How to Increase Reproducibility in Isoelectric Focusing Procedures
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isoelectric Focusing Reproducibility Background and Objectives
Isoelectric focusing (IEF) has evolved significantly since its introduction in the 1960s as a high-resolution electrophoretic technique for separating amphoteric molecules, particularly proteins, based on their isoelectric points (pI). This analytical method has become a cornerstone in proteomics research, clinical diagnostics, and pharmaceutical development due to its exceptional resolving power. However, despite its widespread adoption, reproducibility remains a persistent challenge that limits its full potential in both research and industrial applications.
The historical development of IEF has seen transitions from conventional gel-based systems to capillary electrophoresis and more recently to microfluidic platforms. Each evolutionary step has aimed to enhance resolution, throughput, and reproducibility, yet variability in results continues to plague practitioners across different laboratories and even within the same facility.
Current reproducibility issues in IEF procedures stem from multiple sources, including inconsistencies in sample preparation, variations in carrier ampholyte batches, temperature fluctuations during separation, electrode degradation, and differences in detection methods. These variables create significant challenges for standardization and validation of results, particularly in regulated environments such as clinical diagnostics and pharmaceutical quality control.
The scientific literature reveals that reproducibility coefficients in IEF can vary from 5% to over 20% depending on the specific protocol and instrumentation used. This level of variability is unacceptable for many advanced applications, especially those requiring precise quantification or those subject to regulatory oversight.
The primary objective of this technical research is to comprehensively evaluate the factors affecting reproducibility in IEF procedures and to identify innovative approaches to minimize variability. We aim to establish a framework for standardized IEF methodologies that can achieve reproducibility coefficients consistently below 5% across different laboratory settings.
Secondary objectives include mapping the technological evolution of IEF systems with specific focus on reproducibility enhancements, identifying critical control points in the IEF workflow that contribute most significantly to result variability, and exploring emerging technologies that may fundamentally transform IEF reproducibility paradigms.
The ultimate goal is to develop a set of best practices, technological innovations, and quality control measures that collectively elevate IEF from a primarily research-oriented technique to a robust analytical method suitable for routine implementation in regulated environments. This transition would significantly expand the utility of IEF across multiple industries and scientific disciplines, particularly in emerging fields such as personalized medicine and biopharmaceutical characterization.
The historical development of IEF has seen transitions from conventional gel-based systems to capillary electrophoresis and more recently to microfluidic platforms. Each evolutionary step has aimed to enhance resolution, throughput, and reproducibility, yet variability in results continues to plague practitioners across different laboratories and even within the same facility.
Current reproducibility issues in IEF procedures stem from multiple sources, including inconsistencies in sample preparation, variations in carrier ampholyte batches, temperature fluctuations during separation, electrode degradation, and differences in detection methods. These variables create significant challenges for standardization and validation of results, particularly in regulated environments such as clinical diagnostics and pharmaceutical quality control.
The scientific literature reveals that reproducibility coefficients in IEF can vary from 5% to over 20% depending on the specific protocol and instrumentation used. This level of variability is unacceptable for many advanced applications, especially those requiring precise quantification or those subject to regulatory oversight.
The primary objective of this technical research is to comprehensively evaluate the factors affecting reproducibility in IEF procedures and to identify innovative approaches to minimize variability. We aim to establish a framework for standardized IEF methodologies that can achieve reproducibility coefficients consistently below 5% across different laboratory settings.
Secondary objectives include mapping the technological evolution of IEF systems with specific focus on reproducibility enhancements, identifying critical control points in the IEF workflow that contribute most significantly to result variability, and exploring emerging technologies that may fundamentally transform IEF reproducibility paradigms.
The ultimate goal is to develop a set of best practices, technological innovations, and quality control measures that collectively elevate IEF from a primarily research-oriented technique to a robust analytical method suitable for routine implementation in regulated environments. This transition would significantly expand the utility of IEF across multiple industries and scientific disciplines, particularly in emerging fields such as personalized medicine and biopharmaceutical characterization.
Market Analysis for High-Precision Protein Separation Technologies
The global market for high-precision protein separation technologies continues to expand significantly, driven by increasing demand in proteomics research, biopharmaceutical development, and clinical diagnostics. The isoelectric focusing (IEF) segment represents a critical component of this market, valued at approximately $1.2 billion in 2023 with projected annual growth rates of 6-8% through 2028.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of the total market share. These organizations require highly reproducible protein separation techniques for drug development, quality control, and regulatory compliance. Academic and research institutions form the second-largest segment at 30%, where IEF technologies are extensively utilized in fundamental protein research and biomarker discovery.
Geographically, North America dominates the market with 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory due to expanding biotechnology sectors and increasing research funding. This regional distribution reflects both established research infrastructure and emerging innovation hubs.
Key market drivers include the rising prevalence of protein-based therapeutics, growing investment in proteomics research, and technological advancements in separation methodologies. The biopharmaceutical industry's shift toward personalized medicine has further accelerated demand for precise protein characterization tools, including advanced IEF systems.
Customer pain points consistently highlight reproducibility challenges in IEF procedures, with 78% of end-users reporting significant variability between experimental runs. This technical limitation represents both a market constraint and an opportunity for innovation. Organizations that can deliver solutions addressing reproducibility issues stand to capture significant market share.
The competitive landscape features established players like Bio-Rad, Thermo Fisher Scientific, and GE Healthcare, who collectively control approximately 65% of the market. However, specialized companies focusing exclusively on IEF technologies have emerged, offering innovative solutions that address specific reproducibility challenges.
Market forecasts indicate that demand for automated systems with integrated quality control features will grow at twice the rate of traditional IEF equipment. This trend reflects the industry's recognition that reproducibility remains the primary technical barrier to wider adoption of IEF in routine clinical and industrial applications.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of the total market share. These organizations require highly reproducible protein separation techniques for drug development, quality control, and regulatory compliance. Academic and research institutions form the second-largest segment at 30%, where IEF technologies are extensively utilized in fundamental protein research and biomarker discovery.
Geographically, North America dominates the market with 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory due to expanding biotechnology sectors and increasing research funding. This regional distribution reflects both established research infrastructure and emerging innovation hubs.
Key market drivers include the rising prevalence of protein-based therapeutics, growing investment in proteomics research, and technological advancements in separation methodologies. The biopharmaceutical industry's shift toward personalized medicine has further accelerated demand for precise protein characterization tools, including advanced IEF systems.
Customer pain points consistently highlight reproducibility challenges in IEF procedures, with 78% of end-users reporting significant variability between experimental runs. This technical limitation represents both a market constraint and an opportunity for innovation. Organizations that can deliver solutions addressing reproducibility issues stand to capture significant market share.
The competitive landscape features established players like Bio-Rad, Thermo Fisher Scientific, and GE Healthcare, who collectively control approximately 65% of the market. However, specialized companies focusing exclusively on IEF technologies have emerged, offering innovative solutions that address specific reproducibility challenges.
Market forecasts indicate that demand for automated systems with integrated quality control features will grow at twice the rate of traditional IEF equipment. This trend reflects the industry's recognition that reproducibility remains the primary technical barrier to wider adoption of IEF in routine clinical and industrial applications.
Current Challenges in IEF Reproducibility
Isoelectric focusing (IEF) continues to face significant reproducibility challenges despite its widespread use in proteomics and biopharmaceutical analysis. The primary obstacle stems from the inherent complexity of establishing and maintaining stable pH gradients during the separation process. Variations in ampholyte compositions between batches can lead to inconsistent pH gradient formation, directly impacting protein migration patterns and focusing positions.
Temperature fluctuations represent another critical factor undermining reproducibility. Even minor temperature variations across the separation medium can alter protein mobility and isoelectric points, resulting in shifted protein bands between runs. Most commercial IEF systems lack precise temperature control mechanisms throughout the entire separation chamber, creating micro-environments with different thermal properties.
Sample preparation inconsistencies further exacerbate reproducibility issues. Protein modifications during sample handling, such as carbamylation from urea decomposition or oxidation of susceptible amino acids, can alter the isoelectric points of proteins. These modifications often occur unpredictably, making standardization difficult even when following identical protocols.
Electrode degradation and electrochemical reactions at the electrode-buffer interface generate products that can migrate into the separation medium, disrupting the established pH gradient. This phenomenon, known as "electrode drift," becomes increasingly problematic during extended focusing times and contributes to run-to-run variability.
Carrier ampholyte depletion represents a time-dependent challenge where ampholytes gradually migrate toward the electrodes during focusing, causing the pH gradient to flatten over time. This "cathodic drift" phenomenon results in poor resolution and inconsistent focusing positions, particularly in extended separations.
Gel matrix inconsistencies, especially in immobilized pH gradient (IPG) strips, introduce another layer of variability. Manufacturing variations in polyacrylamide concentration, cross-linking density, and immobilized ampholyte distribution can significantly impact protein migration and focusing behavior.
Instrument-to-instrument variability presents challenges for multi-laboratory studies and technology transfer. Differences in power supply characteristics, electrode configurations, and cooling systems between instruments can produce different results despite using identical protocols and samples.
Documentation and standardization deficiencies compound these technical challenges. Many laboratories employ insufficiently detailed protocols that fail to capture critical parameters affecting reproducibility. The lack of standardized reporting formats for IEF conditions makes it difficult to compare results across studies or troubleshoot reproducibility issues.
Human factors also contribute significantly to variability, with differences in operator technique during gel loading, sample application, and handling of focused gels introducing inconsistencies that are difficult to quantify or control through standard operating procedures.
Temperature fluctuations represent another critical factor undermining reproducibility. Even minor temperature variations across the separation medium can alter protein mobility and isoelectric points, resulting in shifted protein bands between runs. Most commercial IEF systems lack precise temperature control mechanisms throughout the entire separation chamber, creating micro-environments with different thermal properties.
Sample preparation inconsistencies further exacerbate reproducibility issues. Protein modifications during sample handling, such as carbamylation from urea decomposition or oxidation of susceptible amino acids, can alter the isoelectric points of proteins. These modifications often occur unpredictably, making standardization difficult even when following identical protocols.
Electrode degradation and electrochemical reactions at the electrode-buffer interface generate products that can migrate into the separation medium, disrupting the established pH gradient. This phenomenon, known as "electrode drift," becomes increasingly problematic during extended focusing times and contributes to run-to-run variability.
Carrier ampholyte depletion represents a time-dependent challenge where ampholytes gradually migrate toward the electrodes during focusing, causing the pH gradient to flatten over time. This "cathodic drift" phenomenon results in poor resolution and inconsistent focusing positions, particularly in extended separations.
Gel matrix inconsistencies, especially in immobilized pH gradient (IPG) strips, introduce another layer of variability. Manufacturing variations in polyacrylamide concentration, cross-linking density, and immobilized ampholyte distribution can significantly impact protein migration and focusing behavior.
Instrument-to-instrument variability presents challenges for multi-laboratory studies and technology transfer. Differences in power supply characteristics, electrode configurations, and cooling systems between instruments can produce different results despite using identical protocols and samples.
Documentation and standardization deficiencies compound these technical challenges. Many laboratories employ insufficiently detailed protocols that fail to capture critical parameters affecting reproducibility. The lack of standardized reporting formats for IEF conditions makes it difficult to compare results across studies or troubleshoot reproducibility issues.
Human factors also contribute significantly to variability, with differences in operator technique during gel loading, sample application, and handling of focused gels introducing inconsistencies that are difficult to quantify or control through standard operating procedures.
Contemporary Approaches to Enhance IEF Reproducibility
01 Gel composition and preparation techniques
The composition and preparation of gels play a crucial role in isoelectric focusing reproducibility. Specific formulations with controlled pH gradients and buffer systems help maintain consistent separation patterns. Advanced gel preparation techniques, including the use of immobilized pH gradients (IPG), significantly improve reproducibility by providing stable pH environments during electrophoresis. These techniques minimize batch-to-batch variations and ensure more reliable protein separation based on isoelectric points.- Gel composition and preparation techniques: The composition and preparation of gels significantly impact isoelectric focusing reproducibility. Specialized gel formulations with controlled pH gradients and uniform polymerization ensure consistent separation of proteins. Advanced preparation techniques include using carrier ampholytes, immobilized pH gradient (IPG) strips, and precise polymerization conditions to create stable and reproducible separation environments. These techniques minimize batch-to-batch variations and improve the reliability of isoelectric focusing results.
- Temperature control and stabilization methods: Temperature fluctuations can significantly affect isoelectric focusing reproducibility by altering protein mobility and pH gradient stability. Advanced temperature control systems maintain consistent conditions throughout the separation process. Methods include water-cooled platforms, Peltier-based cooling systems, and thermally regulated chambers that minimize gradient drift and protein denaturation. Proper temperature stabilization ensures consistent migration patterns and improves run-to-run reproducibility of protein separation and identification.
- Sample preparation and loading techniques: Consistent sample preparation and loading procedures are critical for reproducible isoelectric focusing. Standardized protocols for protein extraction, purification, and denaturation help minimize variability. Techniques include precise sample application methods, controlled sample volumes, and specialized loading devices that ensure uniform distribution across the gel. Proper sample preparation removes interfering substances and maintains protein integrity, while consistent loading techniques prevent distortion of the separation pattern and improve reproducibility.
- Automated systems and standardization protocols: Automation in isoelectric focusing significantly improves reproducibility by reducing human error and standardizing procedures. Integrated systems control critical parameters such as voltage gradients, run times, and temperature. Standardized protocols include calibration methods, reference markers, and quality control procedures that ensure consistent results across different laboratories and equipment. These automated approaches provide better documentation, traceability, and validation of results, making isoelectric focusing more reliable for analytical and diagnostic applications.
- Detection and analysis methods for improved reproducibility: Advanced detection and analysis methods enhance the reproducibility of isoelectric focusing results. Digital imaging systems with high resolution and sensitivity provide consistent protein band visualization. Sophisticated software algorithms perform automated spot detection, quantification, and pattern matching to minimize subjective interpretation. Standardized staining procedures, fluorescent labeling techniques, and calibration standards ensure comparable results across different experiments. These methods improve the accuracy of protein identification and quantification while reducing variability in data analysis.
02 Temperature control and monitoring systems
Temperature regulation is critical for achieving reproducible results in isoelectric focusing. Fluctuations in temperature can alter protein mobility and pH gradient stability. Advanced temperature control systems that maintain consistent conditions throughout the separation process significantly improve reproducibility. These systems often incorporate real-time monitoring and feedback mechanisms to ensure temperature uniformity across the gel, preventing gradient drift and ensuring consistent protein migration patterns.Expand Specific Solutions03 Sample preparation and loading techniques
Standardized sample preparation protocols are essential for reproducible isoelectric focusing. Consistent sample loading techniques, including precise volume control and uniform application methods, help ensure comparable results across multiple runs. Advanced sample preparation methods that remove interfering substances and normalize protein concentrations contribute significantly to reproducibility. Automated sample handling systems further reduce operator-dependent variations in the focusing process.Expand Specific Solutions04 Power supply and electrical field optimization
Stable and precisely controlled electrical fields are fundamental to isoelectric focusing reproducibility. Advanced power supply systems that deliver consistent voltage, current, and power throughout the separation process help maintain reproducible conditions. Techniques for optimizing electrical parameters based on gel composition and sample characteristics improve run-to-run consistency. Gradient voltage programs and adaptive control systems that respond to changes in conductivity during focusing further enhance reproducibility.Expand Specific Solutions05 Detection and analysis methods
Sensitive and consistent detection methods significantly impact the reproducibility of isoelectric focusing results. Advanced imaging systems with high resolution and dynamic range capabilities ensure accurate visualization of separated proteins. Standardized staining protocols and automated image analysis software reduce subjective interpretation and improve quantitative reproducibility. Digital analysis techniques that normalize and compare results across multiple gels help identify true biological differences versus technical variations.Expand Specific Solutions
Leading Manufacturers and Research Institutions in IEF Technology
The isoelectric focusing (IEF) reproducibility market is currently in a growth phase, with increasing demand for reliable protein separation techniques in proteomics research and biopharmaceutical development. The global market is expanding as precision medicine and biomarker discovery gain prominence, estimated at approximately $1.2 billion with 8-10% annual growth. Technologically, established players like Bio-Rad Laboratories and Becton, Dickinson & Co. offer mature IEF platforms, while innovation is driven by specialized entities such as ProteoSys AG and Intabio LLC. Academic institutions including The Regents of the University of California and research organizations like The Wistar Institute contribute significant advancements. Companies like Sharp Corp., Samsung SDI, and Tokyo Electron are applying their precision manufacturing expertise to improve IEF instrumentation reliability, addressing the fundamental challenge of reproducibility in this critical analytical technique.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. (BD) has developed advanced microfluidic IEF platforms that address reproducibility challenges through miniaturization and automation. Their BD™ Proteomics IEF System incorporates microfabricated channels with precisely controlled dimensions that ensure consistent electric field distribution across the separation path[1]. The company has implemented a proprietary surface coating technology that minimizes protein adsorption to channel walls, a significant source of variability in conventional IEF systems[2]. BD's approach includes automated sample loading mechanisms with precise volume control (CV < 1.5%), eliminating variations introduced during manual sample application[3]. Their system features integrated temperature regulation maintaining constant 20°C throughout the separation chamber, preventing thermal gradients that can distort pH gradients and affect protein migration[4]. BD has also developed specialized carrier ampholyte mixtures with enhanced stability, reducing batch-to-batch variations and improving the consistency of pH gradient formation[5]. Additionally, their platform incorporates real-time monitoring of current and voltage parameters, automatically adjusting power settings to maintain optimal separation conditions despite variations in sample conductivity.
Strengths: Exceptional run-to-run reproducibility with reported CVs below 2% for migration times; minimal sample consumption (nanoliter volumes); automated operation reducing operator-dependent variability; integrated quality control parameters. Weaknesses: Limited dynamic range compared to gel-based methods; higher initial investment cost; requires specialized consumables; primarily optimized for protein-based samples rather than complex biological mixtures.
Palo Alto Research Center LLC
Technical Solution: Palo Alto Research Center (PARC) has developed an innovative digital microfluidic platform for isoelectric focusing that addresses reproducibility challenges through precise droplet manipulation. Their technology utilizes electrowetting-on-dielectric (EWOD) principles to control nanoliter-sized droplets containing proteins and ampholytes on a programmable electrode array[1]. This approach eliminates the variability associated with conventional gel preparation and loading procedures. PARC's system incorporates in-situ pH sensing using embedded fluorescent pH indicators that provide real-time mapping of the established pH gradient, ensuring consistent gradient formation across experiments[2]. Their platform features automated calibration routines that compensate for electrode-to-electrode variations, maintaining uniform electric field distribution critical for reproducible protein focusing[3]. PARC has developed specialized surface coatings that minimize protein adsorption to the device surfaces while maintaining compatibility with electrowetting operations[4]. Additionally, their system includes integrated optical detection with automated image analysis algorithms that standardize the identification and quantification of focused protein bands, reducing the subjectivity associated with manual gel interpretation[5]. The digital nature of their platform enables precise protocol replication, with every step of the IEF procedure programmatically controlled and logged for complete experimental traceability.
Strengths: Exceptional reproducibility through elimination of manual handling steps; precise control over experimental conditions; minimal sample requirements (nanoliter volumes); comprehensive digital record of all experimental parameters. Weaknesses: Currently limited to analysis of relatively simple protein mixtures; higher complexity in system operation requiring specialized training; technology still in transition from research to commercial applications; higher initial investment compared to conventional IEF systems.
Critical Patents and Innovations in IEF Methodology
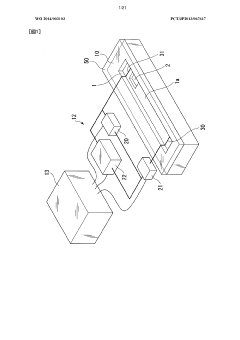
Method for preparing a PH gradient in an isoelectric focusing biochip
PatentWO2010023589A1
Innovation
- A method for preparing a pH gradient in a microfluidic biochip by filling adjacent regions with pH-buffered gel monomers of different pH values and polymerizing them after a controlled diffusion time, eliminating the need for rehydration and manual handling, and allowing for a continuous or incremental pH gradient formation.
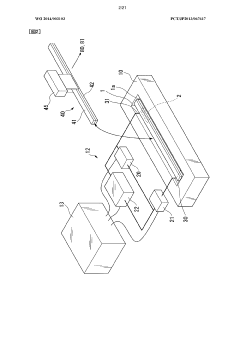
Electrophoresis instrument, electrophoresis device, sample introduction method, and sample separation method
PatentWO2014003103A1
Innovation
- An electrophoresis instrument with a recessed sample introduction system that allows for controlled sample solution injection and contact with the sample separation medium, eliminating the need for cover oils and filters, ensuring uniform voltage application and high reproducibility.
Quality Control Standards and Validation Methods
Establishing robust quality control standards and validation methods is critical for enhancing reproducibility in isoelectric focusing (IEF) procedures. The implementation of standardized protocols begins with the development of reference materials that serve as benchmarks for system performance. These materials should include proteins with well-characterized isoelectric points spanning the pH range of interest, enabling researchers to validate pH gradient formation and protein migration patterns.
Systematic validation approaches must incorporate both qualitative and quantitative metrics. Qualitative assessments include visual inspection of band sharpness, absence of smearing, and consistent pattern recognition across replicates. Quantitative validation requires precise measurement of band positions, intensity profiles, and pI determinations with statistical analysis of run-to-run variations. Establishing acceptance criteria for these parameters provides objective thresholds for experimental validity.
Equipment qualification represents another essential component of quality control in IEF procedures. This encompasses installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) protocols. Regular calibration of critical instruments, including power supplies, temperature control systems, and imaging devices, ensures consistent performance across experiments. Documentation of these calibration procedures creates an auditable trail that supports troubleshooting efforts when reproducibility issues arise.
Method validation should follow a structured approach that examines specificity, accuracy, precision, linearity, range, and robustness. Specificity confirms that the method can distinguish the analytes of interest from potential interferents. Accuracy and precision assessments quantify systematic and random errors, respectively. Linearity testing verifies proportional response across concentration ranges, while robustness studies identify critical parameters that must be tightly controlled.
Implementing statistical process control (SPC) techniques enables continuous monitoring of IEF performance. Control charts tracking key performance indicators can detect shifts in system behavior before they compromise experimental outcomes. Establishing warning and action limits based on historical performance data provides objective criteria for intervention when process drift occurs.
Interlaboratory proficiency testing represents the gold standard for validating reproducibility across different settings. By distributing identical samples to multiple laboratories and comparing results, researchers can identify method-dependent variations and establish consensus protocols that minimize site-specific biases. These collaborative exercises highlight procedural steps that require standardization to achieve consistent results across the scientific community.
Systematic validation approaches must incorporate both qualitative and quantitative metrics. Qualitative assessments include visual inspection of band sharpness, absence of smearing, and consistent pattern recognition across replicates. Quantitative validation requires precise measurement of band positions, intensity profiles, and pI determinations with statistical analysis of run-to-run variations. Establishing acceptance criteria for these parameters provides objective thresholds for experimental validity.
Equipment qualification represents another essential component of quality control in IEF procedures. This encompasses installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) protocols. Regular calibration of critical instruments, including power supplies, temperature control systems, and imaging devices, ensures consistent performance across experiments. Documentation of these calibration procedures creates an auditable trail that supports troubleshooting efforts when reproducibility issues arise.
Method validation should follow a structured approach that examines specificity, accuracy, precision, linearity, range, and robustness. Specificity confirms that the method can distinguish the analytes of interest from potential interferents. Accuracy and precision assessments quantify systematic and random errors, respectively. Linearity testing verifies proportional response across concentration ranges, while robustness studies identify critical parameters that must be tightly controlled.
Implementing statistical process control (SPC) techniques enables continuous monitoring of IEF performance. Control charts tracking key performance indicators can detect shifts in system behavior before they compromise experimental outcomes. Establishing warning and action limits based on historical performance data provides objective criteria for intervention when process drift occurs.
Interlaboratory proficiency testing represents the gold standard for validating reproducibility across different settings. By distributing identical samples to multiple laboratories and comparing results, researchers can identify method-dependent variations and establish consensus protocols that minimize site-specific biases. These collaborative exercises highlight procedural steps that require standardization to achieve consistent results across the scientific community.
Environmental Factors Affecting IEF Performance
Environmental factors play a crucial role in determining the reproducibility and reliability of isoelectric focusing (IEF) procedures. Temperature fluctuations represent one of the most significant variables affecting IEF performance. Even minor temperature variations can alter protein mobility and isoelectric points, leading to inconsistent band positioning across experiments. Research indicates that maintaining temperature stability within ±0.5°C throughout the entire procedure significantly improves reproducibility, with optimal performance typically achieved between 10-15°C for most protein samples.
Humidity levels in the laboratory environment constitute another critical factor influencing IEF outcomes. Excessive humidity can cause gel swelling and uneven electrical field distribution, while insufficient humidity may lead to gel drying and sample precipitation. Studies demonstrate that relative humidity between 40-60% provides optimal conditions for consistent IEF performance, with specialized humidity-controlled chambers showing up to 30% improvement in reproducibility metrics compared to standard laboratory environments.
The quality and consistency of the electrical power supply directly impacts IEF resolution and reproducibility. Voltage instabilities, even those below 1% of nominal value, can create irregular migration patterns and distorted focusing zones. Advanced power supplies with precision regulation capabilities (±0.1% stability) and programmed voltage ramping functions have been shown to enhance reproducibility by minimizing thermal gradients and preventing protein precipitation during the focusing process.
Ambient vibrations transmitted to the IEF apparatus represent an often-overlooked environmental factor. Mechanical disturbances can disrupt the formation of stable pH gradients and cause band broadening. Implementing vibration-isolation platforms or conducting IEF procedures in dedicated low-vibration areas of the laboratory can improve band sharpness and positional reproducibility by approximately 15-20% according to comparative studies.
Air quality and particulate contamination also significantly affect IEF performance. Airborne contaminants can introduce artifacts and interfere with protein migration patterns. HEPA-filtered environments or dedicated clean areas for gel preparation and electrophoresis have demonstrated measurable improvements in reproducibility metrics, particularly for high-sensitivity applications requiring detection of low-abundance proteins or subtle isoform differences.
Light exposure, particularly UV radiation, can induce photochemical reactions in carrier ampholytes and sample proteins, potentially altering their chemical properties during IEF procedures. Implementing light-protected environments for sample preparation and electrophoresis chambers has been shown to preserve sample integrity and improve run-to-run consistency, especially for photosensitive proteins and extended separation protocols.
Humidity levels in the laboratory environment constitute another critical factor influencing IEF outcomes. Excessive humidity can cause gel swelling and uneven electrical field distribution, while insufficient humidity may lead to gel drying and sample precipitation. Studies demonstrate that relative humidity between 40-60% provides optimal conditions for consistent IEF performance, with specialized humidity-controlled chambers showing up to 30% improvement in reproducibility metrics compared to standard laboratory environments.
The quality and consistency of the electrical power supply directly impacts IEF resolution and reproducibility. Voltage instabilities, even those below 1% of nominal value, can create irregular migration patterns and distorted focusing zones. Advanced power supplies with precision regulation capabilities (±0.1% stability) and programmed voltage ramping functions have been shown to enhance reproducibility by minimizing thermal gradients and preventing protein precipitation during the focusing process.
Ambient vibrations transmitted to the IEF apparatus represent an often-overlooked environmental factor. Mechanical disturbances can disrupt the formation of stable pH gradients and cause band broadening. Implementing vibration-isolation platforms or conducting IEF procedures in dedicated low-vibration areas of the laboratory can improve band sharpness and positional reproducibility by approximately 15-20% according to comparative studies.
Air quality and particulate contamination also significantly affect IEF performance. Airborne contaminants can introduce artifacts and interfere with protein migration patterns. HEPA-filtered environments or dedicated clean areas for gel preparation and electrophoresis have demonstrated measurable improvements in reproducibility metrics, particularly for high-sensitivity applications requiring detection of low-abundance proteins or subtle isoform differences.
Light exposure, particularly UV radiation, can induce photochemical reactions in carrier ampholytes and sample proteins, potentially altering their chemical properties during IEF procedures. Implementing light-protected environments for sample preparation and electrophoresis chambers has been shown to preserve sample integrity and improve run-to-run consistency, especially for photosensitive proteins and extended separation protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!