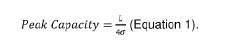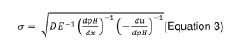How Isoelectric Focusing Enhances Protein Modification Analysis
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Evolution and Objectives
Isoelectric focusing (IEF) has evolved significantly since its inception in the 1960s, transforming from a rudimentary analytical technique to a sophisticated method essential for protein characterization. Initially developed by Svensson and further refined by Vesterberg, IEF leverages the amphoteric nature of proteins to separate them based on their isoelectric points (pI) within a pH gradient. This fundamental principle has remained unchanged, while the technological implementation has undergone remarkable advancements.
The evolution of IEF technology can be traced through several key developmental phases. The first generation utilized carrier ampholytes to establish pH gradients in tube gels, offering limited resolution and reproducibility. The 1980s witnessed the introduction of immobilized pH gradient (IPG) strips by Bjellqvist and colleagues, representing a pivotal innovation that significantly enhanced stability and reproducibility of separations.
Further technological progression led to the integration of IEF with other analytical techniques, particularly mass spectrometry (MS), creating powerful hybrid methodologies. The advent of capillary IEF (cIEF) and microchip-based systems in the 1990s and early 2000s marked another significant advancement, enabling higher throughput and requiring minimal sample volumes.
Recent developments have focused on improving detection sensitivity, automation, and miniaturization. Digital microfluidic systems and lab-on-a-chip technologies have emerged as promising platforms for IEF implementation, offering unprecedented control over separation parameters and integration capabilities with downstream analytical processes.
The primary objective of modern IEF technology in protein modification analysis is to achieve high-resolution separation of protein isoforms that differ subtly in their charge properties due to post-translational modifications (PTMs). These modifications, including phosphorylation, glycosylation, and acetylation, often result in minute pI shifts that can be detected through advanced IEF techniques.
Additional technological goals include enhancing throughput capabilities to accommodate large-scale proteomic studies, improving quantitative accuracy for reliable comparative analyses, and developing more user-friendly systems that require less specialized expertise. The integration of computational tools for data analysis and interpretation represents another critical objective, particularly for complex proteomic datasets.
The ultimate aim of IEF technology development is to provide researchers with comprehensive tools for characterizing the "modificome" – the complete landscape of protein modifications within biological systems. This capability is increasingly recognized as essential for understanding cellular function, disease mechanisms, and developing targeted therapeutic approaches in the era of precision medicine.
The evolution of IEF technology can be traced through several key developmental phases. The first generation utilized carrier ampholytes to establish pH gradients in tube gels, offering limited resolution and reproducibility. The 1980s witnessed the introduction of immobilized pH gradient (IPG) strips by Bjellqvist and colleagues, representing a pivotal innovation that significantly enhanced stability and reproducibility of separations.
Further technological progression led to the integration of IEF with other analytical techniques, particularly mass spectrometry (MS), creating powerful hybrid methodologies. The advent of capillary IEF (cIEF) and microchip-based systems in the 1990s and early 2000s marked another significant advancement, enabling higher throughput and requiring minimal sample volumes.
Recent developments have focused on improving detection sensitivity, automation, and miniaturization. Digital microfluidic systems and lab-on-a-chip technologies have emerged as promising platforms for IEF implementation, offering unprecedented control over separation parameters and integration capabilities with downstream analytical processes.
The primary objective of modern IEF technology in protein modification analysis is to achieve high-resolution separation of protein isoforms that differ subtly in their charge properties due to post-translational modifications (PTMs). These modifications, including phosphorylation, glycosylation, and acetylation, often result in minute pI shifts that can be detected through advanced IEF techniques.
Additional technological goals include enhancing throughput capabilities to accommodate large-scale proteomic studies, improving quantitative accuracy for reliable comparative analyses, and developing more user-friendly systems that require less specialized expertise. The integration of computational tools for data analysis and interpretation represents another critical objective, particularly for complex proteomic datasets.
The ultimate aim of IEF technology development is to provide researchers with comprehensive tools for characterizing the "modificome" – the complete landscape of protein modifications within biological systems. This capability is increasingly recognized as essential for understanding cellular function, disease mechanisms, and developing targeted therapeutic approaches in the era of precision medicine.
Market Demand for Protein Modification Analysis
The global market for protein modification analysis has experienced substantial growth in recent years, driven primarily by advancements in proteomics research and increasing applications in pharmaceutical development. The demand for precise analytical techniques like isoelectric focusing (IEF) has risen significantly as researchers seek to understand post-translational modifications (PTMs) that critically affect protein function and disease mechanisms.
Pharmaceutical and biotechnology sectors represent the largest market segments utilizing protein modification analysis, with an estimated annual growth rate exceeding 8% since 2018. This growth correlates directly with the expanding pipeline of biologic drugs and therapeutic proteins, where understanding modifications is essential for ensuring efficacy and safety. Companies developing monoclonal antibodies, recombinant proteins, and gene therapies particularly rely on advanced analytical methods to characterize their products.
Academic research institutions constitute another significant market segment, with proteomics research funding showing consistent increases across North America, Europe, and Asia-Pacific regions. The need to understand complex protein interactions and modifications in disease pathways has catalyzed demand for high-resolution analytical techniques like IEF combined with mass spectrometry.
Clinical diagnostics represents an emerging market with substantial growth potential. The identification of modified proteins as biomarkers for diseases such as cancer, neurodegenerative disorders, and autoimmune conditions is driving adoption of advanced protein analysis techniques in clinical laboratories. Hospitals and reference laboratories are increasingly incorporating these technologies into their diagnostic workflows.
Geographically, North America dominates the market for protein modification analysis, followed by Europe and Asia-Pacific. However, the fastest growth is observed in China and India, where significant investments in life sciences research and biopharmaceutical manufacturing are creating new demand centers.
End-user requirements are evolving toward more integrated analytical platforms that combine multiple separation techniques with high-resolution detection methods. There is particular demand for systems that can provide comprehensive characterization of protein modifications with minimal sample preparation and increased throughput. Automation and reproducibility have become key purchasing factors as laboratories seek to standardize their analytical workflows.
The consumables segment, including specialized gels, ampholytes, and reagents for IEF, represents a substantial recurring revenue stream within this market. Manufacturers offering complete workflow solutions that integrate sample preparation, separation, and analysis are gaining competitive advantage in this increasingly sophisticated market landscape.
Pharmaceutical and biotechnology sectors represent the largest market segments utilizing protein modification analysis, with an estimated annual growth rate exceeding 8% since 2018. This growth correlates directly with the expanding pipeline of biologic drugs and therapeutic proteins, where understanding modifications is essential for ensuring efficacy and safety. Companies developing monoclonal antibodies, recombinant proteins, and gene therapies particularly rely on advanced analytical methods to characterize their products.
Academic research institutions constitute another significant market segment, with proteomics research funding showing consistent increases across North America, Europe, and Asia-Pacific regions. The need to understand complex protein interactions and modifications in disease pathways has catalyzed demand for high-resolution analytical techniques like IEF combined with mass spectrometry.
Clinical diagnostics represents an emerging market with substantial growth potential. The identification of modified proteins as biomarkers for diseases such as cancer, neurodegenerative disorders, and autoimmune conditions is driving adoption of advanced protein analysis techniques in clinical laboratories. Hospitals and reference laboratories are increasingly incorporating these technologies into their diagnostic workflows.
Geographically, North America dominates the market for protein modification analysis, followed by Europe and Asia-Pacific. However, the fastest growth is observed in China and India, where significant investments in life sciences research and biopharmaceutical manufacturing are creating new demand centers.
End-user requirements are evolving toward more integrated analytical platforms that combine multiple separation techniques with high-resolution detection methods. There is particular demand for systems that can provide comprehensive characterization of protein modifications with minimal sample preparation and increased throughput. Automation and reproducibility have become key purchasing factors as laboratories seek to standardize their analytical workflows.
The consumables segment, including specialized gels, ampholytes, and reagents for IEF, represents a substantial recurring revenue stream within this market. Manufacturers offering complete workflow solutions that integrate sample preparation, separation, and analysis are gaining competitive advantage in this increasingly sophisticated market landscape.
Current IEF Challenges in Proteomics
Despite significant advancements in isoelectric focusing (IEF) technology, several critical challenges persist in contemporary proteomics applications, particularly when analyzing protein modifications. The primary limitation involves resolution capabilities, as current IEF systems struggle to separate proteins with minimal pI differences, especially when post-translational modifications (PTMs) cause only subtle changes in the protein's isoelectric point. This resolution constraint significantly impacts the detection and characterization of important modification events that may have biological significance.
Sample preparation represents another substantial hurdle in IEF-based proteomics. The presence of contaminants, salts, and detergents can dramatically interfere with the establishment of stable pH gradients, leading to inconsistent focusing and poor reproducibility. Additionally, highly hydrophobic proteins and membrane-associated proteins often precipitate at their isoelectric points, creating significant challenges for comprehensive proteome analysis.
Reproducibility issues plague many IEF applications, with run-to-run variations hampering reliable quantitative analysis. These inconsistencies stem from multiple factors including temperature fluctuations, carrier ampholyte batch variations, and electrode degradation over time. Such variability particularly affects modification analysis, where precise quantification is essential for understanding biological significance.
The dynamic range limitation presents another significant challenge, as high-abundance proteins frequently mask the detection of low-abundance modified proteins. This masking effect is especially problematic when studying PTMs, which often occur at substoichiometric levels and can be easily overlooked in complex biological samples.
Integration with downstream analytical techniques remains suboptimal, with protein recovery from IEF gels or strips being incomplete and potentially selective. This recovery bias can lead to systematic underrepresentation of certain protein classes or modifications in subsequent mass spectrometry analysis, creating blind spots in the proteome coverage.
Automation capabilities for IEF remain limited compared to other proteomics techniques, resulting in labor-intensive workflows that are difficult to standardize across laboratories. The manual nature of many IEF protocols introduces operator-dependent variables that further compromise reproducibility and throughput.
Finally, data interpretation presents significant challenges, particularly for modified proteins that may focus at multiple positions due to charge heterogeneity. Current software tools often struggle to deconvolute complex patterns resulting from multiple modification states, hampering the comprehensive characterization of protein modification landscapes in biological systems.
Sample preparation represents another substantial hurdle in IEF-based proteomics. The presence of contaminants, salts, and detergents can dramatically interfere with the establishment of stable pH gradients, leading to inconsistent focusing and poor reproducibility. Additionally, highly hydrophobic proteins and membrane-associated proteins often precipitate at their isoelectric points, creating significant challenges for comprehensive proteome analysis.
Reproducibility issues plague many IEF applications, with run-to-run variations hampering reliable quantitative analysis. These inconsistencies stem from multiple factors including temperature fluctuations, carrier ampholyte batch variations, and electrode degradation over time. Such variability particularly affects modification analysis, where precise quantification is essential for understanding biological significance.
The dynamic range limitation presents another significant challenge, as high-abundance proteins frequently mask the detection of low-abundance modified proteins. This masking effect is especially problematic when studying PTMs, which often occur at substoichiometric levels and can be easily overlooked in complex biological samples.
Integration with downstream analytical techniques remains suboptimal, with protein recovery from IEF gels or strips being incomplete and potentially selective. This recovery bias can lead to systematic underrepresentation of certain protein classes or modifications in subsequent mass spectrometry analysis, creating blind spots in the proteome coverage.
Automation capabilities for IEF remain limited compared to other proteomics techniques, resulting in labor-intensive workflows that are difficult to standardize across laboratories. The manual nature of many IEF protocols introduces operator-dependent variables that further compromise reproducibility and throughput.
Finally, data interpretation presents significant challenges, particularly for modified proteins that may focus at multiple positions due to charge heterogeneity. Current software tools often struggle to deconvolute complex patterns resulting from multiple modification states, hampering the comprehensive characterization of protein modification landscapes in biological systems.
Contemporary IEF Solutions for Protein Analysis
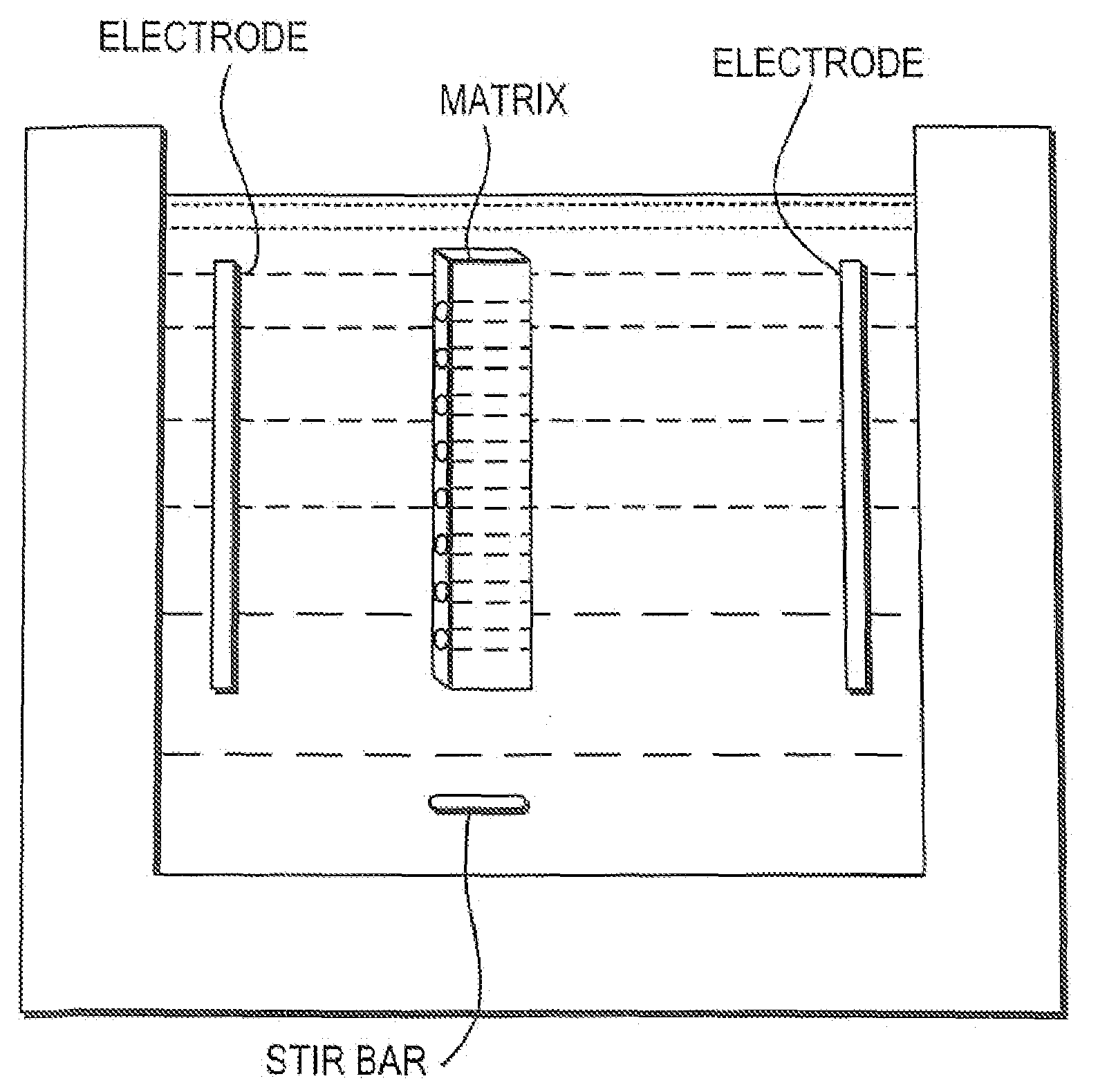
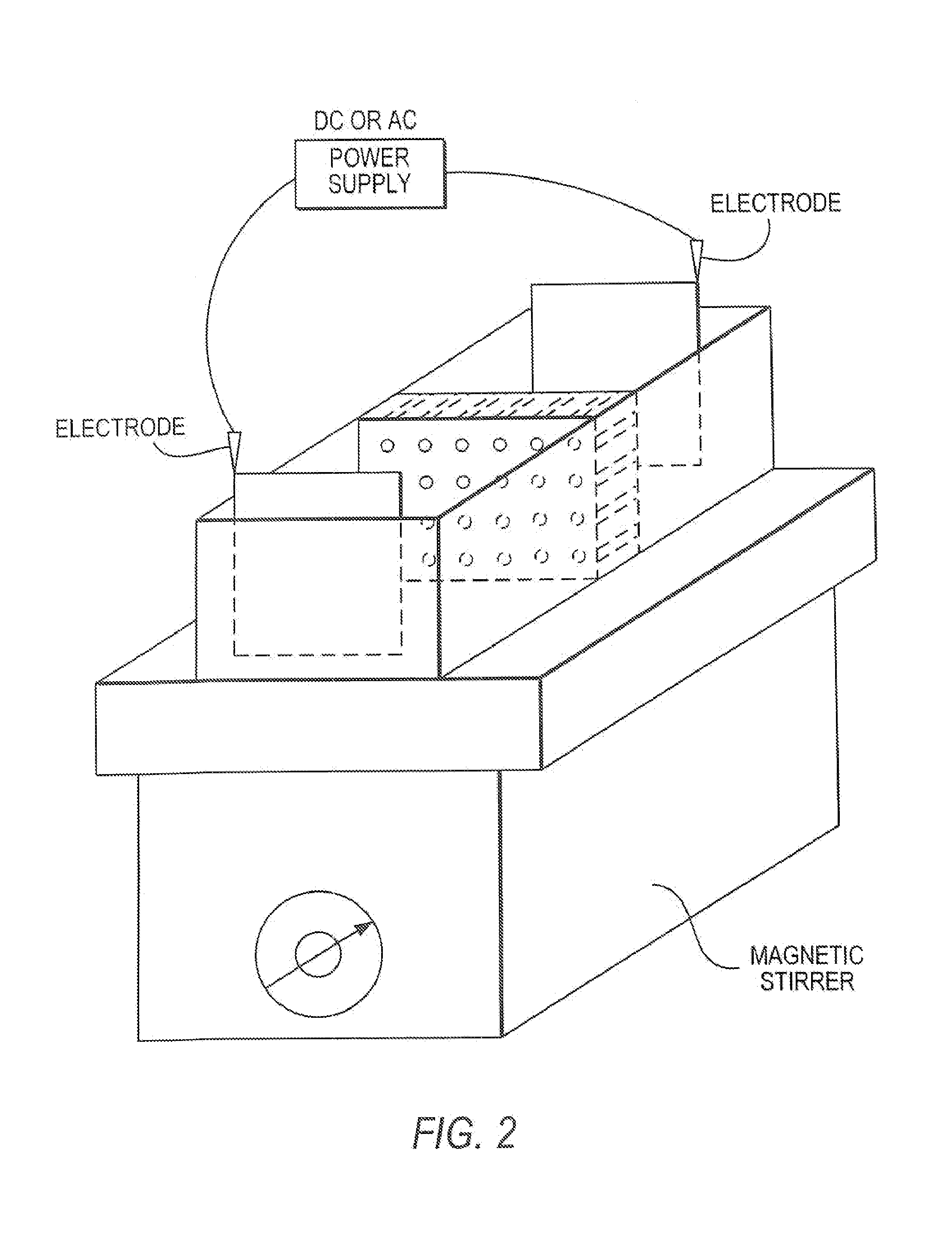
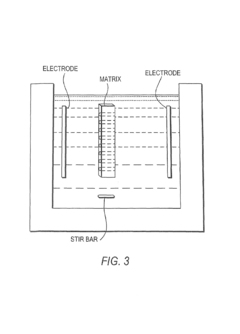
01 Isoelectric focusing apparatus and methods
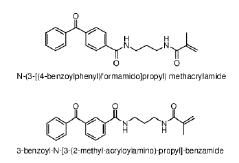
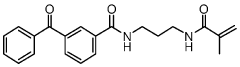
Specialized equipment and methodologies for performing isoelectric focusing (IEF) to separate proteins based on their isoelectric points. These innovations include improved gel systems, electrophoresis chambers, and protocols that enhance resolution and reproducibility for protein modification analysis. The apparatus designs focus on minimizing distortion, improving heat dissipation, and allowing for more precise control of the pH gradient during the focusing process.- Isoelectric focusing techniques for protein analysis: Isoelectric focusing (IEF) is a technique used to separate proteins based on their isoelectric points. This technique allows for high-resolution analysis of protein modifications by separating proteins in a pH gradient until they reach a position where their net charge is zero. The method can be used to detect subtle changes in protein structure resulting from post-translational modifications, which alter the protein's isoelectric point. This approach provides valuable information about protein characteristics and modifications that affect their charge properties.
- Capillary electrophoresis systems for protein modification analysis: Capillary electrophoresis systems offer enhanced resolution for analyzing protein modifications. These systems utilize narrow capillaries to perform isoelectric focusing, allowing for improved separation of proteins with minimal sample consumption. The technique can detect small changes in protein charge resulting from modifications such as phosphorylation, glycosylation, or acetylation. Capillary-based systems also enable automation and high-throughput analysis of protein modifications, making them valuable tools in proteomics research.
- Two-dimensional separation methods for protein modification analysis: Two-dimensional separation methods combine isoelectric focusing with another separation technique, typically gel electrophoresis, to provide comprehensive analysis of protein modifications. In this approach, proteins are first separated based on their isoelectric points and then further separated based on their molecular weight or other properties. This two-dimensional approach significantly increases resolution and allows for the detection of subtle protein modifications that might not be apparent using a single separation method. The technique is particularly useful for complex protein mixtures and for identifying post-translational modifications.
- Microfluidic devices for isoelectric focusing: Microfluidic devices have been developed for performing isoelectric focusing with enhanced efficiency and reduced sample requirements. These devices integrate multiple analytical functions on a single chip, allowing for rapid and automated protein modification analysis. The miniaturized format enables precise control of the separation environment, resulting in improved resolution of protein variants. Microfluidic IEF systems are particularly valuable for analyzing limited samples and can be coupled with detection methods such as fluorescence or mass spectrometry for comprehensive protein characterization.
- Novel detection methods for protein modifications in IEF: Advanced detection methods have been developed to enhance the sensitivity and specificity of identifying protein modifications during isoelectric focusing. These include fluorescent labeling techniques, mass spectrometry integration, and immunodetection approaches that can specifically target modified proteins. Some methods utilize specific dyes or antibodies that recognize particular protein modifications, such as phosphorylation or glycosylation. These detection strategies improve the ability to quantify modified proteins and characterize the nature of the modifications, providing deeper insights into protein function and regulation.
02 Two-dimensional electrophoresis techniques
Combination of isoelectric focusing with other separation methods, particularly SDS-PAGE, to create two-dimensional maps of proteins for comprehensive analysis of post-translational modifications. These techniques allow for the separation of complex protein mixtures first by isoelectric point and then by molecular weight, providing high-resolution visualization of protein modifications such as phosphorylation, glycosylation, and other chemical alterations.Expand Specific Solutions03 Capillary electrophoresis for protein modification analysis
Application of capillary-based isoelectric focusing systems that offer advantages of automation, high throughput, and minimal sample consumption for protein modification analysis. These systems utilize narrow capillaries to perform IEF with enhanced resolution and sensitivity, allowing for detection of subtle changes in protein charge resulting from modifications. The capillary format also enables coupling with mass spectrometry for further characterization.Expand Specific Solutions04 Detection and visualization methods for modified proteins
Specialized staining, labeling, and detection techniques designed to visualize and quantify protein modifications after isoelectric focusing separation. These methods include fluorescent dyes, antibody-based detection systems, and digital imaging technologies that can specifically highlight modified proteins against the background of unmodified forms. Advanced image analysis software helps in quantifying the degree of modification based on spot intensity and position shifts.Expand Specific Solutions05 Microfluidic and chip-based IEF systems
Miniaturized platforms for isoelectric focusing that integrate sample preparation, separation, and detection in compact devices for rapid protein modification analysis. These systems utilize microchannels and chambers fabricated on chips to perform IEF with minimal reagent consumption and faster analysis times. The integration of multiple analytical steps on a single platform allows for streamlined workflows and reduced sample handling, which is particularly beneficial for analyzing labile protein modifications.Expand Specific Solutions
Leading Companies in IEF Instrumentation
Isoelectric Focusing (IEF) technology for protein modification analysis is currently in a growth phase, with the market expected to expand significantly due to increasing demand for precise protein characterization in biopharmaceuticals and proteomics research. The global market size is estimated to reach several billion dollars by 2025, driven by applications in drug development and personalized medicine. Technologically, IEF has reached moderate maturity with continuous innovations from key players. Bio-Rad Laboratories and ProteinSimple lead commercial applications with advanced automated systems, while Agilent Technologies and Regeneron Pharmaceuticals focus on integration with mass spectrometry for enhanced analysis. Academic institutions like MIT and Harvard contribute fundamental research advancements, while pharmaceutical companies such as Novartis and Roche leverage IEF for therapeutic protein development, creating a competitive ecosystem balancing innovation and commercialization.
ProteinSimple
Technical Solution: ProteinSimple has developed advanced capillary isoelectric focusing (cIEF) platforms that automate and enhance protein modification analysis. Their Maurice and Jess systems integrate cIEF with immunodetection to provide high-resolution separation of protein charge variants resulting from post-translational modifications. The technology employs proprietary whole-column imaging detection that captures the entire separation profile simultaneously, rather than waiting for proteins to migrate past a detector. This approach enables quantitative analysis of protein modifications with exceptional reproducibility (CV < 2% for pI determination) and sensitivity (detecting variants comprising <1% of total protein)[1][3]. Their systems can resolve proteins differing by as little as 0.01 pI units, allowing detection of subtle modifications like deamidation, phosphorylation, and glycosylation patterns that affect therapeutic protein efficacy and stability.
Strengths: Exceptional resolution of charge variants with minimal sample consumption (as little as 5μL); fully automated workflow reducing hands-on time by up to 70%; integrated software for quantitative analysis. Weaknesses: Higher initial investment compared to traditional gel-based IEF; limited multiplexing capability; requires specialized consumables that increase per-sample costs.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has pioneered multi-dimensional protein analysis by integrating isoelectric focusing with liquid chromatography and mass spectrometry. Their 2D-PAGE systems remain the gold standard for comprehensive protein modification mapping, while their newer PROTEAN i12 IEF system offers programmable focusing parameters for optimized separation of modified proteins. Bio-Rad's technology employs immobilized pH gradient (IPG) strips with extended pH ranges (3-10, 3-6, 5-8, 7-10) that maintain stable gradients during focusing, preventing protein modification artifacts during analysis[2]. Their patented ReadyStrip IPG technology incorporates specialized ampholytes that enhance resolution of post-translationally modified proteins by creating more uniform pH gradients. The company has also developed specialized staining methods (Pro-Q Diamond for phosphoproteins, Emerald for glycoproteins) that selectively highlight specific modifications following IEF separation, enabling multiplexed visualization of different protein variants[4].
Strengths: Comprehensive ecosystem of compatible products for multi-dimensional analysis; high-capacity IPG strips capable of loading up to 5mg protein for detection of low-abundance modified forms; excellent technical support and established protocols. Weaknesses: Traditional 2D approaches are labor-intensive and time-consuming; some degree of technical expertise required for optimal results; limited automation compared to newer systems.
Key Patents in IEF-Based Protein Modification Detection
Isoelectric focusing arrays and methods of use thereof
PatentWO2017044614A1
Innovation
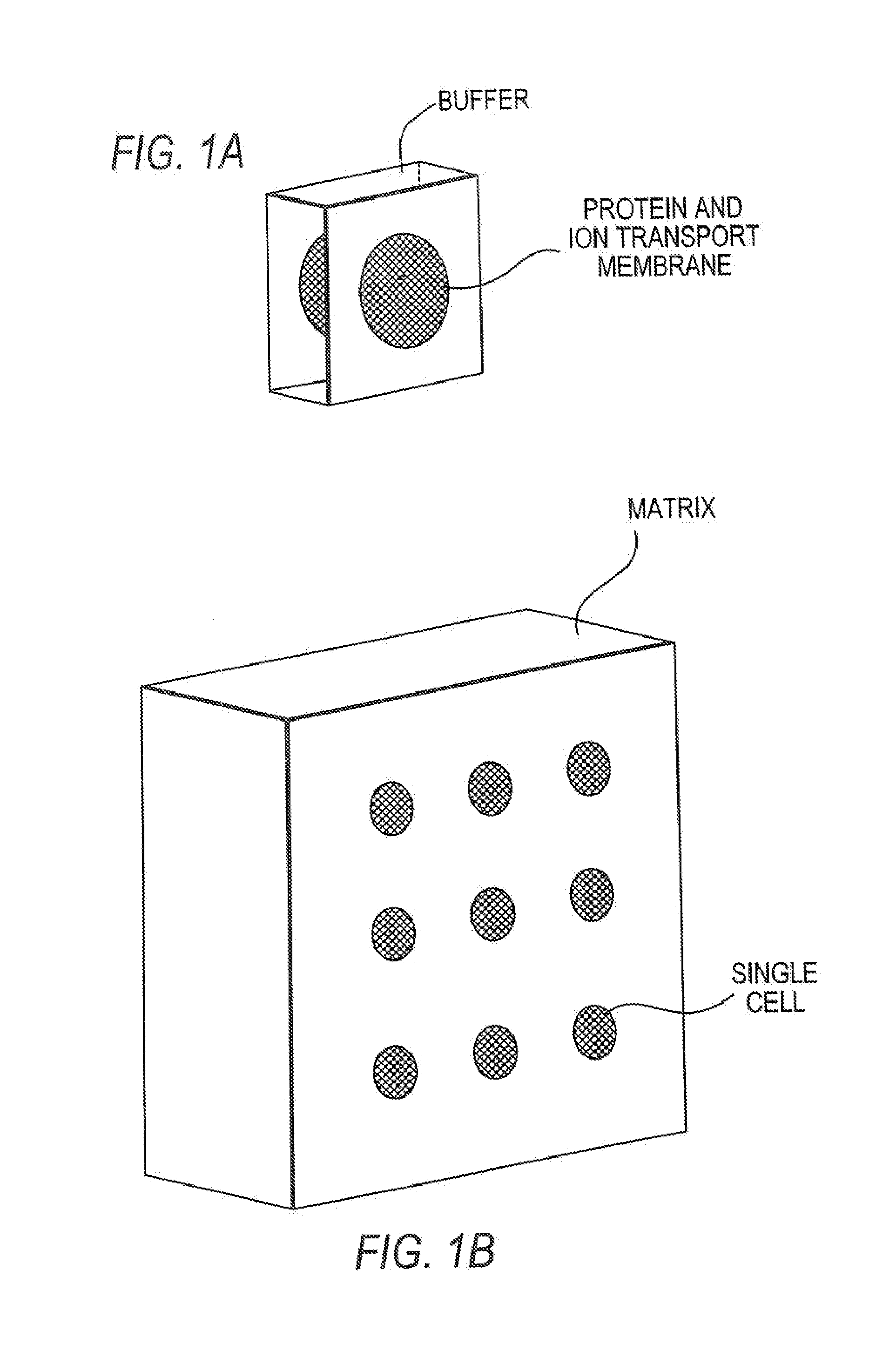
- The development of isoelectric focusing devices with a polymeric separation medium featuring immobilized pH gradients and microwells for parallel separations, where an electric field is applied to separate and immobilize proteins within the medium, enabling multiplex analysis of protein components from multiple samples simultaneously.
Matrixes, arrays, systems and methods
PatentInactiveUS7166202B2
Innovation
- The development of matrixes and systems with discrete, isolated IEF buffers having ultra-narrow pH ranges and reversible electric fields, allowing for high-throughput analysis with minimal manipulation and reduced cooling needs, using circulating buffers and impermeable membranes to enhance protein separation and detection.
Integration with Mass Spectrometry Platforms
The integration of isoelectric focusing (IEF) with mass spectrometry (MS) platforms represents a powerful combination for comprehensive protein modification analysis. This synergistic approach leverages the high-resolution separation capabilities of IEF with the exceptional sensitivity and specificity of mass spectrometry, creating analytical workflows that significantly enhance the detection and characterization of post-translational modifications (PTMs).
Traditional coupling methods involve offline collection of IEF fractions followed by subsequent MS analysis. However, recent technological advancements have enabled more sophisticated integration approaches. Online IEF-MS systems have emerged, where microfluidic IEF devices are directly interfaced with electrospray ionization mass spectrometers, allowing for real-time analysis of separated protein species. This configuration minimizes sample loss and reduces analysis time while maintaining high resolution.
The integration with MALDI-TOF MS platforms has proven particularly valuable for high-throughput proteomics applications. In this setup, IEF-separated proteins are immobilized on specialized plates compatible with matrix-assisted laser desorption/ionization, enabling rapid mass spectral acquisition across the pH gradient. This approach has demonstrated exceptional utility for biomarker discovery and clinical diagnostics where modified protein variants must be distinguished.
LC-MS/MS systems benefit substantially from pre-fractionation by IEF, especially when analyzing complex biological samples. The orthogonal separation mechanisms dramatically increase peak capacity, allowing for detection of low-abundance modified proteins that would otherwise be masked by highly abundant species. Quantitative proteomics workflows incorporating IEF separation prior to MS analysis have shown up to 40% improvement in modified peptide identification compared to conventional approaches.
Software integration represents another critical aspect of IEF-MS platforms. Specialized algorithms have been developed to process the multidimensional data generated by these hybrid systems. These computational tools can correlate isoelectric point information with mass spectral data to improve confidence in PTM identification and localization. Machine learning approaches are increasingly being applied to extract patterns from IEF-MS datasets, enabling more accurate prediction of modification sites.
Miniaturized IEF-MS systems are emerging as next-generation platforms for point-of-care applications. These compact devices integrate microchip-based IEF with miniaturized mass analyzers, offering portable solutions for rapid protein modification analysis. While still in developmental stages, these systems show promise for clinical applications where rapid assessment of protein modifications is crucial for diagnostic or therapeutic decisions.
Traditional coupling methods involve offline collection of IEF fractions followed by subsequent MS analysis. However, recent technological advancements have enabled more sophisticated integration approaches. Online IEF-MS systems have emerged, where microfluidic IEF devices are directly interfaced with electrospray ionization mass spectrometers, allowing for real-time analysis of separated protein species. This configuration minimizes sample loss and reduces analysis time while maintaining high resolution.
The integration with MALDI-TOF MS platforms has proven particularly valuable for high-throughput proteomics applications. In this setup, IEF-separated proteins are immobilized on specialized plates compatible with matrix-assisted laser desorption/ionization, enabling rapid mass spectral acquisition across the pH gradient. This approach has demonstrated exceptional utility for biomarker discovery and clinical diagnostics where modified protein variants must be distinguished.
LC-MS/MS systems benefit substantially from pre-fractionation by IEF, especially when analyzing complex biological samples. The orthogonal separation mechanisms dramatically increase peak capacity, allowing for detection of low-abundance modified proteins that would otherwise be masked by highly abundant species. Quantitative proteomics workflows incorporating IEF separation prior to MS analysis have shown up to 40% improvement in modified peptide identification compared to conventional approaches.
Software integration represents another critical aspect of IEF-MS platforms. Specialized algorithms have been developed to process the multidimensional data generated by these hybrid systems. These computational tools can correlate isoelectric point information with mass spectral data to improve confidence in PTM identification and localization. Machine learning approaches are increasingly being applied to extract patterns from IEF-MS datasets, enabling more accurate prediction of modification sites.
Miniaturized IEF-MS systems are emerging as next-generation platforms for point-of-care applications. These compact devices integrate microchip-based IEF with miniaturized mass analyzers, offering portable solutions for rapid protein modification analysis. While still in developmental stages, these systems show promise for clinical applications where rapid assessment of protein modifications is crucial for diagnostic or therapeutic decisions.
Reproducibility and Standardization Protocols
Establishing robust reproducibility and standardization protocols is critical for the widespread adoption of isoelectric focusing (IEF) in protein modification analysis. Current IEF methodologies often suffer from inter-laboratory variability, which undermines confidence in research findings and limits clinical applications. To address this challenge, standardized sample preparation procedures must be implemented, including consistent protein extraction methods, buffer compositions, and storage conditions that preserve post-translational modifications.
pH gradient formation represents a particularly sensitive aspect of IEF that requires standardization. The development of commercially available immobilized pH gradient (IPG) strips has significantly improved reproducibility compared to carrier ampholyte-based approaches. However, batch-to-batch variations still occur, necessitating rigorous quality control measures and calibration standards. Leading manufacturers now provide certificate of analysis documentation that includes pH gradient validation data, enabling researchers to account for potential variations.
Instrument calibration protocols must be established for both gel-based and capillary IEF systems. Regular performance verification using standard protein mixtures with known isoelectric points allows for system suitability testing and early detection of equipment drift. These reference standards should include proteins with various post-translational modifications to validate the system's ability to resolve modified proteoforms.
Data acquisition parameters require standardization to ensure comparable results across different laboratories. This includes establishing consistent voltage ramping protocols, temperature control specifications, and detection sensitivity settings. The scientific community has begun developing standard operating procedures (SOPs) through collaborative initiatives like the Human Proteome Organization (HUPO) Proteomics Standards Initiative, which aims to establish minimum reporting requirements for IEF experiments.
Statistical validation frameworks must be incorporated into IEF workflows for protein modification analysis. This includes determining appropriate technical and biological replication levels, implementing suitable normalization methods, and establishing acceptance criteria for data quality. Power analysis calculations should guide experimental design to ensure sufficient statistical strength for detecting biologically relevant differences in protein modification states.
Interlaboratory ring trials represent an essential component of standardization efforts. These collaborative studies, where multiple laboratories analyze identical samples using agreed-upon protocols, help identify sources of variability and establish performance benchmarks. Recent ring trials focusing on IEF for protein modification analysis have highlighted the importance of standardized sample handling and data processing algorithms in achieving reproducible results across different research environments.
pH gradient formation represents a particularly sensitive aspect of IEF that requires standardization. The development of commercially available immobilized pH gradient (IPG) strips has significantly improved reproducibility compared to carrier ampholyte-based approaches. However, batch-to-batch variations still occur, necessitating rigorous quality control measures and calibration standards. Leading manufacturers now provide certificate of analysis documentation that includes pH gradient validation data, enabling researchers to account for potential variations.
Instrument calibration protocols must be established for both gel-based and capillary IEF systems. Regular performance verification using standard protein mixtures with known isoelectric points allows for system suitability testing and early detection of equipment drift. These reference standards should include proteins with various post-translational modifications to validate the system's ability to resolve modified proteoforms.
Data acquisition parameters require standardization to ensure comparable results across different laboratories. This includes establishing consistent voltage ramping protocols, temperature control specifications, and detection sensitivity settings. The scientific community has begun developing standard operating procedures (SOPs) through collaborative initiatives like the Human Proteome Organization (HUPO) Proteomics Standards Initiative, which aims to establish minimum reporting requirements for IEF experiments.
Statistical validation frameworks must be incorporated into IEF workflows for protein modification analysis. This includes determining appropriate technical and biological replication levels, implementing suitable normalization methods, and establishing acceptance criteria for data quality. Power analysis calculations should guide experimental design to ensure sufficient statistical strength for detecting biologically relevant differences in protein modification states.
Interlaboratory ring trials represent an essential component of standardization efforts. These collaborative studies, where multiple laboratories analyze identical samples using agreed-upon protocols, help identify sources of variability and establish performance benchmarks. Recent ring trials focusing on IEF for protein modification analysis have highlighted the importance of standardized sample handling and data processing algorithms in achieving reproducible results across different research environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!