SERS Substrates and Their Impact on Regulatory Standards
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SERS Technology Background and Objectives
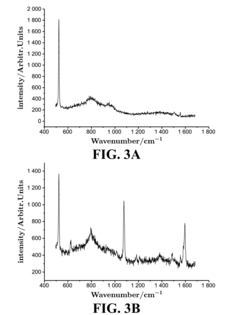
Surface-Enhanced Raman Spectroscopy (SERS) emerged in the 1970s when researchers discovered that Raman signals could be dramatically amplified when molecules were adsorbed on roughened metal surfaces. This phenomenon, initially observed on electrochemically roughened silver electrodes, demonstrated enhancement factors of 105-106, revolutionizing analytical capabilities for trace detection. The subsequent decades witnessed significant technological evolution, from basic metal substrates to sophisticated nanostructured materials designed specifically for SERS applications.
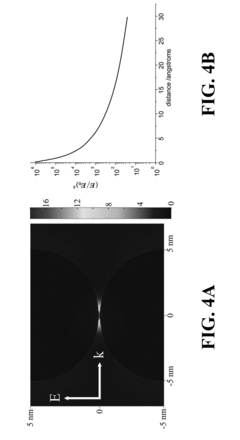
The fundamental principle behind SERS involves two primary enhancement mechanisms: electromagnetic enhancement from localized surface plasmon resonances and chemical enhancement through charge transfer between the analyte and substrate. These mechanisms collectively enable detection sensitivities reaching single-molecule levels under optimal conditions, representing a quantum leap in analytical capabilities compared to conventional spectroscopic techniques.
Current SERS technology encompasses diverse substrate architectures including nanoparticle assemblies, nanopatterned surfaces, and hybrid materials combining metallic structures with other functional components. The field has progressively moved from laboratory curiosities toward standardized, reproducible platforms suitable for routine analytical applications across multiple industries including pharmaceuticals, food safety, environmental monitoring, and security.
The regulatory landscape surrounding SERS has evolved in parallel with technological advancements. Initially, the lack of standardization posed significant challenges for regulatory acceptance. However, recent years have witnessed increasing efforts to establish reference materials, validation protocols, and quality control measures to ensure reliability and comparability of SERS measurements across different laboratories and instrumental setups.
The primary technological objectives in contemporary SERS research focus on addressing several persistent challenges. These include enhancing substrate reproducibility to minimize batch-to-batch variations, improving long-term stability for extended shelf-life, developing cost-effective manufacturing processes for commercial viability, and establishing robust calibration methods for quantitative analysis. Additionally, there is growing emphasis on creating application-specific SERS substrates optimized for particular analytical contexts.
Looking forward, SERS technology aims to achieve seamless integration with existing analytical workflows in regulated industries. This necessitates not only technical improvements but also comprehensive validation studies demonstrating compliance with regulatory requirements. The ultimate goal is to position SERS as a standard analytical tool with clearly defined performance characteristics, validation protocols, and quality assurance measures that satisfy stringent regulatory standards across global markets.
The fundamental principle behind SERS involves two primary enhancement mechanisms: electromagnetic enhancement from localized surface plasmon resonances and chemical enhancement through charge transfer between the analyte and substrate. These mechanisms collectively enable detection sensitivities reaching single-molecule levels under optimal conditions, representing a quantum leap in analytical capabilities compared to conventional spectroscopic techniques.
Current SERS technology encompasses diverse substrate architectures including nanoparticle assemblies, nanopatterned surfaces, and hybrid materials combining metallic structures with other functional components. The field has progressively moved from laboratory curiosities toward standardized, reproducible platforms suitable for routine analytical applications across multiple industries including pharmaceuticals, food safety, environmental monitoring, and security.
The regulatory landscape surrounding SERS has evolved in parallel with technological advancements. Initially, the lack of standardization posed significant challenges for regulatory acceptance. However, recent years have witnessed increasing efforts to establish reference materials, validation protocols, and quality control measures to ensure reliability and comparability of SERS measurements across different laboratories and instrumental setups.
The primary technological objectives in contemporary SERS research focus on addressing several persistent challenges. These include enhancing substrate reproducibility to minimize batch-to-batch variations, improving long-term stability for extended shelf-life, developing cost-effective manufacturing processes for commercial viability, and establishing robust calibration methods for quantitative analysis. Additionally, there is growing emphasis on creating application-specific SERS substrates optimized for particular analytical contexts.
Looking forward, SERS technology aims to achieve seamless integration with existing analytical workflows in regulated industries. This necessitates not only technical improvements but also comprehensive validation studies demonstrating compliance with regulatory requirements. The ultimate goal is to position SERS as a standard analytical tool with clearly defined performance characteristics, validation protocols, and quality assurance measures that satisfy stringent regulatory standards across global markets.
Market Analysis for SERS Substrate Applications
The global market for Surface-Enhanced Raman Spectroscopy (SERS) substrates has experienced significant growth in recent years, driven by increasing applications in analytical chemistry, biomedical diagnostics, food safety, and environmental monitoring. The current market size for SERS substrates is estimated at approximately $125 million, with projections indicating a compound annual growth rate of 10-12% over the next five years, potentially reaching $200-220 million by 2028.
Healthcare and pharmaceutical sectors represent the largest application segments, accounting for nearly 45% of the total market share. The demand in these sectors is primarily fueled by the need for highly sensitive detection methods for biomarkers, pathogens, and drug compounds. The ability of SERS to provide molecular fingerprinting at ultra-low concentrations makes it particularly valuable for clinical diagnostics and pharmaceutical quality control.
Food safety testing represents another rapidly growing segment, currently holding approximately 20% of the market share but expanding at a faster rate than other segments. Regulatory pressures to ensure food safety and detect contaminants at increasingly lower concentrations are driving adoption in this sector. Major food producers and regulatory bodies are investing in SERS technology as a complementary method to traditional testing approaches.
Environmental monitoring applications constitute about 15% of the current market, with particular focus on water quality assessment and detection of pollutants. This segment is expected to grow substantially as environmental regulations become more stringent globally, requiring more sensitive detection methods for emerging contaminants.
Regional analysis reveals North America as the dominant market (38%), followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is demonstrating the highest growth rate, driven by increasing research funding, expanding industrial applications, and strengthening regulatory frameworks in countries like China, Japan, and South Korea.
The market structure is characterized by a mix of specialized substrate manufacturers, analytical instrument companies, and research institutions. Key market players include established analytical instrument manufacturers who have expanded into SERS technology, specialized substrate producers focusing exclusively on SERS applications, and emerging startups introducing innovative substrate designs. This diverse ecosystem has created a competitive landscape where product differentiation is primarily based on sensitivity, reproducibility, shelf-life, and compatibility with existing analytical workflows.
Customer segmentation reveals three primary groups: research institutions (40%), industrial quality control laboratories (35%), and regulatory/compliance testing facilities (25%). Each segment has distinct requirements regarding substrate performance, cost considerations, and implementation complexity.
Healthcare and pharmaceutical sectors represent the largest application segments, accounting for nearly 45% of the total market share. The demand in these sectors is primarily fueled by the need for highly sensitive detection methods for biomarkers, pathogens, and drug compounds. The ability of SERS to provide molecular fingerprinting at ultra-low concentrations makes it particularly valuable for clinical diagnostics and pharmaceutical quality control.
Food safety testing represents another rapidly growing segment, currently holding approximately 20% of the market share but expanding at a faster rate than other segments. Regulatory pressures to ensure food safety and detect contaminants at increasingly lower concentrations are driving adoption in this sector. Major food producers and regulatory bodies are investing in SERS technology as a complementary method to traditional testing approaches.
Environmental monitoring applications constitute about 15% of the current market, with particular focus on water quality assessment and detection of pollutants. This segment is expected to grow substantially as environmental regulations become more stringent globally, requiring more sensitive detection methods for emerging contaminants.
Regional analysis reveals North America as the dominant market (38%), followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is demonstrating the highest growth rate, driven by increasing research funding, expanding industrial applications, and strengthening regulatory frameworks in countries like China, Japan, and South Korea.
The market structure is characterized by a mix of specialized substrate manufacturers, analytical instrument companies, and research institutions. Key market players include established analytical instrument manufacturers who have expanded into SERS technology, specialized substrate producers focusing exclusively on SERS applications, and emerging startups introducing innovative substrate designs. This diverse ecosystem has created a competitive landscape where product differentiation is primarily based on sensitivity, reproducibility, shelf-life, and compatibility with existing analytical workflows.
Customer segmentation reveals three primary groups: research institutions (40%), industrial quality control laboratories (35%), and regulatory/compliance testing facilities (25%). Each segment has distinct requirements regarding substrate performance, cost considerations, and implementation complexity.
Current SERS Substrate Challenges and Limitations
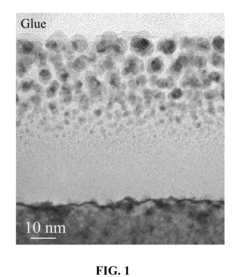
Despite significant advancements in Surface-Enhanced Raman Spectroscopy (SERS) substrate technology, several critical challenges continue to impede widespread adoption and regulatory acceptance. The primary limitation remains reproducibility, with many current substrates exhibiting significant signal variability between batches and even within the same substrate. This inconsistency creates substantial obstacles for establishing standardized protocols and reliable quantitative analysis, particularly problematic for regulatory applications requiring consistent performance metrics.
Stability presents another major challenge, as many high-performance SERS substrates demonstrate rapid degradation under ambient conditions. The active enhancement sites often experience oxidation, contamination, or structural changes that significantly reduce sensitivity over time. This instability necessitates special storage conditions and limits shelf-life, creating logistical complications for commercial applications and regulatory validation processes.
Cost-effectiveness remains a significant barrier, particularly for large-scale implementation. Many high-performance substrates utilize expensive noble metals (primarily gold and silver) with complex nanofabrication techniques requiring specialized equipment. These economic factors restrict accessibility and hinder adoption in routine analytical settings where cost constraints are significant considerations.
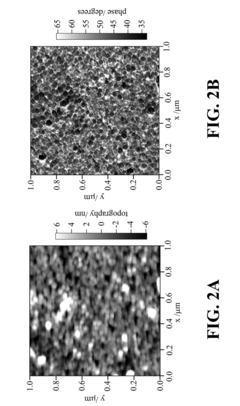
Sensitivity distribution across substrate surfaces frequently exhibits heterogeneity, creating "hot spots" with dramatically enhanced signals alongside areas with minimal enhancement. This spatial inconsistency complicates quantitative analysis and requires sophisticated mapping techniques to ensure reliable measurements, presenting challenges for standardization efforts required by regulatory frameworks.
Substrate selectivity and specificity limitations also persist, with many current platforms demonstrating inadequate discrimination between similar molecular structures or suffering from interference in complex matrices. This deficiency particularly impacts applications in environmental monitoring, food safety, and pharmaceutical analysis where selective detection in complex backgrounds is essential for regulatory compliance.
Scalability challenges further complicate commercial viability, as many laboratory-scale fabrication methods for high-performance substrates prove difficult to translate to industrial production scales without compromising enhancement properties. This manufacturing limitation creates bottlenecks in supply chains and increases costs, hampering widespread implementation in regulated industries.
Integration with existing analytical workflows presents additional hurdles, as many current SERS substrates require specialized handling protocols and instrumentation that deviate from established laboratory practices. This incompatibility creates resistance to adoption and complicates validation procedures necessary for regulatory approval processes.
Stability presents another major challenge, as many high-performance SERS substrates demonstrate rapid degradation under ambient conditions. The active enhancement sites often experience oxidation, contamination, or structural changes that significantly reduce sensitivity over time. This instability necessitates special storage conditions and limits shelf-life, creating logistical complications for commercial applications and regulatory validation processes.
Cost-effectiveness remains a significant barrier, particularly for large-scale implementation. Many high-performance substrates utilize expensive noble metals (primarily gold and silver) with complex nanofabrication techniques requiring specialized equipment. These economic factors restrict accessibility and hinder adoption in routine analytical settings where cost constraints are significant considerations.
Sensitivity distribution across substrate surfaces frequently exhibits heterogeneity, creating "hot spots" with dramatically enhanced signals alongside areas with minimal enhancement. This spatial inconsistency complicates quantitative analysis and requires sophisticated mapping techniques to ensure reliable measurements, presenting challenges for standardization efforts required by regulatory frameworks.
Substrate selectivity and specificity limitations also persist, with many current platforms demonstrating inadequate discrimination between similar molecular structures or suffering from interference in complex matrices. This deficiency particularly impacts applications in environmental monitoring, food safety, and pharmaceutical analysis where selective detection in complex backgrounds is essential for regulatory compliance.
Scalability challenges further complicate commercial viability, as many laboratory-scale fabrication methods for high-performance substrates prove difficult to translate to industrial production scales without compromising enhancement properties. This manufacturing limitation creates bottlenecks in supply chains and increases costs, hampering widespread implementation in regulated industries.
Integration with existing analytical workflows presents additional hurdles, as many current SERS substrates require specialized handling protocols and instrumentation that deviate from established laboratory practices. This incompatibility creates resistance to adoption and complicates validation procedures necessary for regulatory approval processes.
Current SERS Substrate Design Solutions
01 Regulatory compliance for SERS substrate manufacturing
Manufacturing of SERS substrates must adhere to specific regulatory standards to ensure quality and consistency. These regulations cover production processes, material purity requirements, and quality control measures. Compliance with these standards is essential for SERS substrates used in analytical applications, especially those in regulated industries such as healthcare, pharmaceuticals, and environmental monitoring.- FDA and ISO standards for SERS substrate manufacturing: Regulatory standards for the manufacturing of SERS substrates focus on quality control, consistency, and safety. These standards include FDA guidelines for medical applications and ISO certifications for manufacturing processes. Manufacturers must adhere to Good Manufacturing Practices (GMP) and demonstrate batch-to-batch consistency in SERS enhancement factors. Validation protocols and quality management systems are required to ensure compliance with these regulatory frameworks.
- Environmental and safety regulations for SERS materials: SERS substrates often contain nanomaterials and noble metals that are subject to environmental and safety regulations. These regulations govern the handling, disposal, and potential environmental impact of these materials. Manufacturers must provide safety data sheets and risk assessments for their SERS substrates, particularly when they contain silver, gold, or other potentially hazardous nanoparticles. Compliance with REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and similar international frameworks is essential.
- Calibration and validation standards for SERS analytical methods: Standardized protocols for calibration and validation of SERS-based analytical methods are critical for regulatory acceptance. These standards include requirements for reference materials, performance verification, and method validation. Analytical parameters such as limit of detection, reproducibility, and specificity must be established according to guidelines from organizations like ASTM International and USP. Traceability to international standards and inter-laboratory validation studies are often required for regulatory approval of SERS-based analytical methods.
- Clinical and diagnostic application standards: SERS substrates used in clinical diagnostics and medical applications are subject to additional regulatory requirements. These include clinical validation studies, performance specifications, and compliance with medical device regulations. For in vitro diagnostic applications, SERS substrates must meet sensitivity, specificity, and reproducibility criteria established by regulatory bodies. Clinical trials and validation studies must demonstrate the efficacy and safety of SERS-based diagnostic methods before they can receive regulatory approval for patient use.
- Quality control and standardization of SERS enhancement factors: Regulatory standards for SERS substrates require consistent and quantifiable enhancement factors. These standards define acceptable methods for measuring and reporting SERS enhancement, including reference compounds and measurement conditions. Manufacturers must implement quality control procedures to ensure batch-to-batch consistency and stability over time. Standard reference materials and protocols for performance verification are being developed by national metrology institutes to facilitate regulatory compliance and method validation across different laboratories and applications.
02 Standardization of SERS measurement protocols
Standardized protocols for SERS measurements are critical for ensuring reproducibility and reliability across different laboratories and instruments. These standards define specific procedures for sample preparation, instrument calibration, data collection, and analysis. Standardization helps in comparing results from different studies and is particularly important for applications in forensics, clinical diagnostics, and regulatory testing.Expand Specific Solutions03 Quality control standards for SERS substrate performance
Quality control standards for SERS substrates focus on performance metrics such as enhancement factor, reproducibility, stability, and shelf-life. These standards ensure that SERS substrates provide consistent and reliable results across different batches and over time. Performance validation protocols include reference materials, benchmark tests, and statistical analysis methods to verify that substrates meet the required specifications.Expand Specific Solutions04 Safety and biocompatibility regulations for SERS applications
Safety and biocompatibility regulations are particularly important for SERS substrates used in biomedical applications, food safety testing, and environmental monitoring. These standards address potential toxicity concerns, biocompatibility requirements, and environmental impact considerations. Regulatory frameworks may include guidelines for substrate materials, surface coatings, and disposal procedures to ensure safety throughout the product lifecycle.Expand Specific Solutions05 Calibration and validation standards for SERS instrumentation
Calibration and validation standards for SERS instrumentation ensure accurate and reliable measurements across different devices and laboratories. These standards include reference materials with known Raman signatures, calibration protocols, and performance verification methods. Regular calibration and validation procedures are required to maintain compliance with regulatory requirements, particularly in applications such as clinical diagnostics, pharmaceutical quality control, and forensic analysis.Expand Specific Solutions
Key Industry Players in SERS Substrate Development
The SERS substrates market is currently in a growth phase, characterized by increasing adoption across analytical chemistry, biosensing, and medical diagnostics. The global market size is estimated to reach approximately $125 million by 2025, with a CAGR of 15-18%. Technologically, SERS substrates are advancing from early commercial applications toward standardization, with academic institutions (Boston University, Northwestern University, National Taiwan University) driving fundamental research while companies like IBM, Corning, and SICPA lead commercial development. Regulatory standards remain in flux, with organizations like Naval Research Laboratory and UK Research & Innovation working to establish consistent performance metrics and validation protocols. The competitive landscape features collaboration between academic institutions and industry partners to address reproducibility challenges critical for regulatory compliance.
Corning, Inc.
Technical Solution: Corning has developed advanced SERS substrates utilizing their expertise in glass and ceramic materials. Their proprietary technology involves creating highly uniform nanostructured surfaces with precisely controlled geometries that enhance Raman signals by factors exceeding 10^6. Corning's SERS substrates incorporate noble metal nanoparticles (primarily silver and gold) deposited on specialized glass surfaces using controlled deposition techniques. These substrates feature optimized "hot spots" - nanoscale junctions between metallic features where electromagnetic fields are significantly amplified. Corning has focused on manufacturing reproducibility, a critical factor for regulatory compliance, by implementing rigorous quality control processes that ensure batch-to-batch consistency with signal enhancement variations below 10%. Their substrates are designed with extended shelf-life stability and resistance to environmental degradation, addressing key regulatory concerns about reliability in analytical applications.
Strengths: Superior manufacturing consistency and reproducibility critical for regulatory compliance; exceptional shelf-life stability; leverages Corning's extensive materials science expertise. Weaknesses: Higher production costs compared to some competitors; primarily optimized for laboratory rather than field applications; requires specialized handling protocols.
UK Research & Innovation Ltd.
Technical Solution: UKRI has developed advanced SERS substrate technologies through collaborative research across multiple institutions. Their approach focuses on creating regulatory-compliant SERS platforms through precise control of nanostructure morphology and composition. UKRI's substrates utilize novel fabrication techniques including template-assisted electrodeposition and nanosphere lithography to create highly ordered arrays of metallic nanostructures with consistent inter-particle spacing. A key innovation is their development of multi-metal SERS substrates that combine gold, silver, and copper in optimized ratios to achieve broader spectral response while maintaining enhancement factors above 10^6. These substrates incorporate protective coatings that prevent oxidation and contamination, ensuring consistent performance over time - a critical factor for regulatory acceptance. UKRI has pioneered standardized characterization methods for SERS substrates, developing protocols that quantify enhancement factor distribution, spatial uniformity, and batch-to-batch reproducibility. Their research has established correlations between physical characteristics of substrates and analytical performance metrics required by regulatory bodies, creating a framework for quality assessment that supports regulatory compliance.
Strengths: Exceptional durability and environmental stability; comprehensive standardized testing protocols that align with regulatory requirements; versatile spectral response suitable for diverse analytical applications. Weaknesses: Complex manufacturing process increases production costs; requires specialized expertise for optimal implementation; limited scalability for mass production.
Critical Patents and Innovations in SERS Technology
Surface enhanced raman scattering substrate
PatentActiveUS20240118211A1
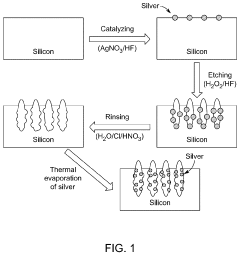
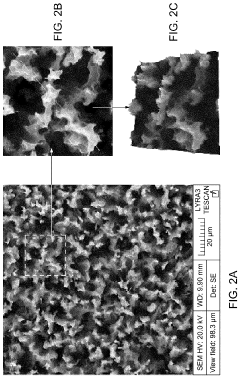
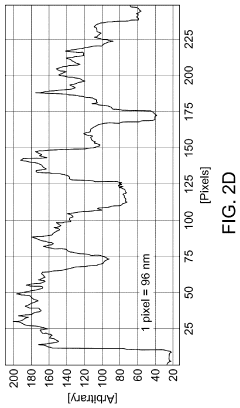
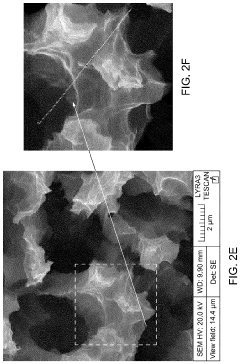
Innovation
- A SERS substrate is developed with a silicon substrate featuring microscale valleys and terraces decorated with silver nanoparticles, fabricated using a method involving metal catalyzed electroless etching and thermal evaporation, to create a platform with controlled nanostructures and enhanced electromagnetic hotspots.
Substrates for surface enhanced raman spectroscopy
PatentInactiveUS20160274031A1
Innovation
- A method involving high dose metal ion implantation into substrates to form metal nano-particle/substrate composites, where the substrate is ion implanted with metal ions to exceed solid solubility limits, followed by thermal annealing and selective etching to expose nano-particles, allowing for controlled particle size and separation, and enabling the use of various substrate materials and geometries.
Regulatory Framework Impact on SERS Implementation
The regulatory landscape surrounding SERS (Surface-Enhanced Raman Spectroscopy) technology is increasingly complex and multifaceted, creating both challenges and opportunities for implementation across various sectors. Current regulatory frameworks in major markets such as the United States, European Union, and Asia Pacific regions have established specific guidelines for analytical methods used in product quality control, environmental monitoring, and clinical diagnostics—all areas where SERS applications are expanding rapidly.
In the pharmaceutical industry, regulatory bodies like the FDA and EMA have implemented stringent requirements for analytical method validation, which directly impacts how SERS technologies can be deployed in drug development and quality control processes. These frameworks typically demand extensive documentation of method reproducibility, accuracy, and precision—parameters that are particularly challenging for SERS due to substrate variability and signal enhancement inconsistencies.
For environmental applications, regulatory standards such as those from the EPA in the US or the Water Framework Directive in the EU establish detection limits for contaminants that SERS methods must reliably achieve to gain acceptance as official monitoring techniques. The current regulatory emphasis on quantitative reliability creates a significant barrier for SERS implementation despite its superior sensitivity for many analytes.
Medical device and diagnostic regulations present another layer of complexity, with frameworks like the EU's IVDR (In Vitro Diagnostic Regulation) and the FDA's medical device approval process requiring extensive clinical validation studies before SERS-based diagnostic tools can enter the market. These regulatory pathways typically require 3-5 years of validation studies, significantly extending time-to-market for innovative SERS applications.
Data integrity requirements across all regulatory frameworks also impact SERS implementation, as the technology generates complex spectral datasets that must be stored, processed, and interpreted according to established compliance standards. This necessitates robust data management systems that can maintain regulatory compliance while handling the unique characteristics of SERS data.
Emerging regulatory trends indicate a gradual shift toward performance-based standards rather than prescriptive methodologies, potentially creating more favorable conditions for novel analytical approaches like SERS. Several regulatory agencies have initiated specialized programs to accelerate the adoption of innovative analytical technologies, providing potential pathways for faster SERS implementation in regulated industries.
In the pharmaceutical industry, regulatory bodies like the FDA and EMA have implemented stringent requirements for analytical method validation, which directly impacts how SERS technologies can be deployed in drug development and quality control processes. These frameworks typically demand extensive documentation of method reproducibility, accuracy, and precision—parameters that are particularly challenging for SERS due to substrate variability and signal enhancement inconsistencies.
For environmental applications, regulatory standards such as those from the EPA in the US or the Water Framework Directive in the EU establish detection limits for contaminants that SERS methods must reliably achieve to gain acceptance as official monitoring techniques. The current regulatory emphasis on quantitative reliability creates a significant barrier for SERS implementation despite its superior sensitivity for many analytes.
Medical device and diagnostic regulations present another layer of complexity, with frameworks like the EU's IVDR (In Vitro Diagnostic Regulation) and the FDA's medical device approval process requiring extensive clinical validation studies before SERS-based diagnostic tools can enter the market. These regulatory pathways typically require 3-5 years of validation studies, significantly extending time-to-market for innovative SERS applications.
Data integrity requirements across all regulatory frameworks also impact SERS implementation, as the technology generates complex spectral datasets that must be stored, processed, and interpreted according to established compliance standards. This necessitates robust data management systems that can maintain regulatory compliance while handling the unique characteristics of SERS data.
Emerging regulatory trends indicate a gradual shift toward performance-based standards rather than prescriptive methodologies, potentially creating more favorable conditions for novel analytical approaches like SERS. Several regulatory agencies have initiated specialized programs to accelerate the adoption of innovative analytical technologies, providing potential pathways for faster SERS implementation in regulated industries.
Compliance Strategies for SERS-Based Analytical Methods
Compliance with regulatory standards is a critical consideration for any analytical method intended for commercial or clinical applications. For SERS-based analytical techniques, developing robust compliance strategies requires a multifaceted approach that addresses both the unique characteristics of SERS substrates and the evolving regulatory landscape.
The foundation of any compliance strategy begins with thorough documentation of substrate manufacturing processes. This includes establishing standard operating procedures (SOPs) that detail every aspect of substrate production, from raw material specifications to quality control parameters. Implementing Good Manufacturing Practices (GMP) for SERS substrate production ensures consistency and reliability, which are paramount for regulatory acceptance.
Validation protocols specific to SERS-based methods must be developed in accordance with guidelines from relevant regulatory bodies such as the FDA, EMA, or ISO. These protocols should address key performance characteristics including sensitivity, specificity, accuracy, precision, reproducibility, and stability under various environmental conditions. Particular attention must be paid to batch-to-batch consistency of SERS substrates, as this has been identified as a significant challenge in regulatory submissions.
Risk assessment frameworks tailored to SERS technology represent another crucial component of compliance strategies. These frameworks should identify potential failure modes in both substrate manufacturing and analytical procedures, evaluating their impact on result integrity. Implementing appropriate control measures for each identified risk creates a robust quality system that satisfies regulatory expectations.
Collaborative approaches with regulatory agencies through pre-submission consultations can significantly streamline the approval process. Early engagement allows for clarification of specific requirements for novel SERS-based methods and provides opportunities to address potential concerns before formal submission. Several companies have successfully utilized this approach to gain regulatory clearance for SERS-based diagnostic tests.
Reference standards and proficiency testing programs specifically designed for SERS applications are emerging as valuable tools for demonstrating method reliability. Participation in interlaboratory comparison studies using standardized SERS substrates helps establish method transferability and robustness across different laboratory settings – a key consideration for regulatory bodies evaluating new analytical approaches.
Data integrity assurance through appropriate electronic systems compliant with 21 CFR Part 11 or equivalent standards ensures the traceability and reliability of results generated using SERS-based methods. This includes implementing audit trails, electronic signatures, and data security measures that meet current regulatory expectations for analytical data management.
The foundation of any compliance strategy begins with thorough documentation of substrate manufacturing processes. This includes establishing standard operating procedures (SOPs) that detail every aspect of substrate production, from raw material specifications to quality control parameters. Implementing Good Manufacturing Practices (GMP) for SERS substrate production ensures consistency and reliability, which are paramount for regulatory acceptance.
Validation protocols specific to SERS-based methods must be developed in accordance with guidelines from relevant regulatory bodies such as the FDA, EMA, or ISO. These protocols should address key performance characteristics including sensitivity, specificity, accuracy, precision, reproducibility, and stability under various environmental conditions. Particular attention must be paid to batch-to-batch consistency of SERS substrates, as this has been identified as a significant challenge in regulatory submissions.
Risk assessment frameworks tailored to SERS technology represent another crucial component of compliance strategies. These frameworks should identify potential failure modes in both substrate manufacturing and analytical procedures, evaluating their impact on result integrity. Implementing appropriate control measures for each identified risk creates a robust quality system that satisfies regulatory expectations.
Collaborative approaches with regulatory agencies through pre-submission consultations can significantly streamline the approval process. Early engagement allows for clarification of specific requirements for novel SERS-based methods and provides opportunities to address potential concerns before formal submission. Several companies have successfully utilized this approach to gain regulatory clearance for SERS-based diagnostic tests.
Reference standards and proficiency testing programs specifically designed for SERS applications are emerging as valuable tools for demonstrating method reliability. Participation in interlaboratory comparison studies using standardized SERS substrates helps establish method transferability and robustness across different laboratory settings – a key consideration for regulatory bodies evaluating new analytical approaches.
Data integrity assurance through appropriate electronic systems compliant with 21 CFR Part 11 or equivalent standards ensures the traceability and reliability of results generated using SERS-based methods. This includes implementing audit trails, electronic signatures, and data security measures that meet current regulatory expectations for analytical data management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!