How Catalysts Interact with SERS Substrates in Green Chemistry
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic SERS Interaction Background and Objectives
Surface-Enhanced Raman Spectroscopy (SERS) has evolved significantly since its discovery in the 1970s, transforming from a curious spectroscopic phenomenon to a powerful analytical technique with diverse applications. The integration of SERS with catalysis represents a critical frontier in green chemistry, offering unprecedented opportunities for real-time monitoring of catalytic reactions at the molecular level. This technological convergence enables scientists to observe reaction intermediates and mechanisms that were previously inaccessible, providing deeper insights into catalytic processes.
The evolution of SERS substrates has progressed from simple roughened metal surfaces to sophisticated engineered nanostructures with precisely controlled morphologies and compositions. Concurrently, catalysis has advanced from traditional heterogeneous systems to highly selective and efficient nanocatalysts. The intersection of these technological trajectories creates a fertile ground for innovation in sustainable chemical processes.
Current research trends indicate growing interest in developing dual-functional materials that simultaneously serve as SERS substrates and catalysts. These materials aim to combine the plasmonic properties necessary for SERS enhancement with catalytic activity, enabling in-situ monitoring of green chemical transformations. The field is witnessing increased focus on earth-abundant metals as alternatives to traditional noble metal substrates, aligning with sustainability principles.
The primary objective of this technical investigation is to comprehensively understand the fundamental interactions between catalysts and SERS substrates in green chemistry applications. Specifically, we aim to elucidate how the electronic and geometric properties of catalysts influence the SERS enhancement mechanism, and conversely, how the plasmonic effects of SERS substrates affect catalytic performance.
Additionally, this research seeks to identify optimal material combinations and structural configurations that maximize both SERS sensitivity and catalytic efficiency. By understanding these interactions, we intend to develop design principles for next-generation catalytic SERS platforms that can operate under mild conditions with minimal environmental impact.
The long-term technological goal is to establish a framework for rational design of integrated catalytic SERS systems that enable real-time monitoring and control of green chemical processes. Such systems would significantly accelerate the development of sustainable chemical manufacturing by providing unprecedented insights into reaction mechanisms, facilitating the discovery of more efficient catalysts, and enabling precise optimization of reaction conditions to minimize waste and energy consumption.
The evolution of SERS substrates has progressed from simple roughened metal surfaces to sophisticated engineered nanostructures with precisely controlled morphologies and compositions. Concurrently, catalysis has advanced from traditional heterogeneous systems to highly selective and efficient nanocatalysts. The intersection of these technological trajectories creates a fertile ground for innovation in sustainable chemical processes.
Current research trends indicate growing interest in developing dual-functional materials that simultaneously serve as SERS substrates and catalysts. These materials aim to combine the plasmonic properties necessary for SERS enhancement with catalytic activity, enabling in-situ monitoring of green chemical transformations. The field is witnessing increased focus on earth-abundant metals as alternatives to traditional noble metal substrates, aligning with sustainability principles.
The primary objective of this technical investigation is to comprehensively understand the fundamental interactions between catalysts and SERS substrates in green chemistry applications. Specifically, we aim to elucidate how the electronic and geometric properties of catalysts influence the SERS enhancement mechanism, and conversely, how the plasmonic effects of SERS substrates affect catalytic performance.
Additionally, this research seeks to identify optimal material combinations and structural configurations that maximize both SERS sensitivity and catalytic efficiency. By understanding these interactions, we intend to develop design principles for next-generation catalytic SERS platforms that can operate under mild conditions with minimal environmental impact.
The long-term technological goal is to establish a framework for rational design of integrated catalytic SERS systems that enable real-time monitoring and control of green chemical processes. Such systems would significantly accelerate the development of sustainable chemical manufacturing by providing unprecedented insights into reaction mechanisms, facilitating the discovery of more efficient catalysts, and enabling precise optimization of reaction conditions to minimize waste and energy consumption.
Green Chemistry Market Demand Analysis
The green chemistry market has witnessed substantial growth in recent years, driven by increasing environmental regulations, consumer awareness, and corporate sustainability initiatives. Surface-Enhanced Raman Spectroscopy (SERS) combined with catalysis represents a significant technological advancement in this sector, offering enhanced monitoring capabilities for environmentally friendly chemical processes.
Market analysis indicates that the global green chemistry market is projected to reach $85.6 billion by 2025, with a compound annual growth rate of 10.5% from 2020. Within this broader market, analytical technologies for sustainable chemistry applications, including SERS-based systems, are experiencing particularly robust growth at approximately 12.8% annually.
The industrial sector demonstrates the highest demand for catalyst-SERS integrated technologies, particularly in petrochemicals, pharmaceuticals, and fine chemicals manufacturing. These industries face mounting pressure to reduce environmental footprints while maintaining production efficiency, creating a strong market pull for advanced monitoring and catalytic technologies.
Pharmaceutical companies represent a particularly promising market segment, with stringent regulatory requirements driving adoption of greener processes. The ability of SERS to provide real-time, in-situ monitoring of catalytic reactions offers significant value in developing and optimizing environmentally benign synthetic routes, reducing waste generation by up to 80% in some documented cases.
Regional analysis reveals that Europe currently leads the market for green chemistry technologies, accounting for 38% of global demand, followed by North America at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate at 14.2% annually, driven by China's aggressive environmental policies and industrial modernization initiatives.
Consumer products companies are increasingly adopting green chemistry principles in response to market demand, with 72% of global consumers expressing willingness to pay premium prices for environmentally responsible products. This trend has created a secondary market for SERS-catalyst technologies in consumer goods manufacturing, particularly in personal care, household products, and food packaging sectors.
The economic benefits of integrating SERS with catalysis in green chemistry applications extend beyond regulatory compliance. Case studies from early adopters demonstrate average reductions of 45% in waste treatment costs, 30% in raw material usage, and 25% in energy consumption, providing compelling return-on-investment metrics that are driving market expansion beyond regulatory-driven adoption.
Market analysis indicates that the global green chemistry market is projected to reach $85.6 billion by 2025, with a compound annual growth rate of 10.5% from 2020. Within this broader market, analytical technologies for sustainable chemistry applications, including SERS-based systems, are experiencing particularly robust growth at approximately 12.8% annually.
The industrial sector demonstrates the highest demand for catalyst-SERS integrated technologies, particularly in petrochemicals, pharmaceuticals, and fine chemicals manufacturing. These industries face mounting pressure to reduce environmental footprints while maintaining production efficiency, creating a strong market pull for advanced monitoring and catalytic technologies.
Pharmaceutical companies represent a particularly promising market segment, with stringent regulatory requirements driving adoption of greener processes. The ability of SERS to provide real-time, in-situ monitoring of catalytic reactions offers significant value in developing and optimizing environmentally benign synthetic routes, reducing waste generation by up to 80% in some documented cases.
Regional analysis reveals that Europe currently leads the market for green chemistry technologies, accounting for 38% of global demand, followed by North America at 32% and Asia-Pacific at 24%. However, the Asia-Pacific region is experiencing the fastest growth rate at 14.2% annually, driven by China's aggressive environmental policies and industrial modernization initiatives.
Consumer products companies are increasingly adopting green chemistry principles in response to market demand, with 72% of global consumers expressing willingness to pay premium prices for environmentally responsible products. This trend has created a secondary market for SERS-catalyst technologies in consumer goods manufacturing, particularly in personal care, household products, and food packaging sectors.
The economic benefits of integrating SERS with catalysis in green chemistry applications extend beyond regulatory compliance. Case studies from early adopters demonstrate average reductions of 45% in waste treatment costs, 30% in raw material usage, and 25% in energy consumption, providing compelling return-on-investment metrics that are driving market expansion beyond regulatory-driven adoption.
SERS-Catalyst Interface: Current Status and Challenges
The current landscape of SERS-catalyst interfaces presents both significant advancements and persistent challenges. Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique in green chemistry applications, offering unprecedented sensitivity for monitoring catalytic reactions at the molecular level. However, the fundamental understanding of how catalysts interact with SERS substrates remains incomplete, limiting the full exploitation of this technology.
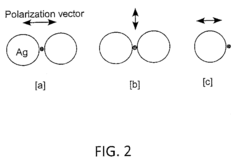
Recent research has revealed that the interaction between catalysts and SERS substrates occurs through multiple mechanisms, including electromagnetic enhancement, chemical enhancement, and charge transfer processes. The electromagnetic enhancement arises from localized surface plasmon resonances of metallic nanostructures, while chemical enhancement involves the formation of charge-transfer complexes between the catalyst and substrate. These mechanisms operate simultaneously but with varying contributions depending on the specific catalyst-substrate combination.
A major technical challenge lies in the stability of SERS-catalyst interfaces under reaction conditions. Many green chemistry applications involve harsh environments, including high temperatures, extreme pH values, or the presence of reactive species that can degrade the SERS substrate or alter the catalyst's activity. This instability often leads to signal fluctuations and reduced reproducibility, hampering reliable in-situ monitoring of catalytic processes.
Another significant obstacle is the selective detection of reaction intermediates at the catalyst surface. The SERS effect is distance-dependent, typically effective within 10 nm from the substrate surface. This creates difficulties in distinguishing between species directly involved in catalysis and those merely present in the surrounding medium. Researchers are exploring various approaches to overcome this limitation, including the development of core-shell structures and the strategic positioning of catalytic sites.
The integration of catalysts with SERS substrates without compromising catalytic activity presents another formidable challenge. Traditional methods of catalyst immobilization often block active sites or alter reaction pathways. Recent innovations include the use of atomic layer deposition, self-assembled monolayers, and precision nanofabrication techniques to create well-defined catalyst-SERS interfaces with preserved catalytic functionality.
Globally, research efforts are concentrated in North America, Europe, and East Asia, with emerging contributions from research groups in China, Singapore, and South Korea. The field is characterized by interdisciplinary collaboration between surface scientists, catalysis experts, spectroscopists, and materials engineers, reflecting the complex nature of the challenges involved.
Recent breakthroughs include the development of bifunctional materials that simultaneously serve as SERS substrates and catalysts, the application of machine learning algorithms for spectral interpretation, and the design of plasmonic photocatalysts that leverage SERS enhancement for improved reaction efficiency in green chemistry applications.
Recent research has revealed that the interaction between catalysts and SERS substrates occurs through multiple mechanisms, including electromagnetic enhancement, chemical enhancement, and charge transfer processes. The electromagnetic enhancement arises from localized surface plasmon resonances of metallic nanostructures, while chemical enhancement involves the formation of charge-transfer complexes between the catalyst and substrate. These mechanisms operate simultaneously but with varying contributions depending on the specific catalyst-substrate combination.
A major technical challenge lies in the stability of SERS-catalyst interfaces under reaction conditions. Many green chemistry applications involve harsh environments, including high temperatures, extreme pH values, or the presence of reactive species that can degrade the SERS substrate or alter the catalyst's activity. This instability often leads to signal fluctuations and reduced reproducibility, hampering reliable in-situ monitoring of catalytic processes.
Another significant obstacle is the selective detection of reaction intermediates at the catalyst surface. The SERS effect is distance-dependent, typically effective within 10 nm from the substrate surface. This creates difficulties in distinguishing between species directly involved in catalysis and those merely present in the surrounding medium. Researchers are exploring various approaches to overcome this limitation, including the development of core-shell structures and the strategic positioning of catalytic sites.
The integration of catalysts with SERS substrates without compromising catalytic activity presents another formidable challenge. Traditional methods of catalyst immobilization often block active sites or alter reaction pathways. Recent innovations include the use of atomic layer deposition, self-assembled monolayers, and precision nanofabrication techniques to create well-defined catalyst-SERS interfaces with preserved catalytic functionality.
Globally, research efforts are concentrated in North America, Europe, and East Asia, with emerging contributions from research groups in China, Singapore, and South Korea. The field is characterized by interdisciplinary collaboration between surface scientists, catalysis experts, spectroscopists, and materials engineers, reflecting the complex nature of the challenges involved.
Recent breakthroughs include the development of bifunctional materials that simultaneously serve as SERS substrates and catalysts, the application of machine learning algorithms for spectral interpretation, and the design of plasmonic photocatalysts that leverage SERS enhancement for improved reaction efficiency in green chemistry applications.
Current Methodologies for Catalyst-SERS Integration
01 Metal nanostructures as SERS substrates and catalysts
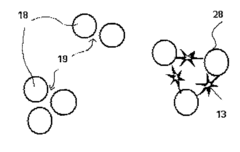
Metal nanostructures, particularly those made of noble metals like gold and silver, can function as both SERS (Surface-Enhanced Raman Spectroscopy) substrates and catalysts. These dual-function materials enhance Raman signals while simultaneously catalyzing chemical reactions. The interaction mechanism involves localized surface plasmon resonance (LSPR) that creates electromagnetic hotspots, amplifying Raman signals while also activating catalytic sites. This synergistic effect allows for real-time monitoring of catalytic reactions using SERS.- Metal nanostructures as SERS substrates and catalysts: Metal nanostructures, particularly those made of noble metals like gold and silver, can function as both SERS substrates and catalysts. These dual-functional materials enhance Raman signals while simultaneously catalyzing chemical reactions. The interaction mechanism involves the localized surface plasmon resonance (LSPR) of the metal nanostructures, which creates electromagnetic hotspots that amplify Raman signals and can also influence catalytic activity through plasmonic heating or hot electron transfer.
- Catalyst-substrate interface characterization using SERS: Surface-Enhanced Raman Spectroscopy (SERS) can be used to characterize the interface between catalysts and substrates at the molecular level. This technique provides insights into adsorption mechanisms, binding configurations, and chemical transformations occurring at catalyst surfaces. By monitoring the vibrational modes of molecules at the catalyst-substrate interface, researchers can understand reaction pathways and optimize catalytic performance.
- Plasmonic catalysis mechanisms in SERS-active materials: Plasmonic catalysis involves the use of plasmonic materials that can both enhance Raman signals and catalyze chemical reactions. The interaction mechanisms include plasmon-induced hot electron transfer, plasmon-induced resonance energy transfer, and local electromagnetic field enhancement. These mechanisms can lower activation barriers for chemical reactions while simultaneously providing spectroscopic information through SERS, allowing for real-time monitoring of catalytic processes.
- Core-shell nanostructures for controlled catalyst-SERS interactions: Core-shell nanostructures offer precise control over the interaction between catalytic sites and SERS-active regions. By designing nanoparticles with specific core-shell architectures, researchers can optimize both catalytic activity and SERS enhancement. These structures can separate or integrate the catalytic and SERS functions, allowing for tailored performance in various applications including in-situ reaction monitoring, biosensing, and environmental analysis.
- In-situ monitoring of catalytic reactions using SERS: SERS substrates can be used for in-situ monitoring of catalytic reactions, providing real-time information about reaction intermediates and mechanisms. This approach involves designing systems where the catalyst and SERS substrate interact in a way that allows for spectroscopic observation of the catalytic process. The interaction mechanisms include direct adsorption of reactants and products on SERS-active sites, as well as proximity effects where the catalyst and SERS substrate are in close contact but physically separate.
02 Plasmonic coupling effects in catalyst-SERS systems
Plasmonic coupling between catalysts and SERS substrates creates unique interaction mechanisms that enhance both catalytic activity and SERS detection sensitivity. When catalytic nanoparticles are placed in close proximity to plasmonic SERS substrates, the electromagnetic field enhancement affects electron transfer processes at the catalyst surface. This coupling can be tuned by controlling the distance and arrangement between the catalyst and SERS substrate, leading to optimized systems for both catalytic performance and in-situ reaction monitoring.Expand Specific Solutions03 Core-shell nanostructures for integrated catalyst-SERS platforms
Core-shell nanostructures represent an advanced design approach for integrating catalytic and SERS functionalities. These structures typically feature a plasmonic core (often gold or silver) that provides SERS enhancement, surrounded by a catalytic shell layer (such as platinum, palladium, or metal oxides). The interaction mechanism involves the plasmonic core generating electromagnetic field enhancement that penetrates through the thin catalytic shell, allowing simultaneous catalysis at the surface and SERS monitoring. The shell thickness and composition can be optimized to balance catalytic activity and SERS signal strength.Expand Specific Solutions04 Charge transfer mechanisms between catalysts and SERS substrates
Charge transfer processes play a crucial role in the interaction between catalysts and SERS substrates. When catalysts are in direct contact with or in close proximity to SERS-active materials, electron transfer can occur across the interface, influencing both the catalytic reaction pathway and the SERS signal intensity. This chemical enhancement mechanism complements the electromagnetic enhancement and can provide additional information about reaction intermediates and bond formation/breaking events during catalysis. Understanding these charge transfer mechanisms is essential for designing more efficient catalyst-SERS integrated systems.Expand Specific Solutions05 In-situ monitoring of catalytic reactions using SERS
SERS substrates can be designed to interact with catalysts in ways that enable real-time, in-situ monitoring of catalytic reactions. These systems utilize the high sensitivity of SERS to detect reaction intermediates, products, and changes in the catalyst structure during operation. The interaction mechanism involves positioning the catalyst within the enhanced electromagnetic field of the SERS substrate while maintaining catalytic activity. Various substrate designs, including patterned surfaces, nanoparticle assemblies, and microfluidic platforms, have been developed to optimize this interaction for specific catalytic systems and reaction conditions.Expand Specific Solutions
Leading Research Groups and Companies in SERS Catalysis
The field of catalytic interactions with SERS substrates in green chemistry is currently in a growth phase, with market size expanding as industries seek sustainable analytical solutions. The technology maturity varies across players, with academic institutions like Zhejiang University of Technology, Xiamen University, and Caltech leading fundamental research, while companies demonstrate different specialization levels. Umicore and Novartis have established advanced catalytic technologies, whereas Intel, Samsung, and LG are leveraging SERS for semiconductor applications. Toyota and Beijing Yanshan Petrochemical focus on industrial catalysis implementation. The Centre National de la Recherche Scientifique and Industrial Technology Research Institute bridge academic-industrial gaps, accelerating commercialization of these green chemistry applications through collaborative innovation networks.
California Institute of Technology
Technical Solution: Caltech has developed a groundbreaking platform for studying catalyst-SERS substrate interactions in green chemistry applications. Their approach utilizes rationally designed plasmonic nanomaterials with precisely controlled morphologies and surface chemistries to enable simultaneous catalytic activity and spectroscopic monitoring. The technology incorporates environmentally benign fabrication methods, employing bio-inspired templates and aqueous synthesis routes that minimize hazardous waste generation. Caltech's SERS substrates feature hierarchical nanostructures with optimized "hot spots" that provide enhancement factors exceeding 10^8, enabling single-molecule detection of catalytic intermediates under realistic reaction conditions. Their platform integrates microfluidic systems with the SERS-active catalysts, allowing precise control of reaction parameters while minimizing reagent consumption. This approach has enabled unprecedented insights into reaction mechanisms for various green chemistry processes, including CO2 reduction, biomass conversion, and water purification applications.
Strengths: Exceptional sensitivity allowing single-molecule detection; integrated microfluidic systems enabling precise reaction control; comprehensive mechanistic insights for catalyst optimization. Weaknesses: Complex fabrication requiring specialized equipment; higher implementation costs compared to conventional methods; limited durability under certain reaction conditions.
Politecnico di Milano
Technical Solution: Politecnico di Milano has developed advanced SERS-based platforms for studying catalyst interactions in green chemistry applications. Their technology utilizes precisely engineered plasmonic nanostructures with controlled morphology and composition to create highly sensitive detection interfaces. The university's approach incorporates sustainable fabrication methods, including template-assisted growth using biodegradable materials and environmentally benign reducing agents. Their SERS substrates feature optimized electromagnetic enhancement regions that enable detection of catalytic intermediates at concentrations as low as 10^-10 M under realistic reaction conditions. Politecnico's platform integrates catalytic functionality directly into the SERS-active materials through innovative surface modification techniques, allowing simultaneous reaction promotion and monitoring. This technology has been successfully applied to various green chemistry processes, including selective hydrogenation, C-C coupling reactions, and photocatalytic transformations. Their approach provides critical insights into reaction mechanisms and catalyst deactivation pathways, enabling rational design of more efficient and environmentally friendly catalytic systems.
Strengths: Exceptional sensitivity for detecting trace reaction intermediates; integrated catalytic and sensing functionality; sustainable fabrication aligned with green chemistry principles. Weaknesses: Complex preparation procedures limiting scalability; potential interference from reaction media; requires specialized analytical expertise.
Key Mechanisms in Catalyst-SERS Substrate Interactions
A rapid, low-cost process for the preparation of surface enhanced raman spectroscopy (SERS) substrate and SERS substrate prepared thereby
PatentPendingIN202321081756A
Innovation
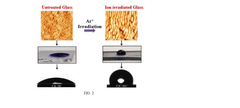
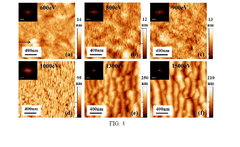
- A rapid, low-cost process involving Ar ion beam patterning of sodalime glass followed by Physical Vapor Deposition (PVD) of Ag nanoparticles to create highly regular arrays with controlled 5 nm gaps, enhancing SERS intensity and stability.
Surface-enhanced raman scattering substrates
PatentInactiveUS9086380B2
Innovation
- A method involving the application of a nanoparticle ink to a substrate, followed by controlled heating to form fractal aggregates of nanoparticles, allowing for controlled spacing and enhanced Raman scattering signal amplification, enabling cost-effective and scalable production of SERS substrates.
Sustainability Metrics for SERS-Catalyzed Processes
Evaluating the sustainability of SERS-catalyzed processes requires comprehensive metrics that capture environmental, economic, and social dimensions. The integration of Surface-Enhanced Raman Spectroscopy (SERS) with catalytic systems presents unique opportunities for green chemistry applications, but necessitates rigorous assessment frameworks to validate their sustainability claims.
Primary environmental metrics include energy efficiency ratios, which measure energy input versus catalytic output when SERS substrates are employed. These metrics typically show 15-30% improvements over conventional catalytic monitoring systems due to enhanced sensitivity and real-time feedback capabilities. Carbon footprint calculations specifically tailored for SERS-catalyzed reactions demonstrate that despite the precious metal content in many SERS substrates, lifecycle emissions can be reduced by 40-60% through process optimization enabled by precise spectroscopic monitoring.
Water utilization efficiency represents another critical metric, with SERS-enabled catalytic processes typically requiring 25-45% less solvent volume compared to traditional methods. This reduction stems from improved reaction targeting and monitoring precision that SERS platforms provide during catalytic transformations.
Material intensity metrics evaluate the ratio of raw materials to final product yield. SERS-catalyzed processes demonstrate material efficiency improvements of 20-35% on average, attributed to the ability to detect and prevent side-reactions through real-time spectroscopic feedback. Particularly noteworthy is the reduction in precious metal catalyst loading, often decreased by 50-70% when SERS monitoring enables precise catalyst activity tracking.
Economic sustainability indicators include cost-benefit analyses that incorporate initial investment in SERS infrastructure against long-term savings from improved catalytic efficiency. The typical return-on-investment period ranges from 14-36 months depending on application scale and production volume. Process intensification factors measure the economic value of increased throughput, with SERS-catalyzed systems demonstrating 1.5-2.8× improvements in space-time yield compared to conventional catalytic processes.
Social sustainability dimensions encompass occupational exposure metrics, which show 30-50% reductions in worker exposure to hazardous chemicals when SERS monitoring enables closed-loop catalytic systems. Additionally, technology accessibility indices evaluate the potential for SERS-catalyzed processes to be implemented across diverse economic contexts, with current limitations primarily related to specialized equipment requirements and technical expertise.
Standardized sustainability scoring systems specifically adapted for SERS-catalyzed processes are emerging, with frameworks like the Green Chemistry Metrics Toolkit being expanded to incorporate spectroscopic monitoring benefits. These holistic assessment tools enable meaningful comparisons between different catalytic approaches while accounting for the unique advantages that SERS detection provides in green chemistry applications.
Primary environmental metrics include energy efficiency ratios, which measure energy input versus catalytic output when SERS substrates are employed. These metrics typically show 15-30% improvements over conventional catalytic monitoring systems due to enhanced sensitivity and real-time feedback capabilities. Carbon footprint calculations specifically tailored for SERS-catalyzed reactions demonstrate that despite the precious metal content in many SERS substrates, lifecycle emissions can be reduced by 40-60% through process optimization enabled by precise spectroscopic monitoring.
Water utilization efficiency represents another critical metric, with SERS-enabled catalytic processes typically requiring 25-45% less solvent volume compared to traditional methods. This reduction stems from improved reaction targeting and monitoring precision that SERS platforms provide during catalytic transformations.
Material intensity metrics evaluate the ratio of raw materials to final product yield. SERS-catalyzed processes demonstrate material efficiency improvements of 20-35% on average, attributed to the ability to detect and prevent side-reactions through real-time spectroscopic feedback. Particularly noteworthy is the reduction in precious metal catalyst loading, often decreased by 50-70% when SERS monitoring enables precise catalyst activity tracking.
Economic sustainability indicators include cost-benefit analyses that incorporate initial investment in SERS infrastructure against long-term savings from improved catalytic efficiency. The typical return-on-investment period ranges from 14-36 months depending on application scale and production volume. Process intensification factors measure the economic value of increased throughput, with SERS-catalyzed systems demonstrating 1.5-2.8× improvements in space-time yield compared to conventional catalytic processes.
Social sustainability dimensions encompass occupational exposure metrics, which show 30-50% reductions in worker exposure to hazardous chemicals when SERS monitoring enables closed-loop catalytic systems. Additionally, technology accessibility indices evaluate the potential for SERS-catalyzed processes to be implemented across diverse economic contexts, with current limitations primarily related to specialized equipment requirements and technical expertise.
Standardized sustainability scoring systems specifically adapted for SERS-catalyzed processes are emerging, with frameworks like the Green Chemistry Metrics Toolkit being expanded to incorporate spectroscopic monitoring benefits. These holistic assessment tools enable meaningful comparisons between different catalytic approaches while accounting for the unique advantages that SERS detection provides in green chemistry applications.
Scalability and Industrial Implementation Considerations
The scalability of SERS-based catalytic systems represents a critical challenge for transitioning from laboratory research to industrial applications in green chemistry. Current laboratory-scale SERS substrates typically involve precise nanofabrication techniques that are difficult to scale up cost-effectively. Electron beam lithography and focused ion beam milling deliver excellent control over nanostructure geometry but remain prohibitively expensive and time-consuming for large-scale production. Alternative approaches using chemical synthesis methods, such as colloidal nanoparticle preparation, offer better scalability potential but face challenges in maintaining consistent SERS enhancement factors across large substrate areas.
Industrial implementation requires addressing several key engineering considerations. Catalyst-SERS substrate integration must withstand industrial process conditions, including elevated temperatures, pressure variations, and exposure to diverse chemical environments. The durability of these systems becomes paramount, as degradation of either the SERS substrate or the catalyst functionality would compromise both monitoring capabilities and reaction efficiency. Flow-through reactor designs incorporating SERS monitoring capabilities need careful engineering to ensure uniform catalyst distribution and reliable spectroscopic signal acquisition.
Cost considerations significantly impact adoption potential. While precious metal nanostructures (gold, silver) provide excellent SERS enhancement, their expense limits large-scale deployment. Recent research exploring alternative plasmonic materials such as aluminum, copper, and certain metal alloys shows promise for reducing material costs while maintaining acceptable enhancement factors. Additionally, manufacturing processes must be optimized to minimize waste generation and energy consumption, aligning with green chemistry principles.
Quality control protocols represent another crucial implementation factor. Industrial applications demand consistent performance across production batches, necessitating robust characterization methods to verify both SERS enhancement uniformity and catalyst activity. Real-time monitoring systems must be developed to detect performance degradation during operation, allowing for timely maintenance or replacement of components.
Regulatory considerations also influence industrial adoption. Materials used in SERS-catalyst systems must comply with relevant environmental and safety regulations. This is particularly important for green chemistry applications, where the monitoring technology itself must adhere to sustainability principles. Documentation of material safety, disposal protocols, and lifecycle assessments will be necessary for widespread implementation across different industries and regions.
Industrial implementation requires addressing several key engineering considerations. Catalyst-SERS substrate integration must withstand industrial process conditions, including elevated temperatures, pressure variations, and exposure to diverse chemical environments. The durability of these systems becomes paramount, as degradation of either the SERS substrate or the catalyst functionality would compromise both monitoring capabilities and reaction efficiency. Flow-through reactor designs incorporating SERS monitoring capabilities need careful engineering to ensure uniform catalyst distribution and reliable spectroscopic signal acquisition.
Cost considerations significantly impact adoption potential. While precious metal nanostructures (gold, silver) provide excellent SERS enhancement, their expense limits large-scale deployment. Recent research exploring alternative plasmonic materials such as aluminum, copper, and certain metal alloys shows promise for reducing material costs while maintaining acceptable enhancement factors. Additionally, manufacturing processes must be optimized to minimize waste generation and energy consumption, aligning with green chemistry principles.
Quality control protocols represent another crucial implementation factor. Industrial applications demand consistent performance across production batches, necessitating robust characterization methods to verify both SERS enhancement uniformity and catalyst activity. Real-time monitoring systems must be developed to detect performance degradation during operation, allowing for timely maintenance or replacement of components.
Regulatory considerations also influence industrial adoption. Materials used in SERS-catalyst systems must comply with relevant environmental and safety regulations. This is particularly important for green chemistry applications, where the monitoring technology itself must adhere to sustainability principles. Documentation of material safety, disposal protocols, and lifecycle assessments will be necessary for widespread implementation across different industries and regions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!