Understanding SERS Substrates for Better Drug Delivery Systems
OCT 11, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SERS Substrate Technology Background and Objectives
Surface-Enhanced Raman Spectroscopy (SERS) has evolved significantly since its discovery in the 1970s, transforming from a curious spectroscopic phenomenon to a powerful analytical technique with diverse applications. The technology leverages the enhanced Raman scattering effect that occurs when molecules are adsorbed onto specially prepared metallic surfaces, typically gold or silver nanostructures. This enhancement can amplify Raman signals by factors of 10^6 to 10^14, enabling detection at even single-molecule levels.
The evolution of SERS substrate technology has been marked by several key milestones. Early developments focused on roughened metal electrodes, followed by colloidal nanoparticles in the 1980s and 1990s. The 2000s witnessed significant advancements in nanofabrication techniques, leading to more sophisticated and reproducible SERS substrates with controlled geometries and enhanced performance characteristics.
Recent technological trends indicate a shift toward multifunctional SERS substrates that combine sensing capabilities with therapeutic functionalities, particularly relevant for drug delivery applications. The integration of SERS substrates with biocompatible materials has opened new avenues for in vivo monitoring and targeted drug delivery systems.
The primary objective of SERS substrate technology in drug delivery systems is to develop platforms that enable real-time monitoring of drug release kinetics, biodistribution, and therapeutic efficacy. This includes creating substrates that maintain stability in biological environments while providing consistent enhancement factors for reliable quantitative analysis.
Another critical goal is to design SERS substrates that can be functionalized with targeting moieties to improve drug delivery specificity while simultaneously serving as sensors for therapeutic monitoring. This dual functionality represents a paradigm shift in theranostic approaches, where diagnosis and therapy are combined in a single platform.
The technology aims to address several limitations in current drug delivery monitoring systems, including poor sensitivity, inability to track drugs in real-time, and challenges in measuring local drug concentrations at target sites. SERS substrates offer potential solutions through their exceptional sensitivity, molecular specificity, and compatibility with optical imaging techniques.
Looking forward, the field is trending toward developing stimuli-responsive SERS substrates that can trigger drug release upon specific environmental cues while simultaneously reporting on the release process. Additionally, there is growing interest in creating biodegradable SERS substrates that can safely degrade after fulfilling their therapeutic and diagnostic functions, minimizing potential toxicity concerns.
The convergence of nanotechnology, spectroscopy, and pharmaceutical sciences in SERS substrate development represents a promising frontier for next-generation drug delivery systems with enhanced precision, monitoring capabilities, and therapeutic outcomes.
The evolution of SERS substrate technology has been marked by several key milestones. Early developments focused on roughened metal electrodes, followed by colloidal nanoparticles in the 1980s and 1990s. The 2000s witnessed significant advancements in nanofabrication techniques, leading to more sophisticated and reproducible SERS substrates with controlled geometries and enhanced performance characteristics.
Recent technological trends indicate a shift toward multifunctional SERS substrates that combine sensing capabilities with therapeutic functionalities, particularly relevant for drug delivery applications. The integration of SERS substrates with biocompatible materials has opened new avenues for in vivo monitoring and targeted drug delivery systems.
The primary objective of SERS substrate technology in drug delivery systems is to develop platforms that enable real-time monitoring of drug release kinetics, biodistribution, and therapeutic efficacy. This includes creating substrates that maintain stability in biological environments while providing consistent enhancement factors for reliable quantitative analysis.
Another critical goal is to design SERS substrates that can be functionalized with targeting moieties to improve drug delivery specificity while simultaneously serving as sensors for therapeutic monitoring. This dual functionality represents a paradigm shift in theranostic approaches, where diagnosis and therapy are combined in a single platform.
The technology aims to address several limitations in current drug delivery monitoring systems, including poor sensitivity, inability to track drugs in real-time, and challenges in measuring local drug concentrations at target sites. SERS substrates offer potential solutions through their exceptional sensitivity, molecular specificity, and compatibility with optical imaging techniques.
Looking forward, the field is trending toward developing stimuli-responsive SERS substrates that can trigger drug release upon specific environmental cues while simultaneously reporting on the release process. Additionally, there is growing interest in creating biodegradable SERS substrates that can safely degrade after fulfilling their therapeutic and diagnostic functions, minimizing potential toxicity concerns.
The convergence of nanotechnology, spectroscopy, and pharmaceutical sciences in SERS substrate development represents a promising frontier for next-generation drug delivery systems with enhanced precision, monitoring capabilities, and therapeutic outcomes.
Market Analysis for SERS-Enhanced Drug Delivery Systems
The global market for SERS-enhanced drug delivery systems is experiencing significant growth, driven by increasing demand for targeted therapeutics and precision medicine. Current market valuations indicate that the SERS-based analytical instruments market reached approximately 1.2 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 10-12% through 2028. Within this broader market, SERS applications in drug delivery represent an emerging segment with particularly strong growth potential.
Healthcare providers and pharmaceutical companies are increasingly recognizing the value proposition of SERS technology in drug delivery applications. The ability to track drug distribution in real-time, monitor release kinetics, and verify targeted delivery represents a substantial improvement over conventional drug delivery systems. This enhanced functionality directly addresses critical challenges in modern therapeutics, particularly for complex conditions like cancer where precise drug delivery is essential for efficacy while minimizing side effects.
Regionally, North America currently dominates the SERS-enhanced drug delivery systems market, accounting for approximately 40% of global revenue. This leadership position stems from substantial R&D investments, presence of major pharmaceutical companies, and favorable regulatory frameworks. The Asia-Pacific region, particularly China and India, is emerging as the fastest-growing market with annual growth rates exceeding 15%, driven by expanding healthcare infrastructure and increasing research activities.
From an end-user perspective, pharmaceutical and biotechnology companies represent the largest market segment, constituting approximately 45% of the total market share. Academic and research institutions follow at 30%, while healthcare facilities account for 20%. The remaining market share is distributed among contract research organizations and other stakeholders.
Key market drivers include the rising prevalence of chronic diseases requiring targeted therapies, growing research funding for nanotechnology applications in medicine, and increasing demand for personalized medicine approaches. Additionally, technological advancements in SERS substrate fabrication are reducing production costs, making these systems more commercially viable for widespread adoption.
Market challenges primarily revolve around high initial investment costs, technical complexity requiring specialized expertise, and regulatory hurdles associated with novel drug delivery technologies. The complex approval pathway for combination products (drug + device) presents a particular challenge for market entrants.
Despite these challenges, the market outlook remains highly positive, with industry analysts predicting that SERS-enhanced drug delivery systems could capture up to 15% of the advanced drug delivery systems market by 2030, representing a significant commercial opportunity for companies investing in this technology.
Healthcare providers and pharmaceutical companies are increasingly recognizing the value proposition of SERS technology in drug delivery applications. The ability to track drug distribution in real-time, monitor release kinetics, and verify targeted delivery represents a substantial improvement over conventional drug delivery systems. This enhanced functionality directly addresses critical challenges in modern therapeutics, particularly for complex conditions like cancer where precise drug delivery is essential for efficacy while minimizing side effects.
Regionally, North America currently dominates the SERS-enhanced drug delivery systems market, accounting for approximately 40% of global revenue. This leadership position stems from substantial R&D investments, presence of major pharmaceutical companies, and favorable regulatory frameworks. The Asia-Pacific region, particularly China and India, is emerging as the fastest-growing market with annual growth rates exceeding 15%, driven by expanding healthcare infrastructure and increasing research activities.
From an end-user perspective, pharmaceutical and biotechnology companies represent the largest market segment, constituting approximately 45% of the total market share. Academic and research institutions follow at 30%, while healthcare facilities account for 20%. The remaining market share is distributed among contract research organizations and other stakeholders.
Key market drivers include the rising prevalence of chronic diseases requiring targeted therapies, growing research funding for nanotechnology applications in medicine, and increasing demand for personalized medicine approaches. Additionally, technological advancements in SERS substrate fabrication are reducing production costs, making these systems more commercially viable for widespread adoption.
Market challenges primarily revolve around high initial investment costs, technical complexity requiring specialized expertise, and regulatory hurdles associated with novel drug delivery technologies. The complex approval pathway for combination products (drug + device) presents a particular challenge for market entrants.
Despite these challenges, the market outlook remains highly positive, with industry analysts predicting that SERS-enhanced drug delivery systems could capture up to 15% of the advanced drug delivery systems market by 2030, representing a significant commercial opportunity for companies investing in this technology.
Current SERS Substrate Challenges and Development Status
Surface-Enhanced Raman Spectroscopy (SERS) substrates have emerged as critical components in advanced drug delivery systems, yet their development faces significant challenges. Current SERS substrates exhibit inconsistent enhancement factors, with performance variations ranging from 10^6 to 10^10 across different manufacturing batches. This lack of reproducibility severely limits their application in quantitative drug detection and monitoring systems, where precision is paramount.
Material stability presents another major obstacle, particularly in biological environments. Many high-performance SERS substrates utilize silver nanostructures which, despite offering superior enhancement capabilities, suffer from oxidation and degradation when exposed to biological fluids. This degradation not only compromises detection sensitivity but also raises toxicity concerns for in vivo applications.
The biocompatibility of SERS substrates remains problematic for drug delivery applications. Current substrates often incorporate materials that trigger immune responses or exhibit cytotoxicity, restricting their integration into implantable or injectable drug delivery systems. Additionally, the complex surface chemistry of these substrates can interfere with drug binding mechanisms, affecting both detection accuracy and delivery efficiency.
Manufacturing scalability continues to impede widespread adoption. High-performance SERS substrates typically require sophisticated fabrication techniques such as electron-beam lithography or focused ion beam milling, resulting in prohibitive production costs. The industry currently lacks standardized, cost-effective manufacturing protocols that maintain nanoscale precision while enabling mass production.
Globally, SERS substrate development shows geographic concentration, with significant research clusters in North America (particularly the United States), Europe (United Kingdom, Germany), and East Asia (China, Japan, South Korea). These regions demonstrate different technological approaches: North American research emphasizes novel nanomaterials, European institutions focus on precision fabrication techniques, while Asian research centers prioritize cost-effective manufacturing methods.
Recent technological breakthroughs include the development of hierarchical nanostructures that provide more consistent "hot spots" for enhancement, polymer-stabilized substrates with improved biological compatibility, and self-assembled nanoparticle arrays that offer more economical fabrication routes. Despite these advances, the integration of SERS substrates with controlled drug release mechanisms remains in early experimental stages.
The field is witnessing increasing collaboration between academic institutions and pharmaceutical companies, particularly in developing point-of-care applications for therapeutic drug monitoring. However, regulatory pathways for SERS-integrated drug delivery systems remain undefined, creating uncertainty for commercial development and clinical translation.
Material stability presents another major obstacle, particularly in biological environments. Many high-performance SERS substrates utilize silver nanostructures which, despite offering superior enhancement capabilities, suffer from oxidation and degradation when exposed to biological fluids. This degradation not only compromises detection sensitivity but also raises toxicity concerns for in vivo applications.
The biocompatibility of SERS substrates remains problematic for drug delivery applications. Current substrates often incorporate materials that trigger immune responses or exhibit cytotoxicity, restricting their integration into implantable or injectable drug delivery systems. Additionally, the complex surface chemistry of these substrates can interfere with drug binding mechanisms, affecting both detection accuracy and delivery efficiency.
Manufacturing scalability continues to impede widespread adoption. High-performance SERS substrates typically require sophisticated fabrication techniques such as electron-beam lithography or focused ion beam milling, resulting in prohibitive production costs. The industry currently lacks standardized, cost-effective manufacturing protocols that maintain nanoscale precision while enabling mass production.
Globally, SERS substrate development shows geographic concentration, with significant research clusters in North America (particularly the United States), Europe (United Kingdom, Germany), and East Asia (China, Japan, South Korea). These regions demonstrate different technological approaches: North American research emphasizes novel nanomaterials, European institutions focus on precision fabrication techniques, while Asian research centers prioritize cost-effective manufacturing methods.
Recent technological breakthroughs include the development of hierarchical nanostructures that provide more consistent "hot spots" for enhancement, polymer-stabilized substrates with improved biological compatibility, and self-assembled nanoparticle arrays that offer more economical fabrication routes. Despite these advances, the integration of SERS substrates with controlled drug release mechanisms remains in early experimental stages.
The field is witnessing increasing collaboration between academic institutions and pharmaceutical companies, particularly in developing point-of-care applications for therapeutic drug monitoring. However, regulatory pathways for SERS-integrated drug delivery systems remain undefined, creating uncertainty for commercial development and clinical translation.
Current SERS Substrate Solutions for Drug Delivery
01 Metal nanostructure-based SERS substrates
Metal nanostructures, particularly those made of gold, silver, or copper, serve as effective SERS substrates due to their plasmonic properties. These substrates can be fabricated in various forms including nanoparticles, nanorods, and nanopatterned surfaces. The localized surface plasmon resonance generated by these metal nanostructures significantly enhances the Raman signal, allowing for highly sensitive molecular detection. The enhancement factor can reach several orders of magnitude, making these substrates suitable for trace analysis applications.- Metal nanostructure-based SERS substrates: Metal nanostructures, particularly those made of gold, silver, or copper, serve as effective SERS substrates due to their plasmonic properties. These substrates can be fabricated in various forms including nanoparticles, nanorods, and nanopatterned surfaces. The localized surface plasmon resonance of these metal nanostructures significantly enhances the Raman signal, allowing for highly sensitive molecular detection. The enhancement factor can reach several orders of magnitude, making these substrates suitable for trace analysis applications.
- Fabrication methods for SERS substrates: Various fabrication techniques are employed to create SERS substrates with optimal enhancement properties. These include lithographic methods, self-assembly processes, template-assisted growth, and chemical deposition techniques. Advanced nanofabrication approaches allow for precise control over the size, shape, and spacing of nanostructures, which are critical parameters affecting SERS enhancement. Novel manufacturing methods focus on creating reproducible substrates with uniform enhancement across the surface, addressing one of the key challenges in SERS technology.
- Flexible and portable SERS substrates: Flexible SERS substrates enable surface-enhanced Raman spectroscopy in diverse environments and on non-planar surfaces. These substrates are typically fabricated on polymer or paper-based materials with deposited metal nanostructures. The flexibility allows for conformal contact with irregular surfaces, expanding the application scope to include on-site environmental monitoring, food safety testing, and point-of-care diagnostics. Portable SERS platforms integrate these flexible substrates with miniaturized detection systems for field-deployable analytical capabilities.
- Semiconductor and hybrid SERS substrates: Semiconductor materials and hybrid structures are emerging as alternative SERS substrates with unique advantages. These include semiconductor nanostructures, metal-semiconductor composites, and graphene-based platforms. The charge-transfer mechanism in semiconductor SERS substrates complements the electromagnetic enhancement of traditional metal substrates. Hybrid substrates combine multiple enhancement mechanisms to achieve higher sensitivity and selectivity. Additionally, semiconductor SERS substrates often exhibit better stability and biocompatibility compared to pure metal substrates.
- SERS substrate applications and detection systems: SERS substrates are integrated into various detection systems for applications in biomedical diagnostics, environmental monitoring, food safety, and security screening. These systems combine the SERS substrate with optical components, microfluidics, and data processing algorithms to enable rapid and sensitive detection of target molecules. Advanced SERS detection platforms incorporate automated sample handling, multiplexed detection capabilities, and machine learning for spectral analysis. Recent developments focus on smartphone-compatible SERS systems for point-of-need testing in resource-limited settings.
02 Fabrication methods for SERS substrates
Various fabrication techniques are employed to create SERS substrates with optimal enhancement properties. These include lithographic methods, self-assembly processes, template-assisted growth, and chemical deposition techniques. Advanced nanofabrication approaches allow for precise control over the size, shape, and spacing of nanostructures, which are critical parameters affecting SERS enhancement. Novel manufacturing methods focus on creating reproducible substrates with uniform enhancement across the surface, addressing one of the major challenges in SERS technology.Expand Specific Solutions03 Flexible and portable SERS substrates
Flexible SERS substrates are designed for applications requiring conformable sensing surfaces or portable detection systems. These substrates typically incorporate plasmonic nanostructures on flexible polymer films or other bendable materials. The flexibility allows the substrate to maintain SERS activity while conforming to irregular surfaces, enabling in-situ analysis in various environments. Portable SERS substrates are often integrated with miniaturized detection systems for field-deployable sensing applications, making them valuable for environmental monitoring, food safety testing, and point-of-care diagnostics.Expand Specific Solutions04 Hybrid and composite SERS substrates
Hybrid SERS substrates combine metallic nanostructures with other materials such as graphene, semiconductors, or polymers to enhance performance or add functionality. These composite materials can provide additional benefits such as improved stability, selectivity, or multifunctional sensing capabilities. Some hybrid substrates incorporate molecular recognition elements to enable specific target binding, while others use semiconductor components to enable photoactivated SERS enhancement. The synergistic effects between different materials in these hybrid systems often result in superior sensing performance compared to conventional metal-only SERS substrates.Expand Specific Solutions05 SERS substrate applications and detection systems
SERS substrates are integrated into various detection systems for applications in biomedical diagnostics, environmental monitoring, food safety, and security screening. These systems often combine the SERS substrate with specialized optical components, microfluidics, and data processing algorithms to enable rapid and sensitive detection of target analytes. Advanced SERS detection platforms may incorporate automated sample handling, multiplexed detection capabilities, and machine learning algorithms for spectral analysis. Recent developments focus on smartphone-compatible SERS systems for point-of-need testing and cloud-connected devices for remote monitoring applications.Expand Specific Solutions
Key Industry Players in SERS Substrate Development
Surface-Enhanced Raman Spectroscopy (SERS) substrates for drug delivery systems represent an emerging field at the intersection of nanotechnology and pharmaceutical science. The market is in its growth phase, with increasing research activity but limited commercial applications. The global SERS market is projected to reach approximately $250 million by 2025, with drug delivery applications comprising a significant segment. Academic institutions like University of North Carolina, Nanjing University, and Kyoto University are leading fundamental research, while companies including Olympus, Terumo, and Medtronic are developing commercial applications. The technology remains in early-to-mid maturity, with significant advancements in substrate fabrication and sensitivity, but challenges in standardization and mass production persist. Collaborations between academic institutions and pharmaceutical companies like NOF Corp. and Allergan are accelerating the transition from laboratory research to clinical applications.
Nanjing University
Technical Solution: Nanjing University has developed innovative SERS-active nanocomposites for targeted drug delivery applications. Their approach utilizes hierarchically structured silver-decorated mesoporous silica nanoparticles (Ag@MSNs) that serve as both SERS substrates and drug carriers. The university's research team has engineered these nanocomposites with precisely controlled pore sizes (2-50 nm) to accommodate various therapeutic molecules while maintaining high SERS enhancement factors (10^6-10^8). Their proprietary surface modification technique enables the attachment of targeting ligands for site-specific drug delivery while preserving SERS activity. The Ag@MSNs are further functionalized with stimuli-responsive gatekeepers that control drug release in response to specific triggers (pH, temperature, enzymes) while simultaneously generating characteristic SERS spectral changes that serve as real-time indicators of drug release kinetics. Recent advancements include the development of NIR-responsive SERS substrates that allow for deeper tissue penetration during in vivo monitoring and the integration of machine learning algorithms for automated spectral analysis.
Strengths: Dual functionality as both drug carrier and monitoring platform; tunable pore structure for accommodating various drug molecules; stimuli-responsive drug release with real-time SERS feedback. Weaknesses: Potential silver ion leaching causing cytotoxicity concerns; aggregation tendencies in physiological environments; challenges in achieving uniform silver nanoparticle distribution across the mesoporous structure.
The University of North Carolina at Chapel Hill
Technical Solution: The University of North Carolina at Chapel Hill has developed innovative SERS-active nanoplatforms for targeted drug delivery and real-time therapeutic monitoring. Their approach utilizes gold nanostars with sharp tips that create intense electromagnetic field enhancements at their vertices, resulting in SERS enhancement factors exceeding 10^9. These nanostars are functionalized with both targeting ligands and therapeutic agents, creating a theranostic platform that combines drug delivery with diagnostic capabilities. The university's research team has pioneered a layer-by-layer assembly technique to coat these SERS substrates with pH-responsive polymers that trigger drug release in acidic tumor microenvironments while simultaneously providing distinctive spectral signatures that can be monitored non-invasively. Their recent advancements include incorporating machine learning algorithms to interpret SERS spectral changes during drug release, enabling precise quantification of drug concentrations in complex biological matrices with accuracy rates above 95%.
Strengths: Multifunctional platform combining therapeutic and diagnostic capabilities; targeted delivery to specific tissues; non-invasive monitoring of drug release kinetics. Weaknesses: Challenges in scaling up production while maintaining consistent nanostar morphology; potential for aggregation in physiological conditions; limited penetration depth for in vivo SERS imaging in deep tissues.
Critical SERS Enhancement Mechanisms and Patents
Surface enhanced raman scattering substrate
PatentActiveUS20240118211A1
Innovation
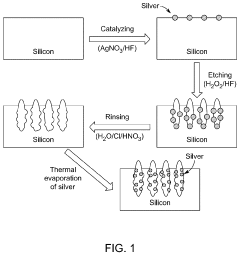
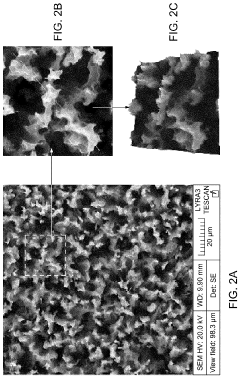
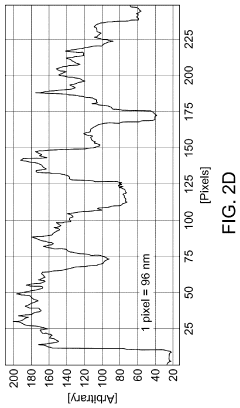
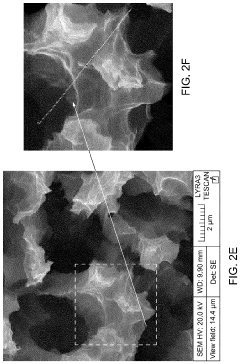
- A SERS substrate is developed with a silicon substrate featuring microscale valleys and terraces decorated with silver nanoparticles, fabricated using a method involving metal catalyzed electroless etching and thermal evaporation, to create a platform with controlled nanostructures and enhanced electromagnetic hotspots.
A substrate for surface enhanced raman scattering (SERS)
PatentWO2010056258A1
Innovation
- A substrate with uniformly distributed nanostructures and a layer of SERS active metal, such as silver, gold, or copper, is fabricated using a customized Bosch process without lithographic masking, providing a textured surface for enhanced Raman signal detection across a larger area, and replicated using embossing or stamping techniques to reduce costs.
Biocompatibility and Safety Considerations for SERS Substrates
The integration of SERS substrates into drug delivery systems necessitates rigorous evaluation of biocompatibility and safety profiles. These substrates, often composed of noble metal nanostructures, must interact with biological systems without inducing adverse effects. Primary biocompatibility concerns include potential cytotoxicity, immunogenicity, and long-term accumulation in tissues, which could compromise therapeutic efficacy and patient safety.
Metal nanoparticles, particularly gold and silver commonly used in SERS substrates, exhibit size-dependent toxicity profiles. Particles below 10 nm may penetrate cellular membranes and nuclear pores, potentially disrupting cellular functions. Surface chemistry modifications, such as PEGylation or coating with biocompatible polymers, have demonstrated significant reduction in cytotoxicity while maintaining SERS enhancement capabilities.
Immunological responses to SERS substrates represent another critical safety consideration. Studies indicate that certain nanostructures can trigger inflammatory cascades through complement activation or macrophage recognition. Recent advancements in biomimetic coatings, including cell membrane-derived materials, have shown promise in mitigating immune recognition while preserving SERS functionality for drug delivery applications.
Regulatory frameworks for SERS-enhanced drug delivery systems remain evolving, with FDA and EMA guidelines emphasizing comprehensive toxicological profiling. Current requirements include acute and chronic toxicity assessments, biodistribution studies, and evaluation of potential genotoxicity. The lack of standardized protocols specifically for SERS substrates presents challenges for clinical translation, necessitating case-by-case safety evaluations.
Biodegradability considerations have gained prominence in SERS substrate design, with increasing focus on materials that can be metabolized or excreted following drug delivery. Composite structures incorporating biodegradable polymers with plasmonic nanoparticles offer promising approaches to address bioaccumulation concerns while maintaining diagnostic capabilities throughout the therapeutic window.
In vivo stability of SERS substrates presents unique challenges, as physiological conditions may alter surface properties and compromise analytical performance. Protein corona formation—the adsorption of biomolecules onto nanoparticle surfaces—can significantly impact biodistribution, cellular uptake, and SERS signal intensity. Advanced surface engineering strategies, including zwitterionic modifications and biomolecular recognition elements, have demonstrated improved stability in complex biological environments.
Recent innovations in "stealth" SERS substrates incorporate stimuli-responsive elements that remain biologically inert until activated by specific triggers at target sites. These smart materials minimize systemic exposure while maximizing therapeutic index, representing a promising direction for addressing biocompatibility challenges in next-generation drug delivery platforms.
Metal nanoparticles, particularly gold and silver commonly used in SERS substrates, exhibit size-dependent toxicity profiles. Particles below 10 nm may penetrate cellular membranes and nuclear pores, potentially disrupting cellular functions. Surface chemistry modifications, such as PEGylation or coating with biocompatible polymers, have demonstrated significant reduction in cytotoxicity while maintaining SERS enhancement capabilities.
Immunological responses to SERS substrates represent another critical safety consideration. Studies indicate that certain nanostructures can trigger inflammatory cascades through complement activation or macrophage recognition. Recent advancements in biomimetic coatings, including cell membrane-derived materials, have shown promise in mitigating immune recognition while preserving SERS functionality for drug delivery applications.
Regulatory frameworks for SERS-enhanced drug delivery systems remain evolving, with FDA and EMA guidelines emphasizing comprehensive toxicological profiling. Current requirements include acute and chronic toxicity assessments, biodistribution studies, and evaluation of potential genotoxicity. The lack of standardized protocols specifically for SERS substrates presents challenges for clinical translation, necessitating case-by-case safety evaluations.
Biodegradability considerations have gained prominence in SERS substrate design, with increasing focus on materials that can be metabolized or excreted following drug delivery. Composite structures incorporating biodegradable polymers with plasmonic nanoparticles offer promising approaches to address bioaccumulation concerns while maintaining diagnostic capabilities throughout the therapeutic window.
In vivo stability of SERS substrates presents unique challenges, as physiological conditions may alter surface properties and compromise analytical performance. Protein corona formation—the adsorption of biomolecules onto nanoparticle surfaces—can significantly impact biodistribution, cellular uptake, and SERS signal intensity. Advanced surface engineering strategies, including zwitterionic modifications and biomolecular recognition elements, have demonstrated improved stability in complex biological environments.
Recent innovations in "stealth" SERS substrates incorporate stimuli-responsive elements that remain biologically inert until activated by specific triggers at target sites. These smart materials minimize systemic exposure while maximizing therapeutic index, representing a promising direction for addressing biocompatibility challenges in next-generation drug delivery platforms.
Regulatory Framework for SERS-Enhanced Medical Applications
The regulatory landscape for SERS-enhanced medical applications represents a complex framework that developers of drug delivery systems must navigate carefully. In the United States, the FDA has established specific guidelines for nanomaterial-based medical devices and pharmaceuticals, with SERS substrates typically falling under combination product regulations when integrated into drug delivery systems. These regulations require comprehensive safety assessments, including biocompatibility testing, stability studies, and evaluation of potential leachables from the SERS substrates.
European regulatory bodies, particularly under the EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), have implemented stricter classification systems for nanomaterial-containing medical products. SERS-enhanced drug delivery systems often fall into higher risk categories, necessitating more rigorous clinical evidence and post-market surveillance. The European Medicines Agency (EMA) has also published specific guidance on the development of nanomedicines that applies to SERS-integrated delivery platforms.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have begun addressing nanotechnology-based medical applications, though specific guidelines for SERS technologies remain under development. The ISO Technical Committee 229 has established several standards for nanotechnology characterization that are applicable to SERS substrate manufacturing and quality control.
Regulatory challenges specific to SERS-enhanced drug delivery systems include demonstrating long-term stability of the SERS substrates in biological environments, validating analytical methods for detecting potential degradation products, and establishing appropriate biocompatibility testing protocols. The unique optical properties of SERS materials present additional regulatory considerations regarding potential phototoxicity when activated by specific wavelengths of light.
Recent regulatory trends indicate movement toward a risk-based approach for evaluating nanomaterial-based medical technologies. This includes consideration of the intended clinical application, patient exposure duration, and potential for systemic distribution of nanomaterials. For SERS-enhanced drug delivery systems, developers must provide evidence that the enhancement in drug targeting or controlled release provides clinical benefits that outweigh potential risks associated with the nanomaterials.
Regulatory pathways for approval typically require extensive preclinical testing, including in vitro and in vivo studies to characterize SERS substrate behavior in physiological conditions. Clinical trials for SERS-enhanced drug delivery systems must address both the safety of the delivery platform and the efficacy of the therapeutic agent, with particular attention to potential interactions between the drug and the SERS substrate.
European regulatory bodies, particularly under the EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), have implemented stricter classification systems for nanomaterial-containing medical products. SERS-enhanced drug delivery systems often fall into higher risk categories, necessitating more rigorous clinical evidence and post-market surveillance. The European Medicines Agency (EMA) has also published specific guidance on the development of nanomedicines that applies to SERS-integrated delivery platforms.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have begun addressing nanotechnology-based medical applications, though specific guidelines for SERS technologies remain under development. The ISO Technical Committee 229 has established several standards for nanotechnology characterization that are applicable to SERS substrate manufacturing and quality control.
Regulatory challenges specific to SERS-enhanced drug delivery systems include demonstrating long-term stability of the SERS substrates in biological environments, validating analytical methods for detecting potential degradation products, and establishing appropriate biocompatibility testing protocols. The unique optical properties of SERS materials present additional regulatory considerations regarding potential phototoxicity when activated by specific wavelengths of light.
Recent regulatory trends indicate movement toward a risk-based approach for evaluating nanomaterial-based medical technologies. This includes consideration of the intended clinical application, patient exposure duration, and potential for systemic distribution of nanomaterials. For SERS-enhanced drug delivery systems, developers must provide evidence that the enhancement in drug targeting or controlled release provides clinical benefits that outweigh potential risks associated with the nanomaterials.
Regulatory pathways for approval typically require extensive preclinical testing, including in vitro and in vivo studies to characterize SERS substrate behavior in physiological conditions. Clinical trials for SERS-enhanced drug delivery systems must address both the safety of the delivery platform and the efficacy of the therapeutic agent, with particular attention to potential interactions between the drug and the SERS substrate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!