Comparing Isoelectric Focusing vs Chromatography for Protein Purity
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Protein Analysis Technology Evolution and Objectives
Protein analysis has evolved significantly over the past century, with major technological breakthroughs transforming our ability to isolate, purify, and characterize proteins. The journey began in the early 20th century with rudimentary precipitation techniques, progressing through increasingly sophisticated separation methods. The 1930s saw the introduction of electrophoresis, while the 1950s marked the development of chromatography techniques that revolutionized protein separation capabilities.
Isoelectric focusing (IEF) emerged in the 1960s as a powerful technique utilizing proteins' unique isoelectric points for separation. This innovation significantly enhanced resolution compared to earlier methods. Concurrently, various chromatography approaches evolved, including size exclusion, ion exchange, affinity, and hydrophobic interaction chromatography, each exploiting different protein properties to achieve separation.
The 1970s and 1980s witnessed the integration of these techniques into more comprehensive analytical platforms, with two-dimensional gel electrophoresis combining IEF with SDS-PAGE to provide unprecedented protein resolution. The late 20th century brought automation and miniaturization, dramatically increasing throughput and reducing sample requirements.
Recent decades have seen remarkable advancements in high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC), offering superior resolution, sensitivity, and speed. Concurrently, capillary electrophoresis has evolved as a high-efficiency alternative to traditional gel-based methods, while mass spectrometry has emerged as an indispensable complementary technique for protein identification and characterization.
The technological evolution continues with microfluidic "lab-on-a-chip" systems and novel hybrid approaches combining multiple separation principles. These innovations address the growing demand for higher resolution, increased sensitivity, and greater throughput in protein analysis across various industries.
The primary objectives in comparing isoelectric focusing and chromatography for protein purity assessment include establishing standardized protocols for consistent purity determination, developing more sensitive methods for detecting trace impurities, and creating integrated analytical workflows that combine complementary techniques for comprehensive purity profiles.
Additional goals include miniaturization for reduced sample consumption, automation for higher throughput, and development of predictive models to optimize separation conditions. The ultimate aim is to establish robust, reproducible methodologies that can reliably determine protein purity with high precision across different laboratory settings, supporting critical applications in biopharmaceutical development, quality control, and fundamental protein research.
Isoelectric focusing (IEF) emerged in the 1960s as a powerful technique utilizing proteins' unique isoelectric points for separation. This innovation significantly enhanced resolution compared to earlier methods. Concurrently, various chromatography approaches evolved, including size exclusion, ion exchange, affinity, and hydrophobic interaction chromatography, each exploiting different protein properties to achieve separation.
The 1970s and 1980s witnessed the integration of these techniques into more comprehensive analytical platforms, with two-dimensional gel electrophoresis combining IEF with SDS-PAGE to provide unprecedented protein resolution. The late 20th century brought automation and miniaturization, dramatically increasing throughput and reducing sample requirements.
Recent decades have seen remarkable advancements in high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC), offering superior resolution, sensitivity, and speed. Concurrently, capillary electrophoresis has evolved as a high-efficiency alternative to traditional gel-based methods, while mass spectrometry has emerged as an indispensable complementary technique for protein identification and characterization.
The technological evolution continues with microfluidic "lab-on-a-chip" systems and novel hybrid approaches combining multiple separation principles. These innovations address the growing demand for higher resolution, increased sensitivity, and greater throughput in protein analysis across various industries.
The primary objectives in comparing isoelectric focusing and chromatography for protein purity assessment include establishing standardized protocols for consistent purity determination, developing more sensitive methods for detecting trace impurities, and creating integrated analytical workflows that combine complementary techniques for comprehensive purity profiles.
Additional goals include miniaturization for reduced sample consumption, automation for higher throughput, and development of predictive models to optimize separation conditions. The ultimate aim is to establish robust, reproducible methodologies that can reliably determine protein purity with high precision across different laboratory settings, supporting critical applications in biopharmaceutical development, quality control, and fundamental protein research.
Market Demand for Protein Purity Assessment Methods
The protein purity assessment market has witnessed substantial growth in recent years, driven primarily by advancements in biopharmaceutical development and increasing regulatory requirements for protein-based therapeutics. The global market for protein analysis, including purity assessment methods, was valued at approximately $3.2 billion in 2022 and is projected to reach $5.8 billion by 2027, representing a compound annual growth rate of 12.6%.
Biopharmaceuticals constitute the largest segment driving demand for protein purity assessment methods. With over 350 protein-based therapeutics approved globally and thousands more in clinical trials, manufacturers require robust analytical techniques to ensure product quality and safety. Monoclonal antibodies alone represent a $150 billion market, with each product requiring extensive purity verification throughout development and manufacturing.
Regulatory agencies worldwide have strengthened requirements for protein characterization and purity assessment. The FDA, EMA, and other regulatory bodies now mandate comprehensive protein purity profiles using orthogonal analytical methods, creating sustained demand for both isoelectric focusing and chromatography technologies. This regulatory landscape has expanded the market for analytical services and equipment.
Academic and research institutions represent another significant market segment, accounting for approximately 25% of the total demand. The increasing focus on proteomics research and structural biology has created consistent demand for high-resolution protein separation and analysis techniques.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) have emerged as major consumers of protein purity assessment technologies. The outsourcing trend in biopharmaceutical development has led to a 15% annual growth in this segment's demand for analytical services, including both isoelectric focusing and chromatography methods.
Regional analysis reveals North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth at 18% annually, driven by expanding biopharmaceutical manufacturing capabilities in China, India, and Singapore.
End-user preferences indicate a growing demand for integrated analytical platforms that combine multiple separation techniques. Market surveys show that 78% of biopharmaceutical companies seek solutions that incorporate both chromatographic and electrophoretic methods for comprehensive protein characterization, suggesting a trend toward complementary rather than competitive positioning of these technologies.
Biopharmaceuticals constitute the largest segment driving demand for protein purity assessment methods. With over 350 protein-based therapeutics approved globally and thousands more in clinical trials, manufacturers require robust analytical techniques to ensure product quality and safety. Monoclonal antibodies alone represent a $150 billion market, with each product requiring extensive purity verification throughout development and manufacturing.
Regulatory agencies worldwide have strengthened requirements for protein characterization and purity assessment. The FDA, EMA, and other regulatory bodies now mandate comprehensive protein purity profiles using orthogonal analytical methods, creating sustained demand for both isoelectric focusing and chromatography technologies. This regulatory landscape has expanded the market for analytical services and equipment.
Academic and research institutions represent another significant market segment, accounting for approximately 25% of the total demand. The increasing focus on proteomics research and structural biology has created consistent demand for high-resolution protein separation and analysis techniques.
Contract research organizations (CROs) and contract manufacturing organizations (CMOs) have emerged as major consumers of protein purity assessment technologies. The outsourcing trend in biopharmaceutical development has led to a 15% annual growth in this segment's demand for analytical services, including both isoelectric focusing and chromatography methods.
Regional analysis reveals North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth at 18% annually, driven by expanding biopharmaceutical manufacturing capabilities in China, India, and Singapore.
End-user preferences indicate a growing demand for integrated analytical platforms that combine multiple separation techniques. Market surveys show that 78% of biopharmaceutical companies seek solutions that incorporate both chromatographic and electrophoretic methods for comprehensive protein characterization, suggesting a trend toward complementary rather than competitive positioning of these technologies.
Current Challenges in Protein Separation Techniques
Protein separation techniques are fundamental in biopharmaceutical development, analytical biochemistry, and proteomics research. Despite significant advancements, both isoelectric focusing (IEF) and chromatography methods face persistent challenges that limit their effectiveness in achieving optimal protein purity. These challenges span technical, operational, and economic dimensions that researchers and industry professionals must navigate.
Resolution limitations remain a primary concern for both techniques. In IEF, achieving stable pH gradients for extended periods is problematic, particularly when separating proteins with closely similar isoelectric points. The phenomenon of cathodic drift often disrupts gradient stability, leading to inconsistent results. Similarly, chromatography techniques struggle with resolving complex protein mixtures containing components with similar physicochemical properties, especially when dealing with post-translational modifications or structural isomers.
Sample preparation complexity presents another significant hurdle. Proteins often require extensive pre-treatment before separation, including denaturation, reduction, and solubilization steps that can alter native conformations. This is particularly challenging for membrane proteins and hydrophobic species that tend to aggregate during preparation. The requirement for specialized buffers and additives further complicates the process and may introduce artifacts.
Scalability issues affect both technologies differently. IEF faces substantial challenges in scaling up from analytical to preparative applications due to heat dissipation problems and difficulties in maintaining uniform electric fields across larger dimensions. Chromatography, while more scalable, encounters issues with column packing consistency, flow distribution, and pressure limitations when scaled to industrial production levels.
Automation and reproducibility challenges persist despite technological advances. IEF suffers from gel-to-gel variability and difficulties in standardizing electrophoretic conditions across different runs. Chromatography systems, though more amenable to automation, still face challenges with column aging, matrix fouling, and the need for frequent calibration to maintain separation performance.
Detection sensitivity limitations affect both techniques when dealing with low-abundance proteins or complex biological samples. The dynamic range of detection often fails to capture the full spectrum of protein concentrations present in biological samples, which can span several orders of magnitude. This is particularly problematic in biomarker discovery and proteomics applications.
Cost considerations remain significant, especially for high-resolution separations. High-quality chromatography media, specialized IEF equipment, and the consumables required for both techniques represent substantial investments. Additionally, the expertise required to optimize separation protocols and interpret results adds to the overall cost burden, particularly for smaller research facilities or startups.
Environmental concerns are increasingly relevant, with both techniques generating significant waste through buffer systems, carrier ampholytes, and chromatography solvents. The pharmaceutical industry faces growing pressure to develop more sustainable separation processes that reduce solvent usage and waste generation while maintaining separation efficiency.
Resolution limitations remain a primary concern for both techniques. In IEF, achieving stable pH gradients for extended periods is problematic, particularly when separating proteins with closely similar isoelectric points. The phenomenon of cathodic drift often disrupts gradient stability, leading to inconsistent results. Similarly, chromatography techniques struggle with resolving complex protein mixtures containing components with similar physicochemical properties, especially when dealing with post-translational modifications or structural isomers.
Sample preparation complexity presents another significant hurdle. Proteins often require extensive pre-treatment before separation, including denaturation, reduction, and solubilization steps that can alter native conformations. This is particularly challenging for membrane proteins and hydrophobic species that tend to aggregate during preparation. The requirement for specialized buffers and additives further complicates the process and may introduce artifacts.
Scalability issues affect both technologies differently. IEF faces substantial challenges in scaling up from analytical to preparative applications due to heat dissipation problems and difficulties in maintaining uniform electric fields across larger dimensions. Chromatography, while more scalable, encounters issues with column packing consistency, flow distribution, and pressure limitations when scaled to industrial production levels.
Automation and reproducibility challenges persist despite technological advances. IEF suffers from gel-to-gel variability and difficulties in standardizing electrophoretic conditions across different runs. Chromatography systems, though more amenable to automation, still face challenges with column aging, matrix fouling, and the need for frequent calibration to maintain separation performance.
Detection sensitivity limitations affect both techniques when dealing with low-abundance proteins or complex biological samples. The dynamic range of detection often fails to capture the full spectrum of protein concentrations present in biological samples, which can span several orders of magnitude. This is particularly problematic in biomarker discovery and proteomics applications.
Cost considerations remain significant, especially for high-resolution separations. High-quality chromatography media, specialized IEF equipment, and the consumables required for both techniques represent substantial investments. Additionally, the expertise required to optimize separation protocols and interpret results adds to the overall cost burden, particularly for smaller research facilities or startups.
Environmental concerns are increasingly relevant, with both techniques generating significant waste through buffer systems, carrier ampholytes, and chromatography solvents. The pharmaceutical industry faces growing pressure to develop more sustainable separation processes that reduce solvent usage and waste generation while maintaining separation efficiency.
Comparative Analysis of IEF and Chromatography Methods
01 Isoelectric focusing techniques for protein purification
Isoelectric focusing (IEF) is a technique used to separate proteins based on their isoelectric points. This method allows for high-resolution separation of proteins by creating a pH gradient in which proteins migrate until they reach the pH at which their net charge is zero. This technique is particularly useful for analyzing protein purity and can be combined with other methods to enhance separation efficiency. Advanced IEF techniques have been developed to improve resolution and reproducibility in protein analysis.- Isoelectric focusing techniques for protein purification: Isoelectric focusing (IEF) is an electrophoretic method that separates proteins based on their isoelectric points. This technique allows for high-resolution separation of proteins by creating a pH gradient in which proteins migrate until they reach the pH at which their net charge is zero. IEF can be used as a standalone technique or combined with other methods to achieve higher purity levels in protein samples. The method is particularly effective for separating proteins with small differences in their isoelectric points.
- Chromatography methods for protein purification: Various chromatography techniques are employed for protein purification, including ion exchange, affinity, size exclusion, and hydrophobic interaction chromatography. These methods separate proteins based on different physical or chemical properties such as charge, binding affinity, size, or hydrophobicity. Chromatography can be used in multiple stages to progressively increase protein purity. Advanced chromatographic techniques allow for the separation of proteins with similar properties, contributing to higher purity levels in the final product.
- Combined IEF and chromatography approaches: The combination of isoelectric focusing and chromatography techniques provides powerful tools for achieving high protein purity. Two-dimensional approaches that use IEF in the first dimension and chromatography in the second dimension can separate complex protein mixtures with high resolution. These combined methods take advantage of different separation principles to resolve proteins that would co-migrate in single-technique approaches. The orthogonal nature of these combined techniques significantly enhances the purity assessment and isolation of target proteins.
- Analytical methods for assessing protein purity: Various analytical methods are used to assess protein purity after isoelectric focusing and chromatography. These include gel electrophoresis, mass spectrometry, spectrophotometric methods, and analytical ultracentrifugation. These techniques provide quantitative and qualitative information about the purity of protein samples, allowing researchers to evaluate the effectiveness of their purification strategies. Advanced imaging and detection systems enhance the sensitivity of these analytical methods, enabling the detection of even minor impurities in purified protein samples.
- Innovations in equipment and materials for protein purification: Recent innovations in equipment and materials have significantly improved protein purification processes. These include advanced gel formulations, capillary electrophoresis systems, microfluidic devices, and automated purification platforms. Novel materials such as specialized membranes, monolithic columns, and functionalized nanoparticles enhance separation efficiency and protein recovery. These technological advancements allow for faster, more efficient, and more reproducible protein purification with higher yields and purity levels.
02 Chromatography methods for protein purification
Various chromatography methods are employed for protein purification, including ion exchange, affinity, size exclusion, and hydrophobic interaction chromatography. These techniques separate proteins based on different physical and chemical properties such as charge, size, binding affinity, and hydrophobicity. Chromatography methods can be used individually or in combination to achieve higher levels of protein purity. Recent advancements have focused on improving separation efficiency, reducing processing time, and increasing yield.Expand Specific Solutions03 Combined IEF and chromatography systems
The integration of isoelectric focusing with chromatography techniques creates powerful two-dimensional separation systems for protein purification. These combined approaches leverage the complementary separation mechanisms to achieve higher resolution and purity than either method alone. Two-dimensional electrophoresis, which often combines IEF with gel electrophoresis or liquid chromatography, has become a standard method for complex protein mixture analysis. These integrated systems are particularly valuable for detecting impurities and ensuring high protein purity in biopharmaceutical applications.Expand Specific Solutions04 Analytical methods for assessing protein purity
Various analytical methods are used to assess protein purity following separation by isoelectric focusing and chromatography. These include spectroscopic techniques, mass spectrometry, electrophoretic methods, and immunoassays. Advanced imaging and detection systems have been developed to visualize separated proteins and quantify their purity. These analytical methods provide critical information about protein homogeneity, the presence of variants or impurities, and overall purity profiles, which is essential for quality control in biopharmaceutical production.Expand Specific Solutions05 Innovations in equipment and materials for protein purification
Recent innovations in equipment and materials have significantly advanced protein purification technologies. These include the development of novel gel materials, capillary electrophoresis systems, microfluidic devices, and automated purification platforms. Improvements in pH gradient stability, resolution capacity, and reproducibility have enhanced the performance of isoelectric focusing. Similarly, advances in chromatography media, column design, and detection systems have increased the efficiency and throughput of protein purification processes. These technological innovations enable more precise control over separation conditions and improved protein purity outcomes.Expand Specific Solutions
Leading Companies in Protein Analysis Instrumentation
The protein purity analysis market is in a mature growth phase, with isoelectric focusing and chromatography representing established complementary techniques. The global market for protein analysis technologies exceeds $5 billion, growing at 7-8% annually. Chromatography dominates with broader applications, while isoelectric focusing offers higher resolution for specific separations. Leading biopharmaceutical companies like Bristol Myers Squibb, Regeneron, and Roche have developed proprietary adaptations of both technologies, with Bio-Rad Laboratories and Cytiva Sweden providing industry-standard equipment and consumables. Academic institutions including MIT and Johns Hopkins contribute significant innovations, while contract research organizations like WuXi AppTec and WuXi Biologics are expanding capabilities to meet growing biopharmaceutical development demands.
Genentech, Inc.
Technical Solution: Genentech has pioneered advanced approaches for protein purity analysis by developing hybrid methodologies that combine the strengths of both isoelectric focusing and chromatography. Their capillary isoelectric focusing (cIEF) platform achieves exceptional resolution for monoclonal antibody charge variants with minimal sample consumption. This automated system incorporates proprietary algorithms for peak identification and quantification, allowing for detection of subtle charge differences in therapeutic proteins. For chromatographic analysis, Genentech employs multi-dimensional chromatography approaches, particularly their patented two-dimensional liquid chromatography (2D-LC) system that couples ion exchange with reversed-phase separation to provide orthogonal selectivity. Their technology includes specialized columns with novel stationary phases designed specifically for biotherapeutic characterization, enabling the separation of product-related impurities that differ by as little as a single amino acid modification.
Strengths: Exceptional sensitivity for detecting low-abundance protein variants; validated methods for regulatory submissions; high-throughput capabilities suitable for manufacturing environments. Weaknesses: Proprietary systems may lack compatibility with other vendors' equipment; significant expertise required for method development and data interpretation; higher operational costs compared to standard analytical techniques.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed comprehensive solutions for both isoelectric focusing (IEF) and chromatography techniques for protein purity analysis. Their IEF technology utilizes immobilized pH gradient (IPG) strips that provide high-resolution separation of proteins based on their isoelectric points. Their patented technology allows for reproducible pH gradients and improved protein loading capacity. For chromatography, Bio-Rad offers various platforms including their NGC™ Chromatography Systems that integrate multiple chromatographic techniques (ion exchange, size exclusion, affinity) with automated sample handling and real-time monitoring capabilities. Their CHT™ Ceramic Hydroxyapatite technology provides unique selectivity by combining cation exchange and calcium metal affinity mechanisms in a single chromatographic medium, allowing for separation of proteins with very similar properties that would be difficult to resolve using conventional methods.
Strengths: Industry-leading resolution in both techniques with established validation protocols; comprehensive software integration for data analysis; extensive range of consumables optimized for different protein types. Weaknesses: Higher initial investment costs compared to some competitors; some of their advanced systems require specialized training; consumables can be proprietary and expensive for routine use.
Key Innovations in Protein Purity Determination
Kit for co-purification and concentration of DNA and proteins using isotachophoresis
PatentActiveUS20140332389A1
Innovation
- A method for simultaneous co-purification and concentration of nucleic acid and protein targets using isotachophoresis, allowing for automated sample preparation in a handheld, disposable device that can be operated by unskilled operators, utilizing a buffer system to separate and purify both nucleic acids and proteins into a single volume.
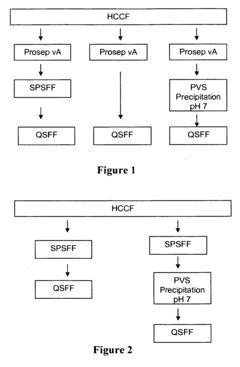
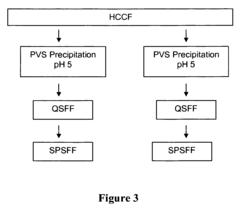
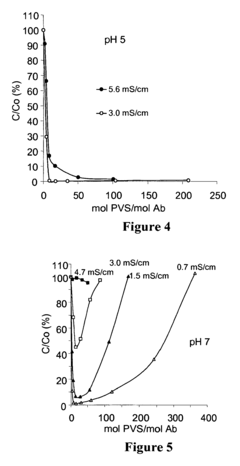
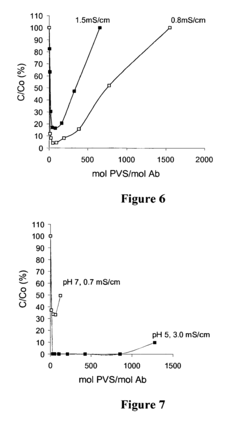
Polyelectrolyte precipitation and purification of antibodies
PatentActiveEP2120915B1
Innovation
- The method involves the use of polyanion polyelectrolytes to precipitate proteins from cell culture fluids, followed by additional chromatography steps, allowing for a non-affinity process that enhances protein purification by forming protein-polyelectrolyte precipitates and separating them from impurities, thereby overcoming resin capacity limitations.
Regulatory Standards for Protein Purity Analysis
Protein purity analysis is subject to stringent regulatory oversight across global markets, with standards varying by region and application domain. The FDA in the United States has established comprehensive guidelines through 21 CFR Part 211 for pharmaceutical proteins, requiring validated analytical methods that demonstrate specificity, accuracy, precision, and robustness. These regulations specifically address both isoelectric focusing (IEF) and chromatography techniques as acceptable methodologies when properly validated.
The European Medicines Agency (EMA) has implemented similar but distinct requirements through ICH Q6B guidelines, which emphasize the need for orthogonal analytical approaches—often recommending the combination of IEF and chromatography methods to ensure comprehensive purity profiles. These guidelines specifically address charge variants detection capabilities of IEF alongside the separation efficiency of various chromatographic techniques.
In Japan, the PMDA follows ICH guidelines but places additional emphasis on method transferability and reproducibility between laboratories, particularly relevant when comparing the more specialized equipment requirements of IEF versus the widely standardized chromatography systems.
ISO standards, particularly ISO 17025 for testing laboratories, establish quality management systems applicable to protein analysis regardless of the chosen methodology. These standards focus on measurement uncertainty, which differs significantly between IEF (typically higher resolution for charge variants) and chromatographic methods (generally better quantitative precision).
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide specific monographs for protein products that often include recommended analytical procedures. Recent updates to these compendia have increasingly recognized high-resolution techniques like capillary IEF and multi-dimensional chromatography as preferred methods for complex protein therapeutics.
Regulatory bodies have established acceptance criteria that vary by protein type and intended use. For therapeutic monoclonal antibodies, purity requirements typically exceed 95% with detailed characterization of impurities required. The FDA's Quality by Design initiative has shifted focus toward understanding critical quality attributes rather than prescribing specific analytical methods, allowing manufacturers flexibility in choosing between IEF and chromatography based on fitness for purpose.
Emerging regulations for biosimilars have created particularly stringent comparative analytical requirements, where manufacturers must demonstrate similarity to reference products using multiple orthogonal methods. This regulatory landscape has driven innovation in analytical technologies that combine the strengths of both IEF and chromatographic approaches, such as chromatofocusing and pH gradient chromatography.
The European Medicines Agency (EMA) has implemented similar but distinct requirements through ICH Q6B guidelines, which emphasize the need for orthogonal analytical approaches—often recommending the combination of IEF and chromatography methods to ensure comprehensive purity profiles. These guidelines specifically address charge variants detection capabilities of IEF alongside the separation efficiency of various chromatographic techniques.
In Japan, the PMDA follows ICH guidelines but places additional emphasis on method transferability and reproducibility between laboratories, particularly relevant when comparing the more specialized equipment requirements of IEF versus the widely standardized chromatography systems.
ISO standards, particularly ISO 17025 for testing laboratories, establish quality management systems applicable to protein analysis regardless of the chosen methodology. These standards focus on measurement uncertainty, which differs significantly between IEF (typically higher resolution for charge variants) and chromatographic methods (generally better quantitative precision).
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide specific monographs for protein products that often include recommended analytical procedures. Recent updates to these compendia have increasingly recognized high-resolution techniques like capillary IEF and multi-dimensional chromatography as preferred methods for complex protein therapeutics.
Regulatory bodies have established acceptance criteria that vary by protein type and intended use. For therapeutic monoclonal antibodies, purity requirements typically exceed 95% with detailed characterization of impurities required. The FDA's Quality by Design initiative has shifted focus toward understanding critical quality attributes rather than prescribing specific analytical methods, allowing manufacturers flexibility in choosing between IEF and chromatography based on fitness for purpose.
Emerging regulations for biosimilars have created particularly stringent comparative analytical requirements, where manufacturers must demonstrate similarity to reference products using multiple orthogonal methods. This regulatory landscape has driven innovation in analytical technologies that combine the strengths of both IEF and chromatographic approaches, such as chromatofocusing and pH gradient chromatography.
Cost-Benefit Analysis of Competing Technologies
When evaluating isoelectric focusing (IEF) and chromatography for protein purification, cost-benefit analysis reveals significant economic and operational differences between these technologies. Initial investment for chromatography systems typically ranges from $50,000 to $500,000 depending on scale and automation level, while IEF equipment generally requires lower capital expenditure ($20,000-$100,000). However, this initial cost advantage for IEF is often offset by other factors in long-term operations.
Operational expenses present a more complex picture. Chromatography consumables (resins, columns) represent substantial recurring costs, with high-quality resins priced between $1,000-$10,000 per liter. These materials typically allow 50-200 purification cycles before replacement. IEF, conversely, requires specialized ampholytes and carrier molecules that, while individually less expensive, may need more frequent replacement, particularly in industrial applications.
Labor requirements differ significantly between technologies. Chromatography benefits from extensive automation capabilities, reducing hands-on time to approximately 1-2 hours per run in modern systems. IEF typically demands more manual intervention, with skilled technician time averaging 3-5 hours per separation. This labor differential becomes particularly significant in high-throughput environments.
Throughput and scalability considerations heavily favor chromatography. Modern chromatography platforms can process sample volumes from milliliters to hundreds of liters, with linear scalability from laboratory to production environments. IEF faces inherent limitations in sample loading capacity and faces challenges in scale-up beyond analytical applications, often requiring significant process redesign for industrial implementation.
Energy consumption metrics indicate chromatography systems typically consume 1.5-3 kWh per run, while IEF systems operate at lower power (0.5-1 kWh) but for extended durations. Water usage presents another operational cost, with chromatography requiring 5-20 liters per gram of purified protein compared to IEF's 2-8 liters, though water quality requirements for IEF are often more stringent.
Return on investment calculations suggest chromatography systems typically achieve ROI within 2-3 years in production environments, while IEF systems may require 3-5 years to reach equivalent financial returns. This difference primarily stems from chromatography's superior throughput capabilities and established position in regulatory frameworks, reducing validation costs and time-to-market for final products.
Operational expenses present a more complex picture. Chromatography consumables (resins, columns) represent substantial recurring costs, with high-quality resins priced between $1,000-$10,000 per liter. These materials typically allow 50-200 purification cycles before replacement. IEF, conversely, requires specialized ampholytes and carrier molecules that, while individually less expensive, may need more frequent replacement, particularly in industrial applications.
Labor requirements differ significantly between technologies. Chromatography benefits from extensive automation capabilities, reducing hands-on time to approximately 1-2 hours per run in modern systems. IEF typically demands more manual intervention, with skilled technician time averaging 3-5 hours per separation. This labor differential becomes particularly significant in high-throughput environments.
Throughput and scalability considerations heavily favor chromatography. Modern chromatography platforms can process sample volumes from milliliters to hundreds of liters, with linear scalability from laboratory to production environments. IEF faces inherent limitations in sample loading capacity and faces challenges in scale-up beyond analytical applications, often requiring significant process redesign for industrial implementation.
Energy consumption metrics indicate chromatography systems typically consume 1.5-3 kWh per run, while IEF systems operate at lower power (0.5-1 kWh) but for extended durations. Water usage presents another operational cost, with chromatography requiring 5-20 liters per gram of purified protein compared to IEF's 2-8 liters, though water quality requirements for IEF are often more stringent.
Return on investment calculations suggest chromatography systems typically achieve ROI within 2-3 years in production environments, while IEF systems may require 3-5 years to reach equivalent financial returns. This difference primarily stems from chromatography's superior throughput capabilities and established position in regulatory frameworks, reducing validation costs and time-to-market for final products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!