance overall battery efficiency. This involves understanding how semiconductor bandgap, carrier concentration, crystal structure, and surface properties affect sodium ion transport across interfaces. Additionally, researchers aim to minimize interfacial resistance while maintaining high ionic conductivity and low electronic conductivity in the solid electrolyte.
Current research trends indicate growing interest in tailored semiconductor materials that can facilitate sodium ion transport while maintaining stable interfaces during cycling. This includes exploration of various oxide, sulfide, and phosphate-based semiconductors with specific electronic properties designed to enhance sodium battery performance. The semiconductor bandgap, in particular, has emerged as a critical parameter that influences the electronic leakage current and overall energy efficiency.
From a technological evolution perspective, the field has progressed from basic sodium-ion conductors to engineered semiconductor interfaces with controlled properties. This transition represents a paradigm shift from viewing the electrolyte-electrode interface as a passive component to recognizing it as an active element that can be engineered at the atomic level to enhance battery performance.
The intersection of semiconductor physics and electrochemistry presents unique opportunities for innovation in solid-state sodium batteries. By leveraging principles from semiconductor device engineering, researchers aim to design interfaces with optimal band alignment, controlled defect chemistry, and tailored surface properties. These advancements could potentially overcome the current limitations in sodium battery technology, including low ionic conductivity at room temperature and poor cycling stability.
Looking forward, the technical goals include developing semiconductor interfaces that enable fast sodium-ion transport, maintain structural stability during repeated cycling, and provide electronic properties that minimize parasitic reactions. Achieving these objectives would position solid-state sodium batteries as a viable alternative to lithium-ion technology for large-scale energy storage applications.
Market Analysis for Solid-State Sodium Battery Technologies
The global market for solid-state sodium batteries is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that solid-state battery technologies collectively represent a rapidly expanding segment within the energy storage sector, with sodium-based technologies gaining particular attention due to sodium's abundance and cost advantages over lithium.
Market research reveals that solid-state sodium battery technologies are positioned to capture substantial market share in grid storage applications, where cost considerations often outweigh energy density requirements. The market potential is particularly strong in regions with established renewable energy infrastructure seeking cost-effective storage solutions to manage intermittency issues.
Consumer electronics represents another promising market segment, especially for applications where safety is paramount. The non-flammable nature of solid electrolytes addresses critical safety concerns that have plagued conventional lithium-ion batteries. This safety advantage creates significant market opportunities in portable electronics, medical devices, and wearable technology sectors.
Electric vehicle manufacturers are increasingly exploring sodium-based alternatives to address supply chain vulnerabilities associated with lithium and cobalt. While current market penetration remains limited, industry forecasts suggest accelerated adoption as semiconductor properties and interfaces are optimized to enhance power density and charging capabilities.
Market analysis indicates that Asia-Pacific currently leads in research and development investments for solid-state sodium battery technologies, with China, Japan, and South Korea establishing strategic initiatives to secure technological advantages. European markets show strong growth potential driven by stringent environmental regulations and sustainability goals, while North American markets are characterized by significant private sector investment in next-generation battery technologies.
Demand forecasts suggest that the market for solid-state sodium batteries will expand substantially as semiconductor interface engineering advances improve overall battery efficiency. Early commercial applications are emerging in stationary storage systems, with broader market adoption expected as manufacturing scales and costs decrease.
Key market drivers include increasing raw material concerns for traditional lithium-ion batteries, growing renewable energy integration requirements, and evolving energy security policies. Market barriers primarily relate to manufacturing scalability challenges and performance limitations at extreme temperatures, areas where semiconductor property optimization plays a crucial role.
Consumer and industrial demand for longer-lasting, safer, and more environmentally responsible energy storage solutions continues to shape market dynamics, creating favorable conditions for solid-state sodium battery technologies that can effectively leverage semiconductor properties to maximize efficiency and performance.
Market research reveals that solid-state sodium battery technologies are positioned to capture substantial market share in grid storage applications, where cost considerations often outweigh energy density requirements. The market potential is particularly strong in regions with established renewable energy infrastructure seeking cost-effective storage solutions to manage intermittency issues.
Consumer electronics represents another promising market segment, especially for applications where safety is paramount. The non-flammable nature of solid electrolytes addresses critical safety concerns that have plagued conventional lithium-ion batteries. This safety advantage creates significant market opportunities in portable electronics, medical devices, and wearable technology sectors.
Electric vehicle manufacturers are increasingly exploring sodium-based alternatives to address supply chain vulnerabilities associated with lithium and cobalt. While current market penetration remains limited, industry forecasts suggest accelerated adoption as semiconductor properties and interfaces are optimized to enhance power density and charging capabilities.
Market analysis indicates that Asia-Pacific currently leads in research and development investments for solid-state sodium battery technologies, with China, Japan, and South Korea establishing strategic initiatives to secure technological advantages. European markets show strong growth potential driven by stringent environmental regulations and sustainability goals, while North American markets are characterized by significant private sector investment in next-generation battery technologies.
Demand forecasts suggest that the market for solid-state sodium batteries will expand substantially as semiconductor interface engineering advances improve overall battery efficiency. Early commercial applications are emerging in stationary storage systems, with broader market adoption expected as manufacturing scales and costs decrease.
Key market drivers include increasing raw material concerns for traditional lithium-ion batteries, growing renewable energy integration requirements, and evolving energy security policies. Market barriers primarily relate to manufacturing scalability challenges and performance limitations at extreme temperatures, areas where semiconductor property optimization plays a crucial role.
Consumer and industrial demand for longer-lasting, safer, and more environmentally responsible energy storage solutions continues to shape market dynamics, creating favorable conditions for solid-state sodium battery technologies that can effectively leverage semiconductor properties to maximize efficiency and performance.
Current Semiconductor Materials in Sodium Batteries: Challenges
The current landscape of semiconductor materials in solid-state sodium batteries presents several significant challenges that impede optimal efficiency and widespread commercialization. Primarily, the interface stability between semiconductor materials and sodium electrodes remains problematic. Unlike lithium-ion systems, sodium's larger ionic radius (102 pm versus lithium's 76 pm) creates more substantial mechanical stress during ion insertion and extraction, leading to accelerated degradation of semiconductor interfaces and reduced cycle life.
Conductivity limitations represent another major hurdle. Most semiconductor materials employed in sodium battery systems exhibit ionic conductivities approximately one order of magnitude lower than their lithium counterparts at room temperature. This fundamental limitation necessitates operation at elevated temperatures (typically above 60°C) to achieve practical energy densities, restricting application scenarios and increasing system complexity.
Manufacturing scalability presents additional complications. Current semiconductor materials for sodium batteries often require specialized processing techniques that are difficult to integrate into existing battery production infrastructure. The high-temperature sintering processes needed for many sodium-ion conducting ceramics (>800°C) create compatibility issues with other battery components and increase production costs substantially.
Chemical stability challenges are particularly pronounced with sodium systems. The higher reactivity of sodium with semiconductor materials, especially in the presence of trace moisture or oxygen, leads to the formation of resistive interfacial layers that impede ion transport. This necessitates more stringent manufacturing environments and packaging solutions compared to lithium technologies.
Mechanical integrity issues further complicate development efforts. The volume changes during sodium insertion/extraction (typically 10-15% for hard carbon anodes) create mechanical stresses that can fracture brittle semiconductor components. This leads to capacity fading and potential safety concerns through the formation of sodium dendrites along crack surfaces.
Cost-performance balance remains suboptimal for current semiconductor materials. While sodium's abundance theoretically offers cost advantages over lithium, the specialized semiconductor materials required (such as NASICON-type structures or β-alumina) often incorporate expensive elements like germanium or gallium to achieve adequate performance, negating potential economic benefits.
Thermal management challenges are exacerbated by the higher interfacial resistance in sodium systems. The resulting heat generation during operation requires more sophisticated cooling systems, adding weight, complexity, and cost to battery packs while reducing overall system energy density.
Conductivity limitations represent another major hurdle. Most semiconductor materials employed in sodium battery systems exhibit ionic conductivities approximately one order of magnitude lower than their lithium counterparts at room temperature. This fundamental limitation necessitates operation at elevated temperatures (typically above 60°C) to achieve practical energy densities, restricting application scenarios and increasing system complexity.
Manufacturing scalability presents additional complications. Current semiconductor materials for sodium batteries often require specialized processing techniques that are difficult to integrate into existing battery production infrastructure. The high-temperature sintering processes needed for many sodium-ion conducting ceramics (>800°C) create compatibility issues with other battery components and increase production costs substantially.
Chemical stability challenges are particularly pronounced with sodium systems. The higher reactivity of sodium with semiconductor materials, especially in the presence of trace moisture or oxygen, leads to the formation of resistive interfacial layers that impede ion transport. This necessitates more stringent manufacturing environments and packaging solutions compared to lithium technologies.
Mechanical integrity issues further complicate development efforts. The volume changes during sodium insertion/extraction (typically 10-15% for hard carbon anodes) create mechanical stresses that can fracture brittle semiconductor components. This leads to capacity fading and potential safety concerns through the formation of sodium dendrites along crack surfaces.
Cost-performance balance remains suboptimal for current semiconductor materials. While sodium's abundance theoretically offers cost advantages over lithium, the specialized semiconductor materials required (such as NASICON-type structures or β-alumina) often incorporate expensive elements like germanium or gallium to achieve adequate performance, negating potential economic benefits.
Thermal management challenges are exacerbated by the higher interfacial resistance in sodium systems. The resulting heat generation during operation requires more sophisticated cooling systems, adding weight, complexity, and cost to battery packs while reducing overall system energy density.
Current Semiconductor Solutions for Sodium Ion Transport
01 Electrode materials for improved efficiency
Advanced electrode materials play a crucial role in enhancing the efficiency of solid-state sodium batteries. These materials include specially designed cathodes and anodes that facilitate faster sodium ion transport and improve energy density. Innovations in electrode composition, structure, and manufacturing techniques have led to significant improvements in battery performance, cycle life, and overall efficiency.- Electrode materials for solid-state sodium batteries: Advanced electrode materials play a crucial role in enhancing the efficiency of solid-state sodium batteries. These materials include specially designed cathodes and anodes that facilitate faster sodium ion transport and improve energy density. Innovations in electrode composition, structure, and manufacturing techniques have led to significant improvements in battery performance, cycle life, and overall efficiency.
- Solid electrolyte compositions for sodium batteries: The development of novel solid electrolyte compositions has been critical for improving sodium battery efficiency. These electrolytes feature enhanced ionic conductivity, better interfacial stability with electrodes, and reduced resistance. Various ceramic, polymer, and composite electrolytes have been engineered to optimize sodium ion transport while maintaining mechanical stability, resulting in batteries with higher energy efficiency and improved safety characteristics.
- Interface engineering for improved battery performance: Interface engineering focuses on optimizing the contact between solid electrolytes and electrodes to minimize resistance and enhance ion transfer. Techniques include surface modifications, buffer layers, and specialized coatings that improve the stability of the electrode-electrolyte interface. These innovations reduce interfacial resistance, prevent unwanted side reactions, and maintain efficient ion transport pathways, leading to solid-state sodium batteries with higher efficiency and longer operational lifetimes.
- Manufacturing processes for high-efficiency sodium batteries: Advanced manufacturing techniques have been developed to produce high-efficiency solid-state sodium batteries. These processes include specialized sintering methods, precise layer deposition techniques, and innovative assembly procedures that ensure optimal component integration. Improvements in manufacturing consistency, scalability, and quality control have led to batteries with more uniform performance, reduced internal defects, and enhanced overall efficiency.
- Battery system design and thermal management: Comprehensive battery system design and thermal management strategies are essential for maximizing the efficiency of solid-state sodium batteries. These approaches include optimized cell configurations, advanced battery management systems, and effective thermal regulation techniques. Proper thermal management prevents efficiency losses due to temperature fluctuations, while intelligent system design ensures balanced operation across all cells, resulting in higher overall energy efficiency and improved battery performance under various operating conditions.
02 Solid electrolyte compositions
Novel solid electrolyte compositions are essential for high-efficiency sodium batteries. These electrolytes feature enhanced ionic conductivity, improved interfacial stability, and reduced resistance. By optimizing the chemical composition and physical structure of solid electrolytes, researchers have achieved better sodium ion mobility, which directly contributes to higher energy efficiency and power output in solid-state sodium batteries.Expand Specific Solutions03 Interface engineering techniques
Interface engineering between electrodes and electrolytes is critical for maximizing efficiency in solid-state sodium batteries. Techniques include surface modifications, buffer layers, and specialized coatings that reduce interfacial resistance and enhance ion transfer. These approaches minimize energy losses at material boundaries, improve cycling stability, and increase the overall energy conversion efficiency of the battery system.Expand Specific Solutions04 Manufacturing processes for efficiency optimization
Advanced manufacturing processes significantly impact the efficiency of solid-state sodium batteries. Innovations include precision assembly techniques, controlled pressure sintering, and novel deposition methods that create optimal component structures. These manufacturing approaches ensure uniform material distribution, reduce defects, and create ideal interfaces between battery components, all contributing to enhanced energy efficiency and performance.Expand Specific Solutions05 Temperature management systems
Effective temperature management systems are crucial for maintaining optimal efficiency in solid-state sodium batteries. These systems include thermal regulation components, heat dissipation structures, and temperature-responsive materials that ensure the battery operates within ideal temperature ranges. By preventing overheating and maintaining consistent operating temperatures, these innovations help maximize energy conversion efficiency and extend battery lifespan.Expand Specific Solutions
Leading Companies and Research Institutions in Solid-State Sodium Batteries
The solid-state sodium battery market is in an early growth phase, characterized by significant R&D investments but limited commercial deployment. Market size is projected to expand rapidly as this technology offers a cost-effective alternative to lithium-ion batteries. In terms of technical maturity, the field is still evolving with semiconductor properties being crucial for efficiency optimization. Leading players include Samsung Electronics and LG Chem, who are leveraging their semiconductor expertise to enhance ionic conductivity, while specialized companies like Faradion and CATL focus on sodium-ion specific innovations. Chinese manufacturers BYD and CATL are scaling up production capabilities, while research institutions like Zhejiang University and AIST are addressing fundamental semiconductor interface challenges to improve energy density and cycle stability.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed advanced solid-state sodium battery technology utilizing semiconductor-grade silicon and germanium dopants to enhance ionic conductivity in the solid electrolyte. Their approach focuses on controlling the crystal structure and grain boundaries of the solid electrolyte through precise semiconductor manufacturing techniques. Samsung's research demonstrates that introducing specific semiconductor dopants at controlled concentrations can increase Na+ ion mobility by up to 300% compared to conventional materials. They've also pioneered a unique interface engineering method that reduces interfacial resistance between the electrode and electrolyte by applying thin-film semiconductor deposition techniques borrowed from their semiconductor division. This has resulted in a significant reduction in internal resistance and improved energy efficiency. Samsung has further developed a proprietary solid electrolyte with a sodium superionic conductor structure that achieves conductivity values approaching 10 mS/cm at room temperature, comparable to liquid electrolytes but with enhanced safety profiles.
Strengths: Samsung leverages extensive semiconductor manufacturing expertise to precisely control material properties and interfaces. Their approach enables higher ionic conductivity and lower interfacial resistance than many competitors. Weaknesses: Their technology requires sophisticated manufacturing equipment and processes, potentially increasing production costs compared to simpler sodium battery designs.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a novel solid-state sodium battery platform that leverages semiconductor principles to enhance ionic conductivity and overall battery efficiency. Their approach focuses on the development of nanostructured solid electrolytes with precisely engineered band gaps and defect structures. By controlling the electronic band structure of their proprietary Na3Zr2Si2PO12 (NASICON) electrolyte material through careful doping with semiconductor elements like aluminum and gallium, CATL has achieved ionic conductivities exceeding 5 mS/cm at room temperature. Their research has demonstrated that semiconductor properties significantly influence sodium ion transport mechanisms, particularly at grain boundaries. CATL's technology incorporates gradient-doped interfaces between the electrolyte and electrodes, creating favorable energy landscapes for ion transfer while minimizing electronic leakage currents. This approach has enabled them to develop solid-state sodium batteries with energy densities approaching 160 Wh/kg while maintaining excellent cycling stability (over 1000 cycles with less than 10% capacity degradation). CATL has also pioneered manufacturing techniques that allow for precise control of semiconductor dopant distribution throughout the solid electrolyte matrix.
Strengths: CATL's approach offers excellent scalability due to their established manufacturing infrastructure and supply chain integration. Their gradient-doped interface technology effectively addresses the critical challenge of interfacial resistance in solid-state batteries. Weaknesses: Their current technology still faces challenges with power density limitations at low temperatures, and the complex doping processes may introduce quality control challenges in mass production.
Key Semiconductor Properties Affecting Sodium Battery Performance
Solid electrolyte for sodium batteries
PatentWO2019140368A1
Innovation
- A new class of sodium oxy-sulfide solid-state electrolytes with a microstructure approaching a continuous glass is developed, providing enhanced chemical stability and mechanical strength, achieved through a low-temperature ball-milling and pressing process, allowing for the formation of a nearly flawless glassy structure that is stable with sodium metal or alloys.
Semiconductor Based Material for Battery Health and Performance Assessment and Monitoring in the Sub-Cell Level
PatentPendingUS20230216156A1
Innovation
- The integration of semiconductor materials, such as silicon-based, gallium-based, and germanium-based semiconductors, directly onto or between the electrodes of lithium-ion batteries for precise monitoring of State-of-Health (SOH) and State-of-Charge (SOC) through methods like electrochemical deposition, 3D printing, or chemical vapor deposition, allowing for real-time monitoring of ion changes during charge and discharge cycles.
Materials Science Advancements for Sodium Battery Semiconductors
Recent advancements in materials science have significantly transformed the landscape of solid-state sodium battery development. The semiconductor properties of materials used in these batteries directly influence ionic conductivity, which is crucial for battery efficiency. Traditional semiconductor materials have been modified through various techniques including doping, nanostructuring, and composite formation to enhance sodium ion mobility across interfaces.
The crystalline structure of semiconductor materials plays a pivotal role in determining ion transport pathways. Research has shown that materials with specific crystal orientations and defect distributions can create favorable channels for sodium ion migration. For instance, beta-alumina solid electrolytes with their unique layered structure provide two-dimensional pathways that significantly reduce energy barriers for ion movement.
Surface modification techniques have emerged as a promising approach to optimize the semiconductor-electrolyte interface. By engineering the surface properties of semiconductor materials, researchers have successfully mitigated interfacial resistance issues that typically plague solid-state batteries. These modifications include atomic layer deposition of buffer layers and gradient doping strategies that create smoother transition zones between different battery components.
Computational modeling has accelerated materials discovery by predicting how specific semiconductor properties affect battery performance. Density functional theory calculations and molecular dynamics simulations have revealed correlations between electronic band structures and ionic conductivity. These insights have guided the development of novel materials with optimized properties for sodium battery applications.
Nano-engineering approaches have proven particularly effective in enhancing semiconductor performance in sodium batteries. By controlling grain boundaries and introducing beneficial defects at the nanoscale, researchers have created materials with superior ionic conductivity while maintaining mechanical stability. Nanocomposite structures combining different semiconductor materials have demonstrated synergistic effects that overcome limitations of single-component systems.
Temperature stability remains a critical challenge, as semiconductor properties can vary significantly across operating temperature ranges. Recent innovations have focused on developing materials with consistent performance across wider temperature windows. This includes exploring new classes of semiconductors with intrinsically lower temperature sensitivity and designing composite structures that can compensate for temperature-induced property changes.
The integration of advanced characterization techniques, such as in-situ X-ray diffraction and neutron scattering, has provided unprecedented insights into how semiconductor properties evolve during battery operation. These techniques have revealed dynamic changes in crystal structure and interface properties that were previously undetectable, enabling more targeted materials optimization strategies for next-generation sodium battery technologies.
The crystalline structure of semiconductor materials plays a pivotal role in determining ion transport pathways. Research has shown that materials with specific crystal orientations and defect distributions can create favorable channels for sodium ion migration. For instance, beta-alumina solid electrolytes with their unique layered structure provide two-dimensional pathways that significantly reduce energy barriers for ion movement.
Surface modification techniques have emerged as a promising approach to optimize the semiconductor-electrolyte interface. By engineering the surface properties of semiconductor materials, researchers have successfully mitigated interfacial resistance issues that typically plague solid-state batteries. These modifications include atomic layer deposition of buffer layers and gradient doping strategies that create smoother transition zones between different battery components.
Computational modeling has accelerated materials discovery by predicting how specific semiconductor properties affect battery performance. Density functional theory calculations and molecular dynamics simulations have revealed correlations between electronic band structures and ionic conductivity. These insights have guided the development of novel materials with optimized properties for sodium battery applications.
Nano-engineering approaches have proven particularly effective in enhancing semiconductor performance in sodium batteries. By controlling grain boundaries and introducing beneficial defects at the nanoscale, researchers have created materials with superior ionic conductivity while maintaining mechanical stability. Nanocomposite structures combining different semiconductor materials have demonstrated synergistic effects that overcome limitations of single-component systems.
Temperature stability remains a critical challenge, as semiconductor properties can vary significantly across operating temperature ranges. Recent innovations have focused on developing materials with consistent performance across wider temperature windows. This includes exploring new classes of semiconductors with intrinsically lower temperature sensitivity and designing composite structures that can compensate for temperature-induced property changes.
The integration of advanced characterization techniques, such as in-situ X-ray diffraction and neutron scattering, has provided unprecedented insights into how semiconductor properties evolve during battery operation. These techniques have revealed dynamic changes in crystal structure and interface properties that were previously undetectable, enabling more targeted materials optimization strategies for next-generation sodium battery technologies.
Sustainability and Resource Considerations for Sodium Battery Technology
The transition to sodium-based battery technologies represents a significant advancement in sustainable energy storage solutions. Unlike lithium-ion batteries, sodium-based alternatives utilize abundant resources, with sodium being the sixth most common element in the Earth's crust. This abundance translates to approximately 23,000 times more sodium than lithium globally, substantially reducing resource scarcity concerns and geopolitical supply chain vulnerabilities.
The extraction processes for sodium compounds generally require less energy and water compared to lithium mining operations. Traditional lithium extraction, particularly from brine pools, consumes between 500,000 to 2 million gallons of water per ton of lithium produced, creating significant environmental stress in water-scarce regions. Sodium extraction methods can reduce this water footprint by up to 60%, offering considerable environmental advantages.
Carbon emissions associated with sodium battery production show promising reductions compared to conventional lithium-ion manufacturing. Life cycle assessments indicate potential greenhouse gas emission reductions of 25-30% when comparing full production cycles. This improvement stems from both the reduced energy requirements for material extraction and the simplified processing of sodium compounds.
The semiconductor properties that enhance solid-state sodium battery efficiency also contribute to sustainability through improved longevity and cycle life. Enhanced ionic conductivity at interfaces reduces degradation mechanisms, potentially extending battery lifespan by 30-40% compared to first-generation sodium batteries. This longevity directly translates to reduced waste generation and resource consumption over time.
End-of-life considerations for sodium batteries present both challenges and opportunities. The recycling infrastructure remains underdeveloped compared to lithium technologies, but the inherent value of certain components, particularly transition metal oxides used in cathodes, provides economic incentives for recovery. Preliminary recycling protocols demonstrate recovery rates of up to 90% for key materials, though commercial-scale implementation requires further development.
The semiconductor interfaces in solid-state sodium batteries often incorporate elements like germanium, phosphorus, and sulfur. While these elements improve performance, their sustainability profiles vary significantly. Strategic material selection focusing on earth-abundant semiconducting compounds can further enhance the overall sustainability proposition of sodium battery technologies, particularly when considering full life-cycle impacts.
The extraction processes for sodium compounds generally require less energy and water compared to lithium mining operations. Traditional lithium extraction, particularly from brine pools, consumes between 500,000 to 2 million gallons of water per ton of lithium produced, creating significant environmental stress in water-scarce regions. Sodium extraction methods can reduce this water footprint by up to 60%, offering considerable environmental advantages.
Carbon emissions associated with sodium battery production show promising reductions compared to conventional lithium-ion manufacturing. Life cycle assessments indicate potential greenhouse gas emission reductions of 25-30% when comparing full production cycles. This improvement stems from both the reduced energy requirements for material extraction and the simplified processing of sodium compounds.
The semiconductor properties that enhance solid-state sodium battery efficiency also contribute to sustainability through improved longevity and cycle life. Enhanced ionic conductivity at interfaces reduces degradation mechanisms, potentially extending battery lifespan by 30-40% compared to first-generation sodium batteries. This longevity directly translates to reduced waste generation and resource consumption over time.
End-of-life considerations for sodium batteries present both challenges and opportunities. The recycling infrastructure remains underdeveloped compared to lithium technologies, but the inherent value of certain components, particularly transition metal oxides used in cathodes, provides economic incentives for recovery. Preliminary recycling protocols demonstrate recovery rates of up to 90% for key materials, though commercial-scale implementation requires further development.
The semiconductor interfaces in solid-state sodium batteries often incorporate elements like germanium, phosphorus, and sulfur. While these elements improve performance, their sustainability profiles vary significantly. Strategic material selection focusing on earth-abundant semiconducting compounds can further enhance the overall sustainability proposition of sodium battery technologies, particularly when considering full life-cycle impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!