Analysis of SERS Substrates in Pharmaceutical Applications
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SERS Technology Evolution and Objectives
Surface-Enhanced Raman Spectroscopy (SERS) has evolved significantly since its discovery in the 1970s when researchers observed enhanced Raman signals from pyridine molecules adsorbed on roughened silver electrodes. This breakthrough laid the foundation for a powerful analytical technique that combines the molecular specificity of Raman spectroscopy with significantly enhanced sensitivity. The evolution of SERS technology has been characterized by continuous improvements in substrate design, fabrication techniques, and theoretical understanding of enhancement mechanisms.
In the 1980s and 1990s, research focused primarily on understanding the fundamental mechanisms behind the SERS effect, with the electromagnetic enhancement mechanism and chemical enhancement mechanism emerging as the two primary contributors. The electromagnetic mechanism, which accounts for the majority of signal enhancement, involves the excitation of localized surface plasmons on metallic nanostructures, while the chemical mechanism involves charge transfer between the analyte and substrate.
The early 2000s witnessed significant advancements in nanofabrication techniques, enabling the production of more controlled and reproducible SERS substrates. These developments included lithographic methods, self-assembly approaches, and template-assisted synthesis, all aimed at creating substrates with optimized "hot spots" - regions of intense electromagnetic field enhancement where SERS signals are maximized.
The pharmaceutical industry began adopting SERS technology more widely in the 2010s, recognizing its potential for high-sensitivity detection of active pharmaceutical ingredients (APIs), excipients, and contaminants. The non-destructive nature of SERS, combined with minimal sample preparation requirements and rapid analysis times, positioned it as an attractive alternative to traditional analytical methods in pharmaceutical quality control and research.
Current technological objectives in SERS substrate development for pharmaceutical applications focus on several key areas: enhancing sensitivity to achieve single-molecule detection capabilities; improving reproducibility to ensure consistent results across batches; expanding the range of detectable compounds, particularly those with weak Raman signals; and developing portable, user-friendly SERS platforms for point-of-need testing in pharmaceutical manufacturing and distribution settings.
Future objectives include the integration of SERS with other analytical techniques such as microfluidics and machine learning algorithms to create comprehensive analytical platforms. Additionally, there is growing interest in developing SERS substrates that can function effectively in complex biological matrices, enabling direct analysis of pharmaceuticals in bodily fluids without extensive sample preparation. The ultimate goal is to establish SERS as a standard analytical tool in pharmaceutical development, manufacturing, and quality control processes, providing rapid, sensitive, and specific molecular information to ensure drug safety and efficacy.
In the 1980s and 1990s, research focused primarily on understanding the fundamental mechanisms behind the SERS effect, with the electromagnetic enhancement mechanism and chemical enhancement mechanism emerging as the two primary contributors. The electromagnetic mechanism, which accounts for the majority of signal enhancement, involves the excitation of localized surface plasmons on metallic nanostructures, while the chemical mechanism involves charge transfer between the analyte and substrate.
The early 2000s witnessed significant advancements in nanofabrication techniques, enabling the production of more controlled and reproducible SERS substrates. These developments included lithographic methods, self-assembly approaches, and template-assisted synthesis, all aimed at creating substrates with optimized "hot spots" - regions of intense electromagnetic field enhancement where SERS signals are maximized.
The pharmaceutical industry began adopting SERS technology more widely in the 2010s, recognizing its potential for high-sensitivity detection of active pharmaceutical ingredients (APIs), excipients, and contaminants. The non-destructive nature of SERS, combined with minimal sample preparation requirements and rapid analysis times, positioned it as an attractive alternative to traditional analytical methods in pharmaceutical quality control and research.
Current technological objectives in SERS substrate development for pharmaceutical applications focus on several key areas: enhancing sensitivity to achieve single-molecule detection capabilities; improving reproducibility to ensure consistent results across batches; expanding the range of detectable compounds, particularly those with weak Raman signals; and developing portable, user-friendly SERS platforms for point-of-need testing in pharmaceutical manufacturing and distribution settings.
Future objectives include the integration of SERS with other analytical techniques such as microfluidics and machine learning algorithms to create comprehensive analytical platforms. Additionally, there is growing interest in developing SERS substrates that can function effectively in complex biological matrices, enabling direct analysis of pharmaceuticals in bodily fluids without extensive sample preparation. The ultimate goal is to establish SERS as a standard analytical tool in pharmaceutical development, manufacturing, and quality control processes, providing rapid, sensitive, and specific molecular information to ensure drug safety and efficacy.
Pharmaceutical Market Demand for SERS Analysis
The pharmaceutical industry is experiencing a significant shift towards more precise analytical methods, with Surface-Enhanced Raman Spectroscopy (SERS) emerging as a critical technology. The global pharmaceutical analytical testing market was valued at approximately $5.6 billion in 2021 and is projected to reach $11.4 billion by 2028, growing at a CAGR of 10.7%. Within this expanding market, SERS technology is gaining substantial traction due to its exceptional sensitivity and specificity.
Pharmaceutical companies face increasing regulatory pressures to ensure drug purity, safety, and efficacy. The FDA and EMA have implemented more stringent requirements for pharmaceutical analysis, creating a strong demand for advanced analytical techniques like SERS. This regulatory landscape has accelerated the adoption of SERS technology across various stages of pharmaceutical development and manufacturing.
The primary market drivers for SERS in pharmaceuticals include the need for trace contaminant detection, counterfeit drug identification, and real-time process monitoring. SERS offers detection limits in the nanogram to picogram range, making it invaluable for identifying impurities that conventional methods might miss. The technology's ability to provide molecular fingerprinting without sample preparation has positioned it as an ideal solution for rapid quality control processes.
Market research indicates that over 70% of pharmaceutical companies are exploring or implementing advanced spectroscopic techniques, with SERS adoption growing at approximately 15% annually. The demand is particularly strong in biologics manufacturing, where complex molecular structures require sophisticated analytical approaches. The biologics segment alone is expected to drive 40% of the SERS substrate market growth in pharmaceuticals over the next five years.
Geographically, North America currently dominates the pharmaceutical SERS market with a 42% share, followed by Europe (31%) and Asia-Pacific (21%). However, the Asia-Pacific region is experiencing the fastest growth rate at 18% annually, driven by expanding pharmaceutical manufacturing capabilities in China and India.
Customer needs analysis reveals that pharmaceutical companies prioritize SERS substrates with high reproducibility, long shelf life, and compatibility with automated systems. There is particular demand for SERS platforms that can be integrated into existing pharmaceutical manufacturing lines for continuous monitoring. Additionally, there is growing interest in portable SERS devices for field testing of raw materials and finished products.
The economic value proposition of SERS technology in pharmaceuticals is compelling, with companies reporting 30-40% reduction in analytical costs and 50-60% decrease in time-to-result compared to traditional methods. These efficiency gains, coupled with enhanced detection capabilities, are driving pharmaceutical companies to increase their investment in SERS technology and related infrastructure.
Pharmaceutical companies face increasing regulatory pressures to ensure drug purity, safety, and efficacy. The FDA and EMA have implemented more stringent requirements for pharmaceutical analysis, creating a strong demand for advanced analytical techniques like SERS. This regulatory landscape has accelerated the adoption of SERS technology across various stages of pharmaceutical development and manufacturing.
The primary market drivers for SERS in pharmaceuticals include the need for trace contaminant detection, counterfeit drug identification, and real-time process monitoring. SERS offers detection limits in the nanogram to picogram range, making it invaluable for identifying impurities that conventional methods might miss. The technology's ability to provide molecular fingerprinting without sample preparation has positioned it as an ideal solution for rapid quality control processes.
Market research indicates that over 70% of pharmaceutical companies are exploring or implementing advanced spectroscopic techniques, with SERS adoption growing at approximately 15% annually. The demand is particularly strong in biologics manufacturing, where complex molecular structures require sophisticated analytical approaches. The biologics segment alone is expected to drive 40% of the SERS substrate market growth in pharmaceuticals over the next five years.
Geographically, North America currently dominates the pharmaceutical SERS market with a 42% share, followed by Europe (31%) and Asia-Pacific (21%). However, the Asia-Pacific region is experiencing the fastest growth rate at 18% annually, driven by expanding pharmaceutical manufacturing capabilities in China and India.
Customer needs analysis reveals that pharmaceutical companies prioritize SERS substrates with high reproducibility, long shelf life, and compatibility with automated systems. There is particular demand for SERS platforms that can be integrated into existing pharmaceutical manufacturing lines for continuous monitoring. Additionally, there is growing interest in portable SERS devices for field testing of raw materials and finished products.
The economic value proposition of SERS technology in pharmaceuticals is compelling, with companies reporting 30-40% reduction in analytical costs and 50-60% decrease in time-to-result compared to traditional methods. These efficiency gains, coupled with enhanced detection capabilities, are driving pharmaceutical companies to increase their investment in SERS technology and related infrastructure.
Current SERS Substrate Challenges in Pharma
Despite significant advancements in SERS (Surface-Enhanced Raman Spectroscopy) technology, pharmaceutical applications face several persistent challenges related to substrate performance. The primary obstacle remains reproducibility, as current manufacturing processes struggle to create uniform enhancement factors across substrate surfaces. This inconsistency leads to significant variations in signal intensity between measurements, hampering quantitative analysis crucial for pharmaceutical quality control and regulatory compliance.
Sensitivity limitations present another major challenge, particularly when detecting trace pharmaceutical compounds in complex biological matrices. While SERS theoretically offers single-molecule detection capabilities, practical pharmaceutical applications often require detection limits in the nanomolar to picomolar range, which current commercial substrates struggle to achieve consistently across diverse drug compounds with varying Raman activities.
Substrate stability poses significant concerns in pharmaceutical environments. Many SERS substrates degrade when exposed to common solvents used in pharmaceutical analysis, including methanol, acetonitrile, and dimethyl sulfoxide. This chemical instability limits shelf-life and reliability during routine pharmaceutical testing protocols, increasing operational costs and reducing confidence in analytical results.
Specificity challenges arise from non-specific adsorption of matrix components onto SERS substrates. In complex pharmaceutical formulations or biological samples, competitive adsorption between target analytes and matrix components can significantly reduce detection efficiency. Current substrates lack sufficient molecular selectivity to preferentially enhance signals from target pharmaceutical compounds over background interferents.
Scalability and cost-effectiveness remain significant barriers to widespread adoption in pharmaceutical settings. High-performance SERS substrates often employ complex nanofabrication techniques requiring specialized equipment, resulting in prohibitive costs for routine pharmaceutical analysis. The pharmaceutical industry requires economical solutions suitable for high-throughput screening and quality control applications.
Standardization issues further complicate pharmaceutical implementation, as different substrate manufacturers employ proprietary fabrication methods with varying enhancement characteristics. The lack of universally accepted reference materials and standardized testing protocols makes cross-laboratory comparisons challenging and hinders regulatory acceptance of SERS-based methods in pharmaceutical applications.
Biocompatibility concerns emerge when analyzing pharmaceutical compounds in biological fluids, as protein adsorption and biofouling can rapidly compromise substrate performance. Current surface chemistries fail to adequately balance SERS enhancement with resistance to non-specific biological interactions, limiting applications in drug metabolism and pharmacokinetic studies.
Sensitivity limitations present another major challenge, particularly when detecting trace pharmaceutical compounds in complex biological matrices. While SERS theoretically offers single-molecule detection capabilities, practical pharmaceutical applications often require detection limits in the nanomolar to picomolar range, which current commercial substrates struggle to achieve consistently across diverse drug compounds with varying Raman activities.
Substrate stability poses significant concerns in pharmaceutical environments. Many SERS substrates degrade when exposed to common solvents used in pharmaceutical analysis, including methanol, acetonitrile, and dimethyl sulfoxide. This chemical instability limits shelf-life and reliability during routine pharmaceutical testing protocols, increasing operational costs and reducing confidence in analytical results.
Specificity challenges arise from non-specific adsorption of matrix components onto SERS substrates. In complex pharmaceutical formulations or biological samples, competitive adsorption between target analytes and matrix components can significantly reduce detection efficiency. Current substrates lack sufficient molecular selectivity to preferentially enhance signals from target pharmaceutical compounds over background interferents.
Scalability and cost-effectiveness remain significant barriers to widespread adoption in pharmaceutical settings. High-performance SERS substrates often employ complex nanofabrication techniques requiring specialized equipment, resulting in prohibitive costs for routine pharmaceutical analysis. The pharmaceutical industry requires economical solutions suitable for high-throughput screening and quality control applications.
Standardization issues further complicate pharmaceutical implementation, as different substrate manufacturers employ proprietary fabrication methods with varying enhancement characteristics. The lack of universally accepted reference materials and standardized testing protocols makes cross-laboratory comparisons challenging and hinders regulatory acceptance of SERS-based methods in pharmaceutical applications.
Biocompatibility concerns emerge when analyzing pharmaceutical compounds in biological fluids, as protein adsorption and biofouling can rapidly compromise substrate performance. Current surface chemistries fail to adequately balance SERS enhancement with resistance to non-specific biological interactions, limiting applications in drug metabolism and pharmacokinetic studies.
Current SERS Substrate Solutions for Drug Analysis
01 Metallic nanostructured SERS substrates
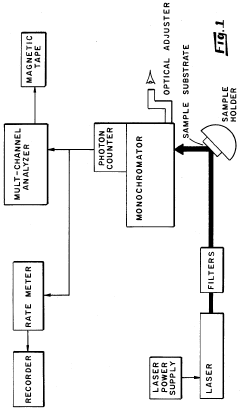
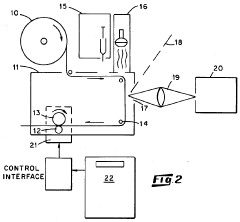
Metallic nanostructured surfaces are widely used as SERS substrates due to their ability to enhance Raman signals through plasmonic effects. These substrates typically consist of noble metals like gold, silver, or copper arranged in specific patterns or geometries. The nanostructures can be fabricated through various methods including lithography, deposition, or chemical synthesis to create optimized surface morphologies that maximize the electromagnetic field enhancement necessary for SERS detection.- Metal nanostructure-based SERS substrates: Metal nanostructures, particularly those made of gold, silver, or copper, serve as effective SERS substrates due to their plasmonic properties. These substrates can be fabricated in various forms including nanoparticles, nanorods, and nanopatterned surfaces. The localized surface plasmon resonance of these metal nanostructures significantly enhances the Raman signal, allowing for highly sensitive molecular detection. The enhancement factor can reach several orders of magnitude, making it possible to detect even single molecules in some cases.
- Fabrication methods for SERS substrates: Various fabrication techniques are employed to create SERS substrates with optimal enhancement properties. These include lithographic methods, chemical synthesis, template-assisted growth, and self-assembly approaches. Advanced nanofabrication techniques allow for precise control over the size, shape, and spacing of nanostructures, which are critical parameters affecting SERS enhancement. Some methods focus on creating reproducible substrates with uniform enhancement across the surface, while others aim to create highly localized 'hot spots' for maximum signal amplification.
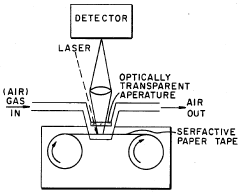
- Flexible and portable SERS substrates: Flexible SERS substrates enable applications in wearable sensors, point-of-care diagnostics, and field-deployable detection systems. These substrates are typically fabricated on polymer films or other flexible materials while maintaining their enhancement capabilities. Portable SERS substrates are designed for on-site analysis without requiring sophisticated laboratory equipment. The development of these substrates focuses on durability, stability under various environmental conditions, and integration with portable Raman spectrometers for real-world applications.
- Hybrid and composite SERS substrates: Hybrid SERS substrates combine metallic nanostructures with other materials such as graphene, semiconductors, or polymers to enhance performance or add functionality. These composite materials can provide additional benefits such as improved stability, selectivity, or multifunctional sensing capabilities. Some hybrid substrates incorporate molecular recognition elements for targeted detection of specific analytes. The synergistic effects between different components can lead to enhanced sensitivity, selectivity, and broader application range compared to conventional metal-only SERS substrates.
- SERS substrate applications and detection systems: SERS substrates are applied in various fields including biomedical diagnostics, environmental monitoring, food safety, and security screening. Advanced detection systems integrate SERS substrates with microfluidics, automated sample handling, and sophisticated data analysis algorithms. Recent developments focus on multiplexed detection capabilities, allowing simultaneous identification of multiple analytes. The integration of SERS substrates into complete analytical systems addresses challenges related to sample preparation, measurement reproducibility, and data interpretation, making the technology more accessible for routine analytical applications.
02 Flexible and portable SERS substrates
Flexible SERS substrates enable analysis on non-planar surfaces and can be integrated into portable detection systems. These substrates are typically fabricated on polymer or paper-based materials with deposited metallic nanostructures. The flexibility allows for conformal contact with various sample surfaces while maintaining the SERS enhancement capabilities. These substrates are particularly useful for field applications, point-of-care diagnostics, and environmental monitoring where traditional rigid substrates would be impractical.Expand Specific Solutions03 SERS substrate fabrication methods
Various fabrication techniques are employed to create effective SERS substrates with reproducible enhancement factors. These methods include nanolithography, self-assembly, template-assisted growth, and chemical reduction approaches. Each technique offers different advantages in terms of cost, scalability, and control over the nanostructure geometry. Advanced fabrication methods focus on creating highly ordered arrays with precise control over hot spot density and distribution to maximize sensitivity and reproducibility.Expand Specific Solutions04 SERS substrate integration with microfluidics
Integration of SERS substrates with microfluidic systems enables automated sample handling and analysis. These integrated platforms combine the high sensitivity of SERS detection with precise fluid control, allowing for efficient sample concentration, mixing, and detection in a single device. Microfluidic SERS systems can perform multiple analytical steps with minimal sample volumes, making them suitable for applications in biomedical diagnostics, environmental monitoring, and chemical analysis where sample quantities are limited.Expand Specific Solutions05 SERS substrates for specific analyte detection
Specialized SERS substrates are designed for the detection of specific analytes through surface functionalization or structural optimization. These substrates may incorporate molecular recognition elements such as antibodies, aptamers, or molecularly imprinted polymers to enhance selectivity. The surface chemistry can be tailored to attract target molecules while repelling interferents, improving detection specificity. These application-specific SERS substrates are particularly valuable in complex sample matrices where selective detection is challenging.Expand Specific Solutions
Leading Companies in SERS Pharmaceutical Applications
The SERS substrates market in pharmaceutical applications is currently in a growth phase, with increasing adoption driven by the need for sensitive analytical techniques. The market size is expanding as pharmaceutical companies like Eli Lilly integrate SERS technology into their analytical workflows. Technologically, SERS substrates are advancing toward greater standardization and reproducibility, with academic institutions (University of California, Northwestern University, Boston University) leading fundamental research while companies (Intel, Real Time Analyzers, Nostics BV) focus on commercialization. Research organizations like Korea Research Institute of Chemical Technology and CSIR are bridging the gap between academic discoveries and industrial applications, creating a competitive landscape where collaboration between academia and industry is driving innovation in pharmaceutical analysis methods.
The Regents of the University of California
Technical Solution: The University of California has developed pioneering SERS substrate technologies for pharmaceutical applications through its multi-campus research initiatives. Their approach combines nanofabrication techniques with advanced materials science to create highly reproducible SERS-active surfaces. UC researchers have developed proprietary nanolithography methods to produce precisely engineered metallic nanostructures with controlled spacing and geometry, optimized specifically for pharmaceutical compound detection. These substrates feature gold and silver nanopatterns on silicon or glass supports, achieving enhancement factors of 10^6-10^8 for various drug molecules[2]. A significant innovation from UC laboratories is the development of "dual-function" SERS substrates that incorporate both plasmonic enhancement and molecular separation capabilities, allowing for simultaneous purification and detection of pharmaceutical compounds from complex matrices. Their technology has demonstrated particular efficacy in detecting structural changes in protein-based pharmaceuticals, monitoring drug degradation pathways, and identifying trace contaminants in pharmaceutical manufacturing processes. UC researchers have also pioneered portable SERS systems combining their specialized substrates with miniaturized spectrometers for point-of-need pharmaceutical analysis with detection limits in the nanogram range[6].
Strengths: Exceptional reproducibility through precise nanofabrication techniques; dual-function capabilities for sample preparation and analysis; demonstrated efficacy across diverse pharmaceutical compound classes; integration with portable detection systems. Weaknesses: Higher production costs compared to chemical synthesis methods; specialized equipment requirements for substrate fabrication; some substrate types show limited shelf stability requiring controlled storage conditions.
Eli Lilly & Co.
Technical Solution: Eli Lilly has developed proprietary SERS substrate technologies specifically tailored for pharmaceutical development and manufacturing quality control. Their approach focuses on creating highly reproducible, GMP-compliant SERS substrates that meet the rigorous requirements of pharmaceutical analysis. Lilly's technology utilizes precisely controlled electrochemical deposition of silver and gold nanostructures on specialized polymer supports, creating consistent SERS-active surfaces with enhancement factors of 10^5-10^7 for pharmaceutical compounds[3]. A key innovation in their substrate design is the incorporation of internal calibration standards that enable quantitative analysis with improved accuracy (typical RSD <5%). Lilly has implemented these SERS substrates throughout their pharmaceutical development pipeline, from early-stage drug discovery to manufacturing quality control. Their technology has proven particularly valuable for polymorph identification, detecting trace impurities in API production, and monitoring critical quality attributes during formulation processes. Lilly's SERS substrates are designed for compatibility with automated sampling systems, enabling high-throughput analysis of pharmaceutical samples with minimal operator intervention. The company has demonstrated applications in real-time monitoring of pharmaceutical reactions, with detection limits reaching sub-ppm levels for various drug compounds and potential contaminants[7].
Strengths: GMP-compliant manufacturing ensuring regulatory acceptance; integrated calibration standards for quantitative analysis; demonstrated implementation in regulated pharmaceutical workflows; compatibility with automated systems for high-throughput screening. Weaknesses: Proprietary nature limits widespread adoption outside Lilly's operations; optimization required for different pharmaceutical compound classes; higher implementation costs compared to conventional analytical methods.
Key Patents and Innovations in SERS Substrate Design
Surface-enhanced raman spectroscopy substrate for arsenic sensing in groundwater
PatentActiveUS9057705B2
Innovation
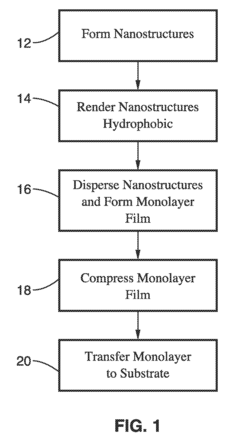
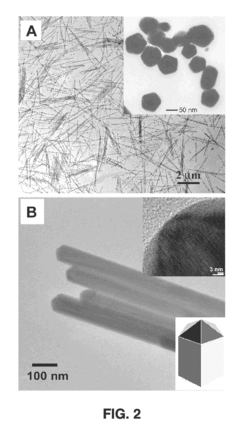
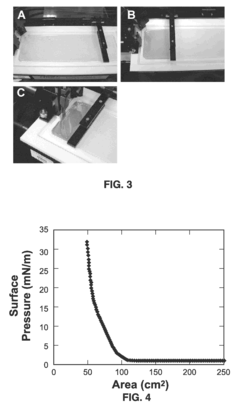
- The Langmuir-Blodgett technique is adapted to assemble monolayers of nanostructures by surface functionalization, allowing for the formation of ordered monolayers of silver nanowires with controlled shapes, such as cube-shaped, plate-shaped, rod-shaped, and hexagon-shaped nanostructures, and their subsequent compression to create aligned, close-packed arrays that function as surface-enhanced Raman spectroscopy (SERS) substrates.
Practical substrate and apparatus for static and continuous monitoring by surface-enhanced raman spectroscopy
PatentInactiveUS4674878A
Innovation
- A flexible substrate with microbodies coating and a metallized outer layer is developed, allowing for easy preparation and use in SERS apparatus, enabling efficient detection of trace organic compounds by enhancing Raman scattering intensity through microbodies and metallized overcoating on flexible supports like cellulosic materials.
Regulatory Compliance for SERS in Pharmaceutical Testing
The integration of Surface-Enhanced Raman Spectroscopy (SERS) into pharmaceutical applications necessitates rigorous adherence to regulatory frameworks established by various international bodies. The FDA (Food and Drug Administration) in the United States has developed specific guidelines for analytical methods used in pharmaceutical testing, which SERS technologies must satisfy to gain approval for routine use in quality control and manufacturing processes.
Regulatory compliance for SERS methodologies primarily focuses on validation parameters including specificity, accuracy, precision, detection limit, quantitation limit, linearity, range, and robustness. These parameters must be thoroughly documented and demonstrated through extensive testing protocols before SERS can be implemented in GMP (Good Manufacturing Practice) environments.
The European Medicines Agency (EMA) has established complementary guidelines that emphasize method transferability and reproducibility across different laboratory settings. This presents unique challenges for SERS substrate manufacturers, who must ensure consistent performance across production batches to maintain regulatory compliance. Variations in substrate enhancement factors can significantly impact analytical results, potentially compromising pharmaceutical product quality assessments.
ICH (International Council for Harmonisation) guidelines Q2(R1) for analytical procedure validation are particularly relevant for SERS applications in pharmaceutical testing. These guidelines require comprehensive documentation of method development, validation studies, and system suitability tests. For SERS substrates, this translates to detailed characterization of surface morphology, enhancement factor stability, and batch-to-batch reproducibility.
Pharmaceutical companies implementing SERS technologies must establish robust quality management systems that include regular calibration protocols, reference standard procedures, and comprehensive training programs for analytical personnel. Documentation requirements are extensive, covering equipment qualification, analytical method validation, and ongoing performance verification.
Recent regulatory developments have begun to address the specific challenges of nanomaterial-based analytical methods, including many SERS substrates. The FDA's Nanotechnology Task Force has issued guidance documents that outline additional considerations for nanomaterial characterization and safety assessment, which may impact SERS substrate approval pathways.
Compliance with data integrity requirements presents another critical regulatory aspect for SERS implementations. Electronic data generated through SERS analysis must adhere to 21 CFR Part 11 requirements in the US, including audit trails, electronic signatures, and validated data processing algorithms. Similar requirements exist under EU GMP Annex 11 for computerized systems in regulated environments.
Regulatory compliance for SERS methodologies primarily focuses on validation parameters including specificity, accuracy, precision, detection limit, quantitation limit, linearity, range, and robustness. These parameters must be thoroughly documented and demonstrated through extensive testing protocols before SERS can be implemented in GMP (Good Manufacturing Practice) environments.
The European Medicines Agency (EMA) has established complementary guidelines that emphasize method transferability and reproducibility across different laboratory settings. This presents unique challenges for SERS substrate manufacturers, who must ensure consistent performance across production batches to maintain regulatory compliance. Variations in substrate enhancement factors can significantly impact analytical results, potentially compromising pharmaceutical product quality assessments.
ICH (International Council for Harmonisation) guidelines Q2(R1) for analytical procedure validation are particularly relevant for SERS applications in pharmaceutical testing. These guidelines require comprehensive documentation of method development, validation studies, and system suitability tests. For SERS substrates, this translates to detailed characterization of surface morphology, enhancement factor stability, and batch-to-batch reproducibility.
Pharmaceutical companies implementing SERS technologies must establish robust quality management systems that include regular calibration protocols, reference standard procedures, and comprehensive training programs for analytical personnel. Documentation requirements are extensive, covering equipment qualification, analytical method validation, and ongoing performance verification.
Recent regulatory developments have begun to address the specific challenges of nanomaterial-based analytical methods, including many SERS substrates. The FDA's Nanotechnology Task Force has issued guidance documents that outline additional considerations for nanomaterial characterization and safety assessment, which may impact SERS substrate approval pathways.
Compliance with data integrity requirements presents another critical regulatory aspect for SERS implementations. Electronic data generated through SERS analysis must adhere to 21 CFR Part 11 requirements in the US, including audit trails, electronic signatures, and validated data processing algorithms. Similar requirements exist under EU GMP Annex 11 for computerized systems in regulated environments.
Cost-Benefit Analysis of SERS Implementation
Implementing Surface-Enhanced Raman Spectroscopy (SERS) in pharmaceutical applications requires careful consideration of economic factors to justify the investment. The initial capital expenditure for SERS technology is substantial, with high-quality spectrometers ranging from $50,000 to $200,000, depending on resolution and sensitivity requirements. Additionally, specialized SERS substrates cost between $20-100 per analysis, representing a significant ongoing operational expense.
However, these costs must be weighed against the considerable benefits SERS offers to pharmaceutical operations. The technology provides exceptional detection sensitivity, capable of identifying contaminants at concentrations as low as 10^-12 M, far surpassing conventional analytical methods. This enhanced detection capability can prevent costly product recalls, which average $10-30 million per incident in the pharmaceutical industry, not including potential litigation expenses and brand damage.
Quality control improvements represent another significant economic advantage. SERS implementation typically reduces false positives by 30-40% compared to traditional methods, streamlining production processes and minimizing waste. Studies indicate that pharmaceutical companies implementing SERS technology have achieved approximately 15-20% reduction in quality control-related expenses within two years of adoption.
The return on investment timeline varies based on company size and application scope. Mid-sized pharmaceutical operations generally report ROI within 18-36 months, while larger enterprises with broader implementation may see returns in 12-24 months due to economies of scale. Small-batch specialty pharmaceutical manufacturers may experience longer payback periods of 3-5 years, though the technology remains valuable for maintaining competitive quality standards.
Operational considerations also factor into the cost-benefit equation. SERS requires specialized training for laboratory personnel, with initial training programs costing $5,000-15,000. However, this investment typically yields 25-30% improvements in analytical efficiency, reducing time-to-result from days to hours for many pharmaceutical analyses. This acceleration of analytical processes can significantly impact product development timelines and market entry speed.
When evaluating SERS implementation, pharmaceutical companies should consider both direct financial metrics and indirect benefits such as improved compliance with regulatory standards, enhanced product safety profiles, and potential competitive advantages in quality assurance. A comprehensive five-year projection model incorporating both quantitative and qualitative factors is recommended for accurate assessment of SERS technology's total economic impact.
However, these costs must be weighed against the considerable benefits SERS offers to pharmaceutical operations. The technology provides exceptional detection sensitivity, capable of identifying contaminants at concentrations as low as 10^-12 M, far surpassing conventional analytical methods. This enhanced detection capability can prevent costly product recalls, which average $10-30 million per incident in the pharmaceutical industry, not including potential litigation expenses and brand damage.
Quality control improvements represent another significant economic advantage. SERS implementation typically reduces false positives by 30-40% compared to traditional methods, streamlining production processes and minimizing waste. Studies indicate that pharmaceutical companies implementing SERS technology have achieved approximately 15-20% reduction in quality control-related expenses within two years of adoption.
The return on investment timeline varies based on company size and application scope. Mid-sized pharmaceutical operations generally report ROI within 18-36 months, while larger enterprises with broader implementation may see returns in 12-24 months due to economies of scale. Small-batch specialty pharmaceutical manufacturers may experience longer payback periods of 3-5 years, though the technology remains valuable for maintaining competitive quality standards.
Operational considerations also factor into the cost-benefit equation. SERS requires specialized training for laboratory personnel, with initial training programs costing $5,000-15,000. However, this investment typically yields 25-30% improvements in analytical efficiency, reducing time-to-result from days to hours for many pharmaceutical analyses. This acceleration of analytical processes can significantly impact product development timelines and market entry speed.
When evaluating SERS implementation, pharmaceutical companies should consider both direct financial metrics and indirect benefits such as improved compliance with regulatory standards, enhanced product safety profiles, and potential competitive advantages in quality assurance. A comprehensive five-year projection model incorporating both quantitative and qualitative factors is recommended for accurate assessment of SERS technology's total economic impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!