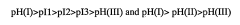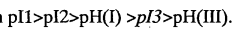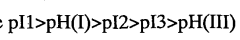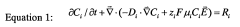Analyzing Isoelectric Focusing's Impact on Protein Characterization
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Evolution and Objectives
Isoelectric focusing (IEF) emerged in the 1960s as a groundbreaking technique for protein separation based on their isoelectric points (pI). The pioneering work of Svensson and Vesterberg established the theoretical foundation and practical implementation of this technique, which revolutionized protein characterization. Over subsequent decades, IEF evolved from conventional gel-based methods to more sophisticated capillary and microchip formats, significantly enhancing resolution and sensitivity.
The evolution of IEF technology has been closely aligned with advances in materials science and instrumentation. Traditional polyacrylamide gels gave way to immobilized pH gradient (IPG) strips in the 1980s, offering improved reproducibility and standardization. The 1990s witnessed the integration of IEF with mass spectrometry, creating powerful hybrid analytical platforms. Recent developments include miniaturized IEF systems and the incorporation of novel carrier ampholytes, expanding the technique's applicability across diverse protein samples.
Digital technology integration represents a significant trend in IEF development, with automated systems and computational tools enhancing data analysis and interpretation. Machine learning algorithms now assist in pattern recognition within complex protein profiles, while simulation software enables prediction of protein behavior under various IEF conditions. These technological advances have transformed IEF from a purely analytical technique to a comprehensive protein characterization platform.
The primary objective of modern IEF technology is to achieve unprecedented resolution in protein separation while maintaining high throughput capabilities. Researchers aim to develop systems capable of distinguishing proteins with minimal pI differences (ΔpI < 0.01), even in complex biological matrices. Another critical goal is to extend the dynamic range of detection, enabling simultaneous analysis of both high-abundance and trace-level proteins within the same sample.
Additional objectives include enhancing the compatibility of IEF with downstream analytical techniques, particularly mass spectrometry and various spectroscopic methods. This integration facilitates comprehensive protein characterization beyond mere separation. The development of environmentally sustainable IEF methodologies represents another important goal, with efforts focused on reducing chemical waste and energy consumption without compromising analytical performance.
Looking forward, the field is moving toward real-time monitoring capabilities, allowing researchers to observe protein behavior during the focusing process rather than analyzing only the end result. The ultimate vision encompasses fully automated, high-throughput IEF platforms capable of processing multiple samples simultaneously while providing detailed structural and functional information about the separated proteins, thereby supporting advances in proteomics, biopharmaceutical development, and clinical diagnostics.
The evolution of IEF technology has been closely aligned with advances in materials science and instrumentation. Traditional polyacrylamide gels gave way to immobilized pH gradient (IPG) strips in the 1980s, offering improved reproducibility and standardization. The 1990s witnessed the integration of IEF with mass spectrometry, creating powerful hybrid analytical platforms. Recent developments include miniaturized IEF systems and the incorporation of novel carrier ampholytes, expanding the technique's applicability across diverse protein samples.
Digital technology integration represents a significant trend in IEF development, with automated systems and computational tools enhancing data analysis and interpretation. Machine learning algorithms now assist in pattern recognition within complex protein profiles, while simulation software enables prediction of protein behavior under various IEF conditions. These technological advances have transformed IEF from a purely analytical technique to a comprehensive protein characterization platform.
The primary objective of modern IEF technology is to achieve unprecedented resolution in protein separation while maintaining high throughput capabilities. Researchers aim to develop systems capable of distinguishing proteins with minimal pI differences (ΔpI < 0.01), even in complex biological matrices. Another critical goal is to extend the dynamic range of detection, enabling simultaneous analysis of both high-abundance and trace-level proteins within the same sample.
Additional objectives include enhancing the compatibility of IEF with downstream analytical techniques, particularly mass spectrometry and various spectroscopic methods. This integration facilitates comprehensive protein characterization beyond mere separation. The development of environmentally sustainable IEF methodologies represents another important goal, with efforts focused on reducing chemical waste and energy consumption without compromising analytical performance.
Looking forward, the field is moving toward real-time monitoring capabilities, allowing researchers to observe protein behavior during the focusing process rather than analyzing only the end result. The ultimate vision encompasses fully automated, high-throughput IEF platforms capable of processing multiple samples simultaneously while providing detailed structural and functional information about the separated proteins, thereby supporting advances in proteomics, biopharmaceutical development, and clinical diagnostics.
Market Demand for Protein Characterization
The protein characterization market has witnessed substantial growth in recent years, driven primarily by advancements in proteomics research and increasing applications in pharmaceutical development. The global protein characterization market was valued at approximately 6.7 billion USD in 2022 and is projected to reach 11.3 billion USD by 2027, representing a compound annual growth rate of 10.9%.
Isoelectric focusing (IEF) has emerged as a critical technique within this expanding market. As a high-resolution analytical method that separates proteins based on their isoelectric points, IEF addresses the growing demand for precise protein characterization in various industries. Pharmaceutical companies, in particular, have shown increased interest in IEF techniques for quality control of biopharmaceuticals, where even minor changes in protein structure can significantly impact drug efficacy and safety.
The biopharmaceutical sector represents the largest market segment utilizing protein characterization technologies, accounting for approximately 45% of the total market share. With over 7,800 biologic drugs in development globally and more than 400 approved for clinical use, the demand for sophisticated protein analysis methods like IEF continues to grow steadily.
Academic research institutions constitute another significant market segment, representing about 30% of the protein characterization market. The increasing focus on proteomics research and the growing number of protein-related studies published annually (over 15,000 in 2022) underscore the expanding demand for advanced protein characterization techniques in academic settings.
Diagnostic applications represent a rapidly growing segment, with a 15% annual growth rate. The integration of protein characterization techniques into clinical diagnostics for diseases such as cancer, autoimmune disorders, and neurodegenerative conditions has created new market opportunities for IEF and related technologies.
Regional analysis reveals that North America dominates the protein characterization market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate at 13.5% annually, driven by increasing research investments in countries like China, Japan, and India.
Key market drivers include the rising prevalence of protein-based drugs, increasing research funding for proteomics, technological advancements in analytical instruments, and growing applications in personalized medicine. The demand for high-resolution protein separation techniques like IEF is particularly strong in research focused on post-translational modifications, protein variants, and charge heterogeneity analysis.
Isoelectric focusing (IEF) has emerged as a critical technique within this expanding market. As a high-resolution analytical method that separates proteins based on their isoelectric points, IEF addresses the growing demand for precise protein characterization in various industries. Pharmaceutical companies, in particular, have shown increased interest in IEF techniques for quality control of biopharmaceuticals, where even minor changes in protein structure can significantly impact drug efficacy and safety.
The biopharmaceutical sector represents the largest market segment utilizing protein characterization technologies, accounting for approximately 45% of the total market share. With over 7,800 biologic drugs in development globally and more than 400 approved for clinical use, the demand for sophisticated protein analysis methods like IEF continues to grow steadily.
Academic research institutions constitute another significant market segment, representing about 30% of the protein characterization market. The increasing focus on proteomics research and the growing number of protein-related studies published annually (over 15,000 in 2022) underscore the expanding demand for advanced protein characterization techniques in academic settings.
Diagnostic applications represent a rapidly growing segment, with a 15% annual growth rate. The integration of protein characterization techniques into clinical diagnostics for diseases such as cancer, autoimmune disorders, and neurodegenerative conditions has created new market opportunities for IEF and related technologies.
Regional analysis reveals that North America dominates the protein characterization market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate at 13.5% annually, driven by increasing research investments in countries like China, Japan, and India.
Key market drivers include the rising prevalence of protein-based drugs, increasing research funding for proteomics, technological advancements in analytical instruments, and growing applications in personalized medicine. The demand for high-resolution protein separation techniques like IEF is particularly strong in research focused on post-translational modifications, protein variants, and charge heterogeneity analysis.
Current IEF Limitations and Challenges
Despite significant advancements in isoelectric focusing (IEF) technology, several critical limitations continue to challenge researchers in protein characterization applications. One of the most persistent issues is resolution constraints, particularly when analyzing complex protein mixtures containing components with closely similar isoelectric points (pIs). Current IEF systems struggle to reliably separate proteins with pI differences smaller than 0.05 pH units, creating significant barriers for detailed proteomic analysis of post-translational modifications.
Sample preparation remains another major hurdle, as proteins with extreme pI values (below 3 or above 10) often precipitate at their isoelectric points, leading to poor recovery and potential loss of critical information. This precipitation phenomenon particularly affects membrane proteins and highly hydrophobic proteins, which represent important therapeutic targets and biomarkers.
Reproducibility challenges continue to plague IEF applications, with inter-laboratory variations often exceeding acceptable thresholds for clinical and industrial applications. Environmental factors such as temperature fluctuations, ampholyte batch variations, and subtle differences in equipment calibration contribute to these inconsistencies, making standardization difficult across different research settings.
The time-intensive nature of traditional IEF methods presents another significant limitation. Conventional gel-based IEF procedures typically require 6-12 hours for complete separation, creating bottlenecks in high-throughput proteomics workflows. This extended processing time increases the risk of protein degradation and modification during analysis, potentially compromising data integrity.
Carrier ampholyte limitations represent another technical challenge, as current commercial ampholytes exhibit batch-to-batch variations that can significantly impact pH gradient formation and stability. These inconsistencies directly affect the reproducibility of protein migration patterns and pI determinations, complicating comparative analyses across experiments.
Automation integration remains problematic for many IEF platforms. While significant progress has been made in developing automated systems, many still require substantial manual intervention for sample loading, gel handling, and data interpretation. This human-dependent workflow introduces variability and limits throughput capacity in industrial and clinical settings.
Detection sensitivity presents ongoing challenges, particularly for low-abundance proteins that may be present at concentrations below current detection thresholds. This limitation is especially problematic in biomarker discovery applications, where proteins of interest may be present at picogram levels within complex biological matrices.
Finally, quantification accuracy remains suboptimal with current IEF technologies. While qualitative protein identification has improved dramatically, precise quantification of separated proteins continues to face technical barriers related to staining linearity, protein-to-protein staining variations, and challenges in standardization across different experimental conditions.
Sample preparation remains another major hurdle, as proteins with extreme pI values (below 3 or above 10) often precipitate at their isoelectric points, leading to poor recovery and potential loss of critical information. This precipitation phenomenon particularly affects membrane proteins and highly hydrophobic proteins, which represent important therapeutic targets and biomarkers.
Reproducibility challenges continue to plague IEF applications, with inter-laboratory variations often exceeding acceptable thresholds for clinical and industrial applications. Environmental factors such as temperature fluctuations, ampholyte batch variations, and subtle differences in equipment calibration contribute to these inconsistencies, making standardization difficult across different research settings.
The time-intensive nature of traditional IEF methods presents another significant limitation. Conventional gel-based IEF procedures typically require 6-12 hours for complete separation, creating bottlenecks in high-throughput proteomics workflows. This extended processing time increases the risk of protein degradation and modification during analysis, potentially compromising data integrity.
Carrier ampholyte limitations represent another technical challenge, as current commercial ampholytes exhibit batch-to-batch variations that can significantly impact pH gradient formation and stability. These inconsistencies directly affect the reproducibility of protein migration patterns and pI determinations, complicating comparative analyses across experiments.
Automation integration remains problematic for many IEF platforms. While significant progress has been made in developing automated systems, many still require substantial manual intervention for sample loading, gel handling, and data interpretation. This human-dependent workflow introduces variability and limits throughput capacity in industrial and clinical settings.
Detection sensitivity presents ongoing challenges, particularly for low-abundance proteins that may be present at concentrations below current detection thresholds. This limitation is especially problematic in biomarker discovery applications, where proteins of interest may be present at picogram levels within complex biological matrices.
Finally, quantification accuracy remains suboptimal with current IEF technologies. While qualitative protein identification has improved dramatically, precise quantification of separated proteins continues to face technical barriers related to staining linearity, protein-to-protein staining variations, and challenges in standardization across different experimental conditions.
Contemporary IEF Implementation Strategies
01 Isoelectric focusing apparatus and systems
Specialized equipment and systems designed for isoelectric focusing of proteins, including gel electrophoresis chambers, capillary systems, and microfluidic devices. These systems enable the separation of proteins based on their isoelectric points by establishing pH gradients. Advanced apparatus may incorporate automated sample loading, temperature control, and integrated detection systems to enhance the precision and reproducibility of protein characterization.- Isoelectric focusing apparatus and methods: Specialized equipment and methodologies for isoelectric focusing (IEF) that enable precise protein separation based on their isoelectric points. These systems include gel-based platforms, capillary electrophoresis systems, and microfluidic devices designed specifically for high-resolution protein characterization. The apparatus often incorporates pH gradient formation capabilities, temperature control systems, and detection mechanisms to ensure accurate and reproducible protein separation.
- pH gradient formation techniques: Methods for creating stable and reproducible pH gradients essential for effective isoelectric focusing. These techniques include the use of carrier ampholytes, immobilized pH gradients (IPGs), and specialized buffer systems that maintain gradient stability during the separation process. Advanced approaches involve the development of novel ampholytic compounds and gradient stabilizers that enhance resolution and prevent protein precipitation at their isoelectric points.
- Integration with other analytical techniques: Combination of isoelectric focusing with complementary analytical methods to enhance protein characterization capabilities. These integrated approaches include coupling IEF with mass spectrometry, liquid chromatography, or gel electrophoresis in multidimensional separation systems. Such combinations provide comprehensive protein analysis by separating proteins based on multiple physicochemical properties, enabling more detailed characterization of complex protein mixtures.
- Detection and visualization methods: Techniques for detecting and visualizing proteins after isoelectric focusing separation. These methods include staining protocols using dyes like Coomassie Blue or silver stain, fluorescent labeling approaches, and label-free detection systems. Advanced detection technologies incorporate real-time monitoring capabilities, digital imaging systems, and automated analysis software to quantify protein abundance and determine isoelectric points with high precision.
- Applications in protein characterization: Specific applications of isoelectric focusing for comprehensive protein characterization in various fields. These applications include analysis of post-translational modifications, determination of protein purity, assessment of protein stability, and identification of protein variants or isoforms. IEF is particularly valuable in biopharmaceutical development for characterizing therapeutic proteins, monitoring batch consistency, and detecting charge variants that may affect efficacy or immunogenicity.
02 pH gradient formation techniques
Methods for creating stable pH gradients essential for effective isoelectric focusing. This includes the use of carrier ampholytes, immobilized pH gradients (IPGs), and specialized buffer systems. The formation of precise and stable pH gradients is crucial for accurate determination of protein isoelectric points and high-resolution separation of complex protein mixtures with similar physicochemical properties.Expand Specific Solutions03 Detection and analysis methods
Techniques for visualizing and analyzing proteins after isoelectric focusing separation. These include staining methods, fluorescent labeling, mass spectrometry coupling, and digital imaging systems. Advanced detection methods enable quantitative analysis of protein abundance, post-translational modifications, and isoform distributions, enhancing the characterization of complex protein samples.Expand Specific Solutions04 Sample preparation techniques
Methods for preparing protein samples prior to isoelectric focusing to improve separation quality and resolution. This includes protein extraction, purification, denaturation, reduction, and alkylation procedures. Proper sample preparation minimizes interference from contaminants, prevents protein aggregation, and ensures complete solubilization, leading to more accurate and reproducible protein characterization results.Expand Specific Solutions05 Multi-dimensional protein separation
Integration of isoelectric focusing with other separation techniques to achieve comprehensive protein characterization. This includes two-dimensional electrophoresis (combining IEF with SDS-PAGE), liquid chromatography coupling, and other orthogonal separation methods. Multi-dimensional approaches significantly enhance the resolving power for complex protein mixtures, enabling the separation of thousands of proteins and identification of minor protein species that might be missed in single-dimension analyses.Expand Specific Solutions
Leading Companies in IEF Instrumentation
Isoelectric Focusing (IEF) technology for protein characterization is currently in a mature growth phase, with an estimated global market size of $1.5-2 billion and expanding at 6-8% annually. The competitive landscape features established leaders like Bio-Rad Laboratories and ProteinSimple (Bio-Techne) who have developed comprehensive IEF platforms with high resolution capabilities. Major pharmaceutical companies including Regeneron, Novartis, and WuXi Biologics are driving adoption through implementation in their protein therapeutic development workflows. Academic institutions (University of California, Shanghai Jiao Tong University) continue advancing fundamental research, while emerging players like Intabio are introducing innovations in automation and integration with mass spectrometry. The technology has reached significant maturity with standardized protocols, though continuous improvements in throughput, sensitivity, and integration with other analytical methods represent ongoing development areas.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed comprehensive IEF solutions through their Criterion and Mini-PROTEAN systems that have significantly advanced protein characterization capabilities. Their patented ReadyStrip IPG strips incorporate immobilized pH gradient technology with precise pH intervals (as narrow as 0.1 pH unit) that enable high-resolution separation of complex protein mixtures. Bio-Rad's V3 Western Workflow integrates IEF with downstream immunoblotting, allowing researchers to characterize proteins based on both isoelectric point and molecular weight. Their PROTEAN i12 IEF system provides programmable voltage ramping and temperature control (18-25°C) that minimizes protein aggregation during focusing. Bio-Rad has also pioneered specialized ampholytes (Bio-Lyte) that create exceptionally linear pH gradients, improving reproducibility with reported inter-assay CVs of 3-5%. Their recent innovations include microfluidic IEF chips that require only nanoliter sample volumes while maintaining resolution comparable to traditional methods.
Strengths: Comprehensive ecosystem of compatible products spanning sample preparation through analysis; exceptional pH gradient stability reducing drift during extended runs; versatile platform supporting both analytical and preparative applications; extensive validation across diverse protein types. Weaknesses: Traditional gel-based formats can be labor-intensive compared to fully automated systems; longer run times (4-12 hours) for optimal resolution; requires specialized training for consistent results; limited throughput for high-volume applications.
Life Technologies Corp.
Technical Solution: Life Technologies (now part of Thermo Fisher Scientific) has developed innovative IEF technologies that have significantly advanced protein characterization capabilities. Their ZOOM IEF Fractionator system utilizes a multi-compartment electrophoresis approach that enables high-resolution pre-fractionation of complex protein samples across customizable pH ranges. This technology incorporates specialized immobilized pH gradient (IPG) membranes that create discrete pH chambers, allowing for collection of proteins in solution rather than embedded in gels. Life Technologies' E-PAGE system combines IEF with SDS-PAGE in a unique horizontal format that reduces separation time by up to 80% compared to traditional methods. Their NuPAGE Novex pH 3-10 IEF gels incorporate proprietary buffer systems that minimize protein precipitation during focusing, particularly for hydrophobic membrane proteins that traditionally perform poorly in IEF. The company has also developed specialized ampholytes (ZOOM Carrier Ampholytes) that create exceptionally stable pH gradients, improving reproducibility with reported inter-assay CVs below 4%.
Strengths: Exceptional versatility across diverse protein types including challenging membrane proteins; seamless integration with downstream proteomics workflows; ability to recover proteins in native state for functional studies; high-throughput capabilities for processing multiple samples simultaneously; comprehensive ecosystem of compatible reagents and instruments. Weaknesses: Some platforms require specialized consumables creating vendor dependence; higher complexity in experimental setup compared to fully automated systems; requires optimization for specific sample types; limited resolution in extremely basic pH ranges.
Critical Patents in IEF Technology
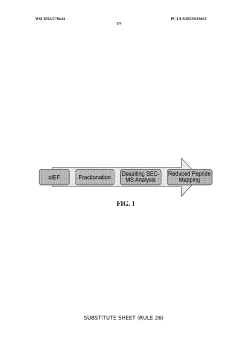
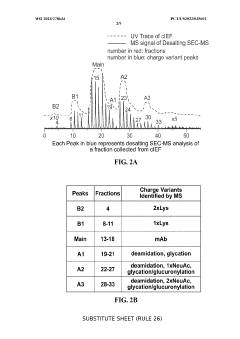
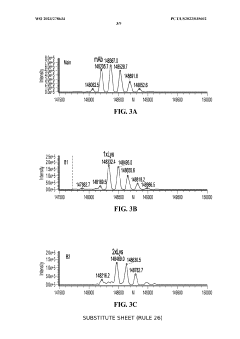
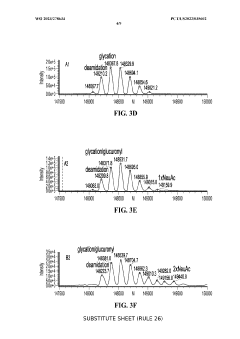
Coupling isoelectric focusing-based fractionation with mass spectrometry analysis
PatentWO2023278634A1
Innovation
- A method involving capillary isoelectric focusing with high-throughput fraction collection, followed by desalting size exclusion chromatography to modify the buffer for mass spectrometry compatibility, and subsequent mass spectrometry analysis for identifying site-specific protein modifications, allowing for high-resolution and high-throughput characterization of charge variants.
Methods and devices of separating molecular analytes
PatentWO2011021195A1
Innovation
- A method and device that generate a dynamic pH profile with multiple zones, allowing for the gradual adjustment of pH levels to induce the migration of molecular analytes based on their isoelectric points, enabling spatial and temporal separation of molecules with different isoelectric points by applying an electric field and controlling ion flows.
Regulatory Considerations for IEF Applications
Regulatory frameworks governing Isoelectric Focusing (IEF) applications vary significantly across global markets, creating a complex compliance landscape for manufacturers and researchers. The FDA in the United States classifies IEF equipment and reagents under medical devices or laboratory developed tests (LDTs) depending on their intended use, with specific requirements outlined in 21 CFR Part 820 for quality management systems. When IEF is employed for clinical diagnostics, compliance with Clinical Laboratory Improvement Amendments (CLIA) becomes mandatory, ensuring test reliability and accuracy through stringent laboratory standards.
In the European Union, IEF technologies must conform to the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVD Directive in May 2022. This transition introduced more rigorous clinical evidence requirements, post-market surveillance, and risk classification systems. Notably, IEF applications for protein characterization typically fall under Class B or C, necessitating conformity assessment by notified bodies and comprehensive technical documentation.
The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q6B, provide specific recommendations for physicochemical characterization of proteins using techniques like IEF. These guidelines emphasize validation parameters including specificity, linearity, range, accuracy, precision, and robustness when implementing IEF methods for protein characterization in pharmaceutical development.
Regulatory bodies increasingly focus on method validation and standardization for IEF applications. The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) have established specific monographs detailing acceptance criteria and procedural standards for IEF when used in quality control of biopharmaceuticals. These standards address critical aspects such as resolution requirements, pH gradient stability, and system suitability tests.
Data integrity considerations present another significant regulatory challenge, with agencies requiring comprehensive documentation of raw data, analysis methods, and results traceability. Electronic records from modern IEF systems must comply with 21 CFR Part 11 (FDA) or Annex 11 (EU GMP), which mandate audit trails, electronic signatures, and validated computer systems.
Looking forward, regulatory trends indicate movement toward harmonized global standards for IEF applications, with increased emphasis on real-time release testing and continuous manufacturing processes. Manufacturers developing IEF-based technologies must implement proactive regulatory strategies, including early engagement with authorities through scientific advice meetings and careful monitoring of evolving guidelines to ensure compliance throughout product lifecycles.
In the European Union, IEF technologies must conform to the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVD Directive in May 2022. This transition introduced more rigorous clinical evidence requirements, post-market surveillance, and risk classification systems. Notably, IEF applications for protein characterization typically fall under Class B or C, necessitating conformity assessment by notified bodies and comprehensive technical documentation.
The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q6B, provide specific recommendations for physicochemical characterization of proteins using techniques like IEF. These guidelines emphasize validation parameters including specificity, linearity, range, accuracy, precision, and robustness when implementing IEF methods for protein characterization in pharmaceutical development.
Regulatory bodies increasingly focus on method validation and standardization for IEF applications. The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) have established specific monographs detailing acceptance criteria and procedural standards for IEF when used in quality control of biopharmaceuticals. These standards address critical aspects such as resolution requirements, pH gradient stability, and system suitability tests.
Data integrity considerations present another significant regulatory challenge, with agencies requiring comprehensive documentation of raw data, analysis methods, and results traceability. Electronic records from modern IEF systems must comply with 21 CFR Part 11 (FDA) or Annex 11 (EU GMP), which mandate audit trails, electronic signatures, and validated computer systems.
Looking forward, regulatory trends indicate movement toward harmonized global standards for IEF applications, with increased emphasis on real-time release testing and continuous manufacturing processes. Manufacturers developing IEF-based technologies must implement proactive regulatory strategies, including early engagement with authorities through scientific advice meetings and careful monitoring of evolving guidelines to ensure compliance throughout product lifecycles.
Quality Control Standards in IEF Analysis
Quality control standards in IEF analysis represent a critical framework for ensuring reliable and reproducible protein characterization results. The establishment of robust quality control measures begins with the implementation of standardized protocols for sample preparation, focusing particularly on protein concentration determination and buffer composition optimization. These protocols must be meticulously documented and consistently followed to minimize variability between analyses.
Performance qualification of IEF equipment constitutes another essential component of quality control standards. Regular calibration of pH gradients using certified standard proteins with known isoelectric points enables accurate pI determination and system validation. Equipment performance should be monitored through routine testing with reference materials to detect any drift or deterioration in separation efficiency over time.
The development and utilization of internal control samples serves as a cornerstone of effective quality assurance in IEF analysis. These controls, typically consisting of well-characterized proteins with known isoelectric points spanning the pH range of interest, provide a means to verify system suitability before analyzing experimental samples. Tracking the migration patterns of these control proteins across multiple runs facilitates the identification of systematic errors or inconsistencies.
Statistical process control methodologies play an increasingly important role in modern IEF quality standards. Establishing acceptance criteria for key performance indicators—such as resolution between adjacent protein bands, signal-to-noise ratio, and reproducibility of pI values—enables objective assessment of analytical performance. Implementation of control charts to monitor these parameters over time helps identify trends that may indicate developing problems before they significantly impact results.
Interlaboratory comparison programs represent the gold standard for external quality assessment in IEF analysis. Participation in such programs allows laboratories to benchmark their performance against peers and identify potential systematic biases in their methodologies. These collaborative exercises typically involve the analysis of identical samples across multiple facilities, with subsequent statistical evaluation of the consistency and accuracy of reported results.
Documentation requirements constitute a fundamental aspect of quality control standards in IEF. Comprehensive record-keeping should encompass instrument maintenance logs, reagent preparation details, calibration data, and complete experimental conditions for each analysis. This documentation not only supports troubleshooting efforts when anomalies occur but also facilitates regulatory compliance in environments where IEF data informs critical decisions about product quality or patient care.
Performance qualification of IEF equipment constitutes another essential component of quality control standards. Regular calibration of pH gradients using certified standard proteins with known isoelectric points enables accurate pI determination and system validation. Equipment performance should be monitored through routine testing with reference materials to detect any drift or deterioration in separation efficiency over time.
The development and utilization of internal control samples serves as a cornerstone of effective quality assurance in IEF analysis. These controls, typically consisting of well-characterized proteins with known isoelectric points spanning the pH range of interest, provide a means to verify system suitability before analyzing experimental samples. Tracking the migration patterns of these control proteins across multiple runs facilitates the identification of systematic errors or inconsistencies.
Statistical process control methodologies play an increasingly important role in modern IEF quality standards. Establishing acceptance criteria for key performance indicators—such as resolution between adjacent protein bands, signal-to-noise ratio, and reproducibility of pI values—enables objective assessment of analytical performance. Implementation of control charts to monitor these parameters over time helps identify trends that may indicate developing problems before they significantly impact results.
Interlaboratory comparison programs represent the gold standard for external quality assessment in IEF analysis. Participation in such programs allows laboratories to benchmark their performance against peers and identify potential systematic biases in their methodologies. These collaborative exercises typically involve the analysis of identical samples across multiple facilities, with subsequent statistical evaluation of the consistency and accuracy of reported results.
Documentation requirements constitute a fundamental aspect of quality control standards in IEF. Comprehensive record-keeping should encompass instrument maintenance logs, reagent preparation details, calibration data, and complete experimental conditions for each analysis. This documentation not only supports troubleshooting efforts when anomalies occur but also facilitates regulatory compliance in environments where IEF data informs critical decisions about product quality or patient care.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!