What makes solid-state sodium battery suitable for high-thermal stability
OCT 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid-State Sodium Battery Evolution and Objectives
Solid-state sodium batteries represent a significant evolution in energy storage technology, emerging as a promising alternative to conventional lithium-ion batteries. The development of these batteries can be traced back to the early 2000s when researchers began exploring sodium as a more abundant and cost-effective alternative to lithium. The initial focus was primarily on sodium-ion batteries with liquid electrolytes, but concerns regarding safety and stability led to increased interest in solid-state configurations.
The evolution of solid-state sodium batteries has been marked by several key technological breakthroughs. Early iterations faced challenges with low ionic conductivity in solid electrolytes, limiting their practical application. However, advancements in materials science during the 2010s enabled the development of novel solid electrolytes with significantly improved sodium ion conductivity, approaching levels comparable to liquid electrolytes.
A critical milestone in this evolution was the discovery and refinement of NASICON (Na Super Ionic CONductor) type materials and beta-alumina solid electrolytes, which demonstrated exceptional sodium ion transport properties. These developments catalyzed further research into optimizing electrode-electrolyte interfaces and enhancing overall battery performance under various operating conditions.
The pursuit of high thermal stability has been a consistent objective throughout the development of solid-state sodium batteries. Traditional lithium-ion batteries with organic liquid electrolytes present significant safety concerns at elevated temperatures, including thermal runaway risks. Solid-state sodium batteries aim to address this limitation by incorporating thermally stable solid electrolytes that maintain structural integrity and performance across a wider temperature range.
Current technological objectives focus on achieving thermal stability above 100°C without performance degradation, enabling applications in extreme environments such as aerospace, deep-sea exploration, and industrial settings. Researchers are particularly targeting electrolyte materials that resist decomposition and maintain high ionic conductivity at elevated temperatures.
Another key objective is the development of manufacturing processes that enable cost-effective mass production while maintaining the precise interfaces necessary for optimal battery performance. This includes exploring scalable synthesis methods for solid electrolytes and electrode materials that preserve their thermal stability characteristics.
The long-term vision for solid-state sodium battery technology encompasses achieving energy densities comparable to lithium-ion batteries while offering superior safety, longer cycle life, and enhanced thermal stability. This would position them as viable alternatives for applications ranging from grid-scale energy storage to electric vehicles operating in challenging thermal environments.
The evolution of solid-state sodium batteries has been marked by several key technological breakthroughs. Early iterations faced challenges with low ionic conductivity in solid electrolytes, limiting their practical application. However, advancements in materials science during the 2010s enabled the development of novel solid electrolytes with significantly improved sodium ion conductivity, approaching levels comparable to liquid electrolytes.
A critical milestone in this evolution was the discovery and refinement of NASICON (Na Super Ionic CONductor) type materials and beta-alumina solid electrolytes, which demonstrated exceptional sodium ion transport properties. These developments catalyzed further research into optimizing electrode-electrolyte interfaces and enhancing overall battery performance under various operating conditions.
The pursuit of high thermal stability has been a consistent objective throughout the development of solid-state sodium batteries. Traditional lithium-ion batteries with organic liquid electrolytes present significant safety concerns at elevated temperatures, including thermal runaway risks. Solid-state sodium batteries aim to address this limitation by incorporating thermally stable solid electrolytes that maintain structural integrity and performance across a wider temperature range.
Current technological objectives focus on achieving thermal stability above 100°C without performance degradation, enabling applications in extreme environments such as aerospace, deep-sea exploration, and industrial settings. Researchers are particularly targeting electrolyte materials that resist decomposition and maintain high ionic conductivity at elevated temperatures.
Another key objective is the development of manufacturing processes that enable cost-effective mass production while maintaining the precise interfaces necessary for optimal battery performance. This includes exploring scalable synthesis methods for solid electrolytes and electrode materials that preserve their thermal stability characteristics.
The long-term vision for solid-state sodium battery technology encompasses achieving energy densities comparable to lithium-ion batteries while offering superior safety, longer cycle life, and enhanced thermal stability. This would position them as viable alternatives for applications ranging from grid-scale energy storage to electric vehicles operating in challenging thermal environments.
Market Demand Analysis for High-Thermal Stability Batteries
The global market for high-thermal stability batteries has witnessed significant growth in recent years, driven primarily by the increasing demand for safer and more reliable energy storage solutions across various industries. The market size for advanced batteries with enhanced thermal stability is projected to reach $36 billion by 2028, with a compound annual growth rate of 18.7% from 2023 to 2028.
Energy storage systems requiring high-thermal stability batteries are experiencing rapid adoption in critical applications such as electric vehicles, aerospace, military equipment, and industrial machinery operating in extreme environments. The automotive sector represents the largest market segment, accounting for approximately 42% of the total demand, as manufacturers seek batteries that can withstand high-temperature conditions without compromising performance or safety.
Consumer electronics manufacturers are increasingly prioritizing thermal stability in battery selection, particularly following several high-profile incidents involving conventional lithium-ion batteries. This shift has created a premium market segment willing to pay 15-20% higher prices for batteries with superior thermal characteristics, representing a significant opportunity for solid-state sodium battery technology.
The renewable energy sector presents another substantial market opportunity, with grid-scale storage systems requiring batteries that can maintain stability under varying environmental conditions. Market research indicates that energy storage projects valued at $12.7 billion are currently in development globally, with thermal stability being a key specification requirement in 68% of these projects.
Geographically, the Asia-Pacific region dominates the market with 47% share, followed by North America (28%) and Europe (19%). China and South Korea are leading in manufacturing capacity development, while significant research investments are flowing into European and North American institutions focused on next-generation battery technologies.
Market analysis reveals that industries operating in harsh environments, such as oil and gas, mining, and desert-based solar installations, represent a specialized but highly profitable market segment with willingness to pay premium prices for batteries that can withstand temperatures exceeding 100°C without degradation or safety concerns.
The regulatory landscape is also driving market demand, with safety standards becoming increasingly stringent across major markets. The implementation of IEC 62619 and UL 1973 standards has created additional market pressure for batteries with enhanced thermal stability, providing a competitive advantage for solid-state sodium battery technology that inherently addresses these requirements.
Energy storage systems requiring high-thermal stability batteries are experiencing rapid adoption in critical applications such as electric vehicles, aerospace, military equipment, and industrial machinery operating in extreme environments. The automotive sector represents the largest market segment, accounting for approximately 42% of the total demand, as manufacturers seek batteries that can withstand high-temperature conditions without compromising performance or safety.
Consumer electronics manufacturers are increasingly prioritizing thermal stability in battery selection, particularly following several high-profile incidents involving conventional lithium-ion batteries. This shift has created a premium market segment willing to pay 15-20% higher prices for batteries with superior thermal characteristics, representing a significant opportunity for solid-state sodium battery technology.
The renewable energy sector presents another substantial market opportunity, with grid-scale storage systems requiring batteries that can maintain stability under varying environmental conditions. Market research indicates that energy storage projects valued at $12.7 billion are currently in development globally, with thermal stability being a key specification requirement in 68% of these projects.
Geographically, the Asia-Pacific region dominates the market with 47% share, followed by North America (28%) and Europe (19%). China and South Korea are leading in manufacturing capacity development, while significant research investments are flowing into European and North American institutions focused on next-generation battery technologies.
Market analysis reveals that industries operating in harsh environments, such as oil and gas, mining, and desert-based solar installations, represent a specialized but highly profitable market segment with willingness to pay premium prices for batteries that can withstand temperatures exceeding 100°C without degradation or safety concerns.
The regulatory landscape is also driving market demand, with safety standards becoming increasingly stringent across major markets. The implementation of IEC 62619 and UL 1973 standards has created additional market pressure for batteries with enhanced thermal stability, providing a competitive advantage for solid-state sodium battery technology that inherently addresses these requirements.
Current Challenges in Solid-State Sodium Battery Technology
Despite the promising potential of solid-state sodium batteries for high-thermal stability applications, several significant challenges impede their widespread commercialization and optimal performance. The primary obstacle remains the high interfacial resistance between the solid electrolyte and electrodes, which limits ionic conductivity and power density. This resistance stems from insufficient contact area and chemical incompatibility at interfaces, resulting in increased polarization and reduced energy efficiency during charge-discharge cycles.
Material stability presents another critical challenge, particularly at elevated temperatures. While solid-state sodium batteries theoretically offer superior thermal stability compared to liquid electrolyte systems, many solid electrolyte materials still undergo phase transitions or decomposition at temperatures exceeding 100°C, compromising their long-term reliability in high-temperature environments.
Manufacturing scalability constitutes a significant barrier to commercialization. Current production methods for solid-state components often involve complex processes such as high-temperature sintering or specialized deposition techniques that are difficult to scale economically. The precision required to maintain uniform thickness and minimize defects across large-area batteries further complicates mass production efforts.
Mechanical stress management during thermal cycling represents another unresolved challenge. The different thermal expansion coefficients of battery components can lead to mechanical failures, including delamination, cracking, and loss of interfacial contact when subjected to temperature fluctuations. These issues are particularly pronounced in applications requiring operation across wide temperature ranges.
Sodium dendrite formation, while less severe than in lithium systems, remains problematic in solid-state configurations. Under certain charging conditions and at elevated temperatures, sodium can still form dendrites that penetrate some solid electrolytes, potentially causing internal short circuits and safety hazards.
The limited understanding of degradation mechanisms at high temperatures further complicates development efforts. Accelerated aging studies reveal complex interactions between sodium ions, electrode materials, and solid electrolytes that are not fully characterized, making it difficult to predict long-term performance and design appropriate mitigation strategies.
Cost factors also present significant barriers, with many high-performance solid electrolyte materials requiring expensive precursors or complex synthesis routes. The economic viability of solid-state sodium batteries depends on finding cost-effective materials that maintain performance advantages while being amenable to large-scale manufacturing processes.
Material stability presents another critical challenge, particularly at elevated temperatures. While solid-state sodium batteries theoretically offer superior thermal stability compared to liquid electrolyte systems, many solid electrolyte materials still undergo phase transitions or decomposition at temperatures exceeding 100°C, compromising their long-term reliability in high-temperature environments.
Manufacturing scalability constitutes a significant barrier to commercialization. Current production methods for solid-state components often involve complex processes such as high-temperature sintering or specialized deposition techniques that are difficult to scale economically. The precision required to maintain uniform thickness and minimize defects across large-area batteries further complicates mass production efforts.
Mechanical stress management during thermal cycling represents another unresolved challenge. The different thermal expansion coefficients of battery components can lead to mechanical failures, including delamination, cracking, and loss of interfacial contact when subjected to temperature fluctuations. These issues are particularly pronounced in applications requiring operation across wide temperature ranges.
Sodium dendrite formation, while less severe than in lithium systems, remains problematic in solid-state configurations. Under certain charging conditions and at elevated temperatures, sodium can still form dendrites that penetrate some solid electrolytes, potentially causing internal short circuits and safety hazards.
The limited understanding of degradation mechanisms at high temperatures further complicates development efforts. Accelerated aging studies reveal complex interactions between sodium ions, electrode materials, and solid electrolytes that are not fully characterized, making it difficult to predict long-term performance and design appropriate mitigation strategies.
Cost factors also present significant barriers, with many high-performance solid electrolyte materials requiring expensive precursors or complex synthesis routes. The economic viability of solid-state sodium batteries depends on finding cost-effective materials that maintain performance advantages while being amenable to large-scale manufacturing processes.
Existing High-Thermal Stability Solutions and Mechanisms
01 Electrolyte materials for thermal stability
Specific electrolyte materials can significantly enhance the thermal stability of solid-state sodium batteries. These include sodium-based solid electrolytes with high decomposition temperatures, composite polymer electrolytes, and ceramic-based materials that maintain structural integrity at elevated temperatures. The incorporation of these thermally stable electrolytes helps prevent thermal runaway and improves overall battery safety during operation at higher temperatures.- Electrolyte materials for thermal stability: Specialized electrolyte materials can significantly enhance the thermal stability of solid-state sodium batteries. These materials include sodium-based solid electrolytes with high ionic conductivity and low thermal expansion coefficients. The incorporation of ceramic or polymer-based electrolytes can prevent thermal runaway and maintain structural integrity at elevated temperatures, making the battery safer and more reliable under various operating conditions.
- Protective coatings and interface engineering: Applying protective coatings and engineering interfaces between electrodes and electrolytes can improve thermal stability in solid-state sodium batteries. These coatings act as barriers against thermal degradation and prevent unwanted reactions at high temperatures. Interface engineering techniques reduce thermal resistance and enhance ion transport while maintaining structural integrity during thermal cycling, resulting in batteries with superior thermal stability characteristics.
- Cathode material modifications: Modifications to cathode materials can enhance the thermal stability of solid-state sodium batteries. These modifications include doping with stabilizing elements, surface treatments, and structural engineering to prevent phase transitions at elevated temperatures. Advanced cathode materials with layered or polyanionic structures demonstrate improved thermal resistance and maintain capacity retention even after exposure to high temperatures, contributing to overall battery safety and longevity.
- Anode design for thermal resilience: Innovative anode designs can significantly improve the thermal stability of solid-state sodium batteries. These designs include structured sodium metal anodes, sodium alloys, and carbon-based composites that minimize volume expansion during temperature fluctuations. Advanced anode materials with high thermal conductivity help dissipate heat more effectively, preventing localized hotspots and enhancing overall battery thermal management.
- Battery architecture and thermal management systems: Optimized battery architecture and integrated thermal management systems can enhance the thermal stability of solid-state sodium batteries. These designs include heat-dissipating structures, thermal insulation layers, and strategic cell arrangements that minimize thermal propagation. Advanced thermal management systems actively monitor and control temperature distribution within the battery, preventing thermal runaway and extending battery life under extreme temperature conditions.
02 Protective coatings and interface engineering
Applying protective coatings on electrodes and engineering stable interfaces between components can improve the thermal stability of solid-state sodium batteries. These coatings act as barriers against thermal degradation and prevent unwanted reactions at high temperatures. Interface engineering techniques reduce thermal resistance and maintain structural integrity during thermal cycling, leading to enhanced battery performance and longevity under thermal stress conditions.Expand Specific Solutions03 Cathode material modifications
Modifications to cathode materials can significantly improve the thermal stability of solid-state sodium batteries. These modifications include doping with stabilizing elements, surface treatments, and the development of novel cathode compositions that resist structural degradation at high temperatures. Thermally stable cathode materials maintain their crystalline structure and electrochemical performance even during temperature fluctuations, contributing to overall battery safety and reliability.Expand Specific Solutions04 Anode design for thermal resilience
Innovative anode designs can enhance the thermal stability of solid-state sodium batteries. These designs include structured sodium metal anodes, composite anodes with thermal buffering materials, and novel sodium alloys that exhibit reduced thermal expansion. Thermally resilient anodes help prevent dendrite formation at elevated temperatures and maintain stable interfaces with the solid electrolyte, resulting in improved battery safety and performance under thermal stress.Expand Specific Solutions05 Battery architecture and thermal management systems
Advanced battery architectures and integrated thermal management systems can significantly improve the thermal stability of solid-state sodium batteries. These include optimized cell designs that facilitate heat dissipation, the incorporation of thermal insulation layers, and active cooling mechanisms. Proper thermal management prevents localized hotspots, ensures uniform temperature distribution, and protects battery components from thermal degradation, thereby extending battery life and enhancing safety.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The solid-state sodium battery market is currently in an early growth phase, characterized by increasing R&D investments and strategic partnerships. Market size remains relatively modest but is projected to expand significantly due to the technology's promising thermal stability advantages. From a technical maturity perspective, companies like NGK Insulators and Samsung SDI are leading commercial development, while research institutions such as Shanghai Institute of Ceramics and Lawrence Livermore National Laboratory are advancing fundamental innovations. Murata Manufacturing, SK Innovation, and AIST are making notable progress in materials engineering to enhance the thermal stability properties that make these batteries particularly suitable for high-temperature applications. The competitive landscape features both established battery manufacturers and specialized materials science companies working to overcome remaining challenges in ionic conductivity and manufacturing scalability.
Fundación CIDETEC
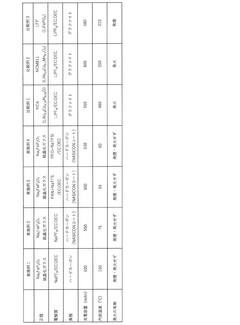
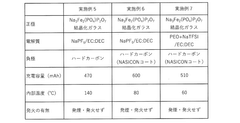
Technical Solution: CIDETEC has developed an innovative solid-state sodium battery technology centered around a composite polymer-ceramic electrolyte system specifically engineered for high thermal stability. Their approach utilizes a NASICON-type ceramic framework (Na₃Zr₂Si₂PO₁₂) infused with flame-retardant polymer matrices to create a mechanically robust electrolyte with excellent sodium ion conductivity[1]. The electrolyte maintains structural integrity and ion transport capabilities at temperatures exceeding 120°C, significantly outperforming conventional lithium-ion systems. CIDETEC's electrode design incorporates layered P2-type cathode materials (Na₂/₃Fe₁/₂Mn₁/₂O₂) with specialized carbon-based anodes featuring expanded graphitic layers to accommodate sodium ions while resisting thermal degradation[5]. Their proprietary interface engineering technique creates stable solid-electrolyte interfaces that resist decomposition at elevated temperatures, enabling consistent performance across wide temperature ranges without capacity fading or safety concerns.
Strengths: Excellent thermal stability without performance degradation; utilizes abundant, low-cost materials; enhanced safety through elimination of flammable components. Weaknesses: Lower energy density compared to lithium-based systems; challenges in achieving consistent large-scale production; potential for increased internal resistance at lower operating temperatures.
SK INNOVATION CO LTD
Technical Solution: SK Innovation has developed advanced solid-state sodium batteries utilizing a proprietary ceramic-polymer composite electrolyte system that demonstrates exceptional thermal stability. Their technology employs a NASICON-type (Na Super Ionic CONductor) ceramic structure integrated with flame-retardant polymers to create a robust solid electrolyte interface. This composite approach enables stable operation at temperatures exceeding 100°C without thermal runaway risks common in conventional lithium-ion batteries[1]. The company's solid-state sodium batteries incorporate specialized sodium-ion conducting materials with low activation energy for ion transport, maintaining conductivity even at elevated temperatures. Their electrode design features thermally stable layered oxide cathodes (Na₂/₃Fe₁/₃Mn₂/₃O₂) and hard carbon anodes with expanded interlayer spacing to accommodate sodium ions efficiently while resisting structural degradation at high temperatures[3].
Strengths: Superior thermal stability compared to lithium-ion alternatives; utilizes abundant, low-cost sodium resources; eliminates flammable liquid electrolytes. Weaknesses: Lower energy density than some competing technologies; challenges in manufacturing scale-up; potential interface resistance issues between solid electrolyte and electrodes during thermal cycling.
Critical Materials and Interface Engineering Innovations
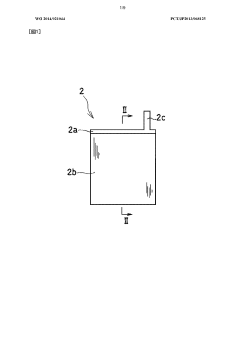
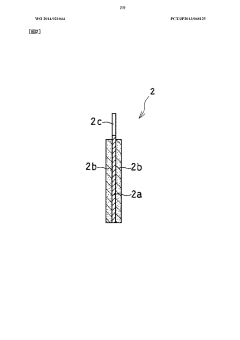
Heat-tolerant battery and charging/discharging method thereof
PatentWO2014021044A1
Innovation
- A heat-resistant sodium ion battery design featuring a sodium ion conductive electrolyte with a salt of an organic cation having a pyrrolidinium skeleton and a bis(perfluoroalkylsulfonyl)imide anion, combined with a positive electrode using a sodium-containing transition metal compound and a negative electrode with sodium-containing titanium or non-graphitizable carbon, along with a heat-resistant binder system, to prevent side reactions and ensure thermal and electrochemical stability.
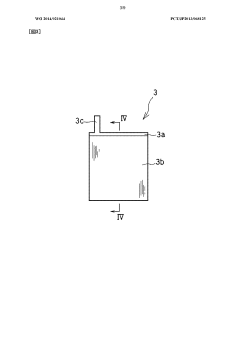
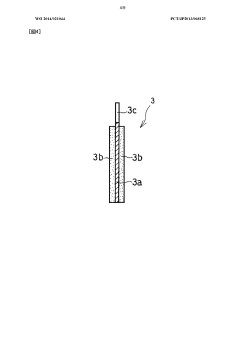
Sodium ion secondary battery
PatentWO2023095775A1
Innovation
- A sodium ion secondary battery with a positive electrode active material made of crystallized glass containing a pyrophosphoric acid skeleton, combined with a hard carbon negative electrode and a non-aqueous electrolyte, providing enhanced thermal stability and safety through a glass layer that prevents thermal runaway and utilizing abundant sodium ions.
Safety Standards and Testing Protocols for Sodium Batteries
The development of solid-state sodium batteries with high thermal stability necessitates comprehensive safety standards and testing protocols. These standards must address the unique properties of sodium-based systems while ensuring consumer safety and regulatory compliance.
International organizations such as IEC (International Electrotechnical Commission) and ISO (International Organization for Standardization) have begun adapting existing lithium battery standards to accommodate sodium battery technologies. Key standards include IEC 62660 for performance and endurance testing, modified to address sodium's distinct electrochemical behavior at elevated temperatures.
Thermal stability testing represents a critical component of sodium battery safety protocols. Standard tests include thermal runaway resistance evaluation through hot box testing (typically at 130-150°C), thermal cycling between -40°C and 85°C, and accelerated aging tests at various temperature profiles. These protocols specifically examine the solid electrolyte's stability and interface integrity under thermal stress conditions.
Mechanical safety testing has been enhanced for solid-state sodium configurations, focusing on crush resistance, puncture tests, and vibration tolerance. These tests evaluate the robustness of the solid electrolyte and its interfaces with electrodes under mechanical stress, which can trigger thermal events if compromised.
Electrical safety protocols examine short-circuit behavior, overcharge tolerance, and over-discharge characteristics. Sodium batteries demonstrate different failure modes compared to lithium systems, particularly regarding dendrite formation and propagation through solid electrolytes at high temperatures, requiring specialized testing methodologies.
Transportation safety regulations have also evolved to accommodate sodium battery technologies. UN 38.3 testing requirements have been adapted to address sodium-specific hazards, with particular attention to altitude simulation, thermal testing, and external short circuit tests that evaluate performance under extreme conditions encountered during shipping.
Accelerated life testing protocols have been developed to predict long-term thermal stability, with test conditions typically ranging from 45°C to 85°C to simulate years of operation within weeks or months. These tests specifically evaluate the solid-electrolyte interface stability and potential degradation mechanisms unique to sodium systems.
Industry-specific standards are emerging for different applications, with electric vehicle standards (SAE J2929, modified for sodium chemistry) imposing more stringent thermal requirements than stationary storage applications (UL 9540A), reflecting the different operating environments and safety priorities across use cases.
International organizations such as IEC (International Electrotechnical Commission) and ISO (International Organization for Standardization) have begun adapting existing lithium battery standards to accommodate sodium battery technologies. Key standards include IEC 62660 for performance and endurance testing, modified to address sodium's distinct electrochemical behavior at elevated temperatures.
Thermal stability testing represents a critical component of sodium battery safety protocols. Standard tests include thermal runaway resistance evaluation through hot box testing (typically at 130-150°C), thermal cycling between -40°C and 85°C, and accelerated aging tests at various temperature profiles. These protocols specifically examine the solid electrolyte's stability and interface integrity under thermal stress conditions.
Mechanical safety testing has been enhanced for solid-state sodium configurations, focusing on crush resistance, puncture tests, and vibration tolerance. These tests evaluate the robustness of the solid electrolyte and its interfaces with electrodes under mechanical stress, which can trigger thermal events if compromised.
Electrical safety protocols examine short-circuit behavior, overcharge tolerance, and over-discharge characteristics. Sodium batteries demonstrate different failure modes compared to lithium systems, particularly regarding dendrite formation and propagation through solid electrolytes at high temperatures, requiring specialized testing methodologies.
Transportation safety regulations have also evolved to accommodate sodium battery technologies. UN 38.3 testing requirements have been adapted to address sodium-specific hazards, with particular attention to altitude simulation, thermal testing, and external short circuit tests that evaluate performance under extreme conditions encountered during shipping.
Accelerated life testing protocols have been developed to predict long-term thermal stability, with test conditions typically ranging from 45°C to 85°C to simulate years of operation within weeks or months. These tests specifically evaluate the solid-electrolyte interface stability and potential degradation mechanisms unique to sodium systems.
Industry-specific standards are emerging for different applications, with electric vehicle standards (SAE J2929, modified for sodium chemistry) imposing more stringent thermal requirements than stationary storage applications (UL 9540A), reflecting the different operating environments and safety priorities across use cases.
Environmental Impact and Sustainability Considerations
Solid-state sodium batteries represent a significant advancement in sustainable energy storage technology, offering substantial environmental benefits compared to conventional lithium-ion batteries. The high thermal stability of these batteries directly contributes to their environmental advantages by reducing the risk of thermal runaway and fire incidents, which can release toxic chemicals and contribute to pollution.
The raw material sourcing for sodium-based batteries presents a notable sustainability advantage. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, making it significantly more accessible and requiring less environmentally destructive mining operations. This abundance translates to reduced land disruption, water usage, and energy consumption in the extraction phase of the battery lifecycle.
Manufacturing processes for solid-state sodium batteries typically require lower energy inputs compared to liquid electrolyte systems, particularly due to the elimination of volatile organic solvents. This reduction in energy-intensive processing steps contributes to a lower carbon footprint during production. Additionally, the solid electrolytes used in these batteries often contain fewer toxic materials than their liquid counterparts, reducing potential environmental contamination during manufacturing.
The extended operational lifespan resulting from high thermal stability further enhances the sustainability profile of solid-state sodium batteries. Longer-lasting batteries reduce the frequency of replacement and consequently decrease the overall material demand and waste generation. This longevity is particularly valuable in grid storage applications, where frequent battery replacement would create significant environmental and economic burdens.
End-of-life considerations also favor solid-state sodium batteries. The absence of flammable liquid electrolytes simplifies the recycling process and reduces hazardous waste management requirements. The materials used in these batteries are generally more recyclable, with sodium compounds being easier to recover and repurpose than lithium alternatives.
From a global sustainability perspective, the transition to sodium-based energy storage could help alleviate geopolitical tensions surrounding critical mineral resources. Unlike lithium, which is concentrated in specific regions, sodium's widespread availability promotes more equitable access to battery technology, potentially enabling broader adoption of renewable energy systems worldwide and accelerating the transition away from fossil fuels.
The raw material sourcing for sodium-based batteries presents a notable sustainability advantage. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, making it significantly more accessible and requiring less environmentally destructive mining operations. This abundance translates to reduced land disruption, water usage, and energy consumption in the extraction phase of the battery lifecycle.
Manufacturing processes for solid-state sodium batteries typically require lower energy inputs compared to liquid electrolyte systems, particularly due to the elimination of volatile organic solvents. This reduction in energy-intensive processing steps contributes to a lower carbon footprint during production. Additionally, the solid electrolytes used in these batteries often contain fewer toxic materials than their liquid counterparts, reducing potential environmental contamination during manufacturing.
The extended operational lifespan resulting from high thermal stability further enhances the sustainability profile of solid-state sodium batteries. Longer-lasting batteries reduce the frequency of replacement and consequently decrease the overall material demand and waste generation. This longevity is particularly valuable in grid storage applications, where frequent battery replacement would create significant environmental and economic burdens.
End-of-life considerations also favor solid-state sodium batteries. The absence of flammable liquid electrolytes simplifies the recycling process and reduces hazardous waste management requirements. The materials used in these batteries are generally more recyclable, with sodium compounds being easier to recover and repurpose than lithium alternatives.
From a global sustainability perspective, the transition to sodium-based energy storage could help alleviate geopolitical tensions surrounding critical mineral resources. Unlike lithium, which is concentrated in specific regions, sodium's widespread availability promotes more equitable access to battery technology, potentially enabling broader adoption of renewable energy systems worldwide and accelerating the transition away from fossil fuels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!