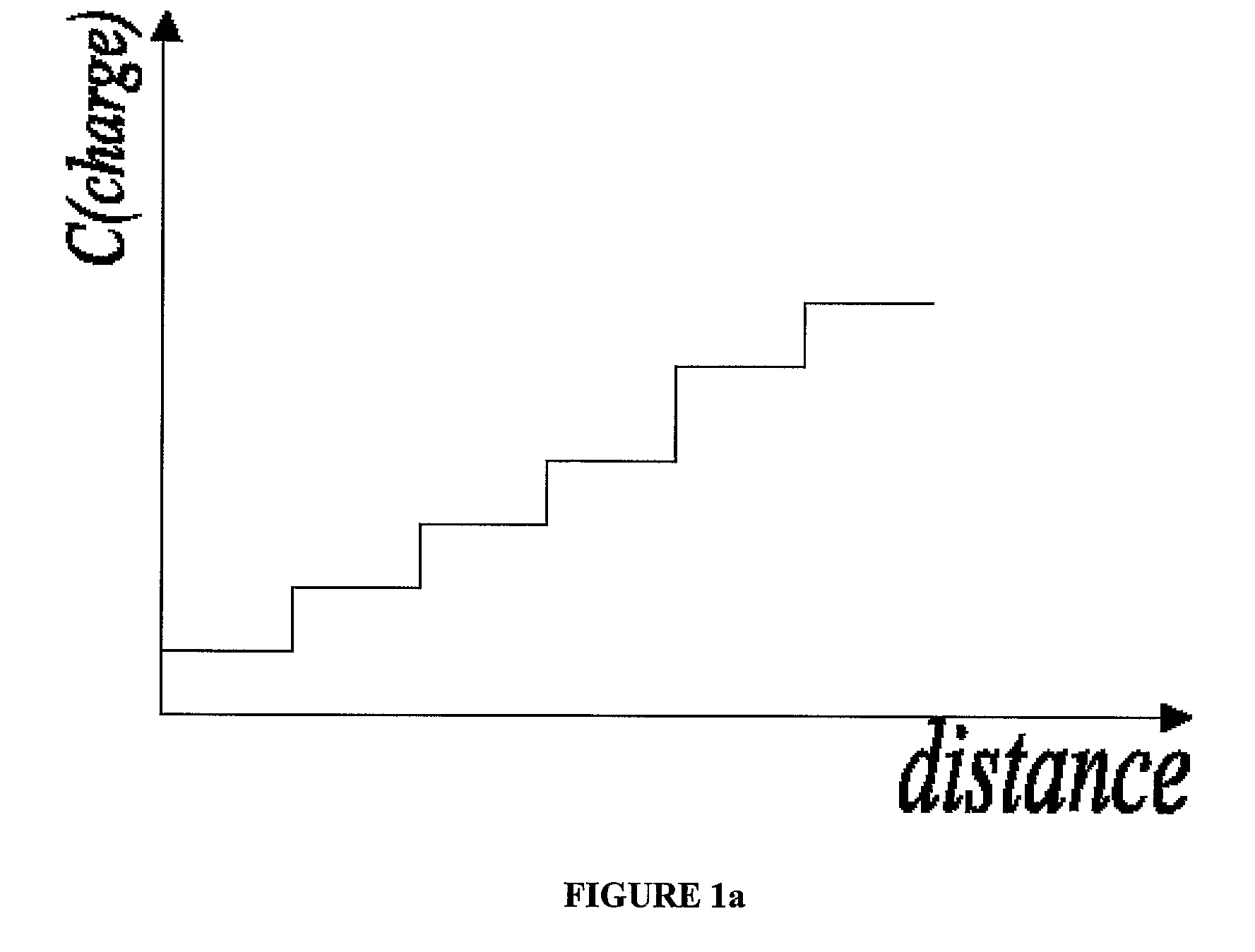
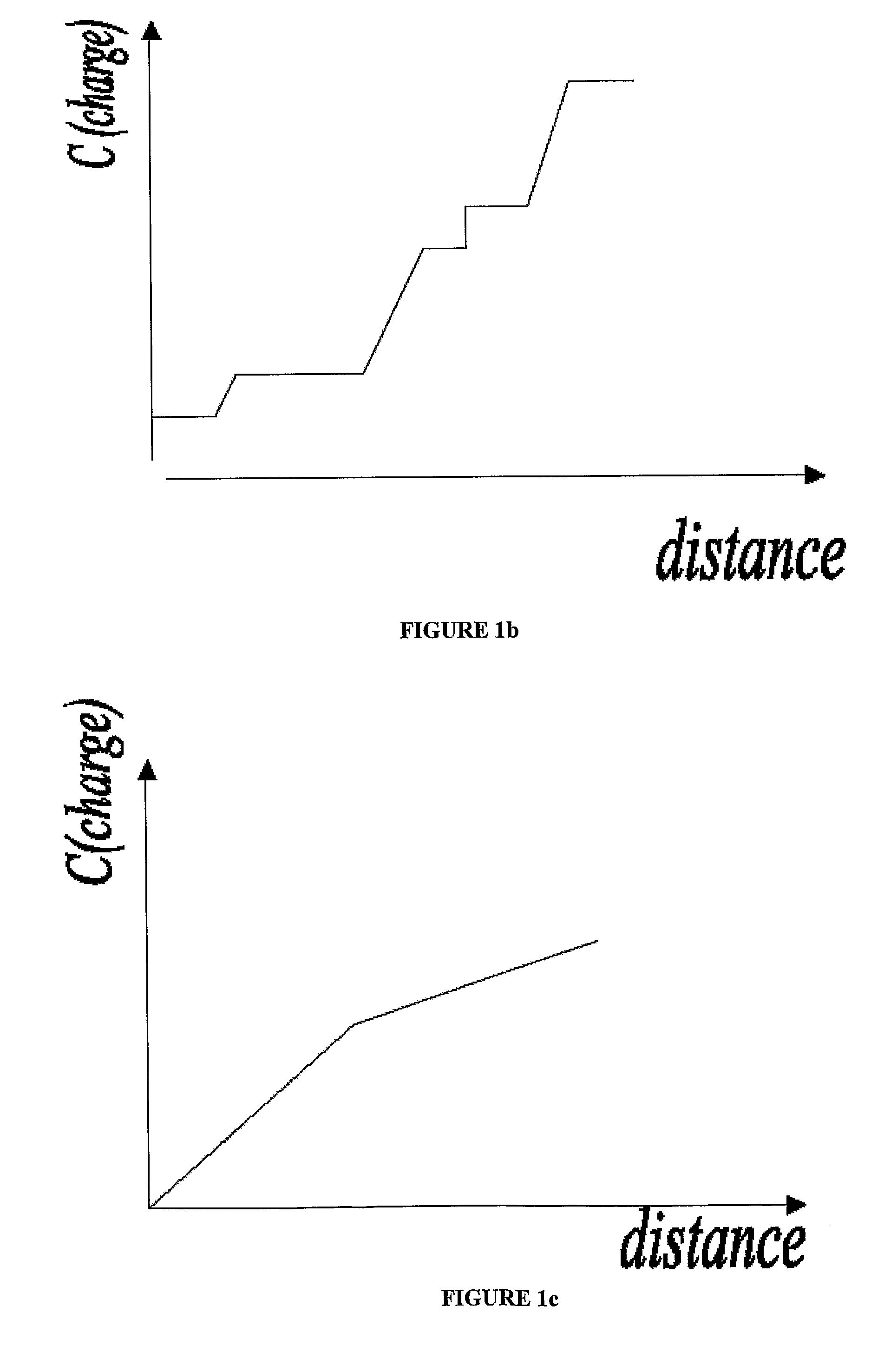
How to Achieve Better pH Stability in Isoelectric Focusing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF pH Stability Background and Objectives
Isoelectric focusing (IEF) represents one of the most powerful analytical techniques in protein separation and characterization, with applications spanning from fundamental research to industrial bioprocessing. The technique, first developed in the 1960s, leverages the amphoteric nature of proteins to separate them based on their isoelectric points (pI) within a pH gradient. Despite its widespread adoption, pH stability remains a persistent challenge that limits the reproducibility, resolution, and overall effectiveness of IEF separations.
The evolution of IEF technology has seen significant advancements from carrier ampholyte-based systems to immobilized pH gradient (IPG) approaches. However, the fundamental issue of pH gradient instability continues to plague researchers and industrial practitioners alike. Cathode drift, anodic drift, and gradient flattening phenomena frequently compromise experimental outcomes, necessitating repeated analyses and increasing operational costs.
Current technological trends in IEF are moving toward more robust, reproducible systems with enhanced pH stability characteristics. This includes the development of novel carrier ampholytes, improved IPG formulations, and innovative electrode designs that minimize electrochemical disturbances. Additionally, computational modeling of electrophoretic processes has emerged as a valuable tool for predicting and mitigating pH instability issues.
The primary objective of this technical investigation is to comprehensively evaluate existing approaches to pH stability enhancement in IEF and identify promising new directions for technological innovation. Specifically, we aim to address the root causes of pH gradient instability, including electrochemical reactions at electrodes, protein-ampholyte interactions, and temperature-induced perturbations.
Furthermore, this research seeks to establish quantitative metrics for assessing pH stability in IEF systems, enabling objective comparison between different methodologies. By developing standardized evaluation protocols, we intend to facilitate more systematic improvement efforts across the field and accelerate the adoption of best practices.
Beyond immediate technical improvements, this investigation aims to explore how enhanced pH stability can expand the application scope of IEF to previously challenging sample types, including membrane proteins, extremely basic or acidic proteins, and complex biological mixtures. The potential impact extends to proteomics research, biopharmaceutical manufacturing, clinical diagnostics, and emerging fields such as synthetic biology.
By establishing a clear understanding of the technical landscape and identifying key innovation opportunities, this research will provide a roadmap for achieving significantly improved pH stability in IEF systems, ultimately enhancing the reliability and utility of this critical analytical technique across multiple scientific and industrial domains.
The evolution of IEF technology has seen significant advancements from carrier ampholyte-based systems to immobilized pH gradient (IPG) approaches. However, the fundamental issue of pH gradient instability continues to plague researchers and industrial practitioners alike. Cathode drift, anodic drift, and gradient flattening phenomena frequently compromise experimental outcomes, necessitating repeated analyses and increasing operational costs.
Current technological trends in IEF are moving toward more robust, reproducible systems with enhanced pH stability characteristics. This includes the development of novel carrier ampholytes, improved IPG formulations, and innovative electrode designs that minimize electrochemical disturbances. Additionally, computational modeling of electrophoretic processes has emerged as a valuable tool for predicting and mitigating pH instability issues.
The primary objective of this technical investigation is to comprehensively evaluate existing approaches to pH stability enhancement in IEF and identify promising new directions for technological innovation. Specifically, we aim to address the root causes of pH gradient instability, including electrochemical reactions at electrodes, protein-ampholyte interactions, and temperature-induced perturbations.
Furthermore, this research seeks to establish quantitative metrics for assessing pH stability in IEF systems, enabling objective comparison between different methodologies. By developing standardized evaluation protocols, we intend to facilitate more systematic improvement efforts across the field and accelerate the adoption of best practices.
Beyond immediate technical improvements, this investigation aims to explore how enhanced pH stability can expand the application scope of IEF to previously challenging sample types, including membrane proteins, extremely basic or acidic proteins, and complex biological mixtures. The potential impact extends to proteomics research, biopharmaceutical manufacturing, clinical diagnostics, and emerging fields such as synthetic biology.
By establishing a clear understanding of the technical landscape and identifying key innovation opportunities, this research will provide a roadmap for achieving significantly improved pH stability in IEF systems, ultimately enhancing the reliability and utility of this critical analytical technique across multiple scientific and industrial domains.
Market Analysis for Stable IEF Applications
The global market for isoelectric focusing (IEF) applications has been experiencing steady growth, primarily driven by increasing demand in proteomics research, pharmaceutical development, and clinical diagnostics. The market size for electrophoresis equipment and consumables, which includes IEF technology, was valued at approximately $1.8 billion in 2022 and is projected to reach $2.5 billion by 2027, growing at a CAGR of 6.8%.
The pharmaceutical and biotechnology sectors represent the largest market segments for stable IEF applications, accounting for nearly 45% of the total market share. These industries rely heavily on IEF for protein characterization, quality control, and drug development processes. The academic and research institutions segment follows closely, contributing about 30% to the market revenue.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to increasing investments in life sciences research and expanding biotechnology sectors.
A significant market driver is the growing focus on personalized medicine and biomarker discovery, which requires precise protein separation techniques. The demand for improved pH stability in IEF is particularly acute in these applications, as researchers seek more reliable and reproducible results for complex biological samples.
The clinical diagnostics segment presents substantial growth opportunities, with IEF being increasingly utilized for disease biomarker identification and monitoring. This application area is expected to grow at a CAGR of 8.5% through 2027, outpacing the overall market growth.
Key customer pain points include inconsistent results due to pH gradient instability, lengthy procedure times, and difficulties in standardization across laboratories. Market surveys indicate that 78% of end-users consider pH stability as a critical factor when selecting IEF systems, highlighting the significant commercial potential for innovations addressing this challenge.
The consumables segment, including carrier ampholytes and specialized buffers for enhanced pH stability, generates recurring revenue streams and represents approximately 65% of the total market value. This segment is projected to grow faster than the equipment segment, indicating increasing demand for solutions that improve IEF reliability.
Emerging applications in proteogenomics, structural biology, and biopharmaceutical characterization are creating new market opportunities for stable IEF technologies. The rising adoption of automated and high-throughput systems is also reshaping market dynamics, with integrated solutions commanding premium pricing and gaining market share from traditional manual systems.
The pharmaceutical and biotechnology sectors represent the largest market segments for stable IEF applications, accounting for nearly 45% of the total market share. These industries rely heavily on IEF for protein characterization, quality control, and drug development processes. The academic and research institutions segment follows closely, contributing about 30% to the market revenue.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to increasing investments in life sciences research and expanding biotechnology sectors.
A significant market driver is the growing focus on personalized medicine and biomarker discovery, which requires precise protein separation techniques. The demand for improved pH stability in IEF is particularly acute in these applications, as researchers seek more reliable and reproducible results for complex biological samples.
The clinical diagnostics segment presents substantial growth opportunities, with IEF being increasingly utilized for disease biomarker identification and monitoring. This application area is expected to grow at a CAGR of 8.5% through 2027, outpacing the overall market growth.
Key customer pain points include inconsistent results due to pH gradient instability, lengthy procedure times, and difficulties in standardization across laboratories. Market surveys indicate that 78% of end-users consider pH stability as a critical factor when selecting IEF systems, highlighting the significant commercial potential for innovations addressing this challenge.
The consumables segment, including carrier ampholytes and specialized buffers for enhanced pH stability, generates recurring revenue streams and represents approximately 65% of the total market value. This segment is projected to grow faster than the equipment segment, indicating increasing demand for solutions that improve IEF reliability.
Emerging applications in proteogenomics, structural biology, and biopharmaceutical characterization are creating new market opportunities for stable IEF technologies. The rising adoption of automated and high-throughput systems is also reshaping market dynamics, with integrated solutions commanding premium pricing and gaining market share from traditional manual systems.
Current Challenges in pH Gradient Stability
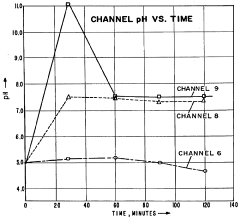
Despite significant advancements in isoelectric focusing (IEF) technology, pH gradient stability remains one of the most persistent challenges limiting the technique's reproducibility and resolution. The primary issue stems from the phenomenon known as "cathodic drift," where ampholytes migrate toward the cathode during extended focusing periods, causing a progressive flattening of the pH gradient. This drift significantly compromises protein separation efficiency and makes reproducible results difficult to achieve, particularly in extended runs exceeding 24 hours.
Carrier ampholyte-based systems, while widely used, exhibit inherent instability due to their heterogeneous composition. The varying molecular weights and charge characteristics of these ampholytes lead to differential migration rates, resulting in gradient distortion over time. Commercial carrier ampholytes typically contain hundreds of different species with varying isoelectric points, creating complex interactions that are difficult to control precisely during separation.
Temperature fluctuations represent another critical destabilizing factor. Even minor temperature gradients across the separation medium can cause localized changes in ampholyte behavior, leading to inconsistent pH values at supposedly identical positions. This temperature sensitivity is particularly problematic in high-voltage applications where Joule heating becomes significant, creating a feedback loop that further destabilizes the gradient.
Electroendosmosis effects contribute substantially to gradient instability, especially in gel-based systems. The movement of water molecules toward the cathode due to fixed charges in the separation matrix causes physical displacement of the gradient, compressing it in certain regions while expanding it in others. This mechanical distortion alters the effective resolution of the separation and introduces positional variability in protein focusing.
Sample-induced perturbations present additional complications. High-concentration protein samples can locally overwhelm the buffering capacity of ampholytes, creating "plateau effects" that distort the linearity of the gradient. Furthermore, proteins with strong buffering properties may themselves alter the local pH environment, creating micro-heterogeneities in the gradient that affect reproducibility.
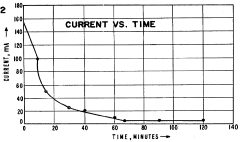
Chemical instability of ampholytes during prolonged exposure to electric fields represents another significant challenge. Electrolysis at the electrodes generates acid at the anode and base at the cathode, which can progressively degrade ampholytes through oxidation or reduction reactions. This chemical degradation alters the buffering properties of the ampholytes, further contributing to gradient drift over time.
Recent research has highlighted the role of electrode reactions in gradient instability. The production of hydrogen and oxygen gases at the electrodes creates localized pH extremes that can penetrate the separation medium, particularly in systems with insufficient electrode buffers. These extreme pH zones can irreversibly alter ampholyte chemistry and disrupt the carefully established gradient.
Carrier ampholyte-based systems, while widely used, exhibit inherent instability due to their heterogeneous composition. The varying molecular weights and charge characteristics of these ampholytes lead to differential migration rates, resulting in gradient distortion over time. Commercial carrier ampholytes typically contain hundreds of different species with varying isoelectric points, creating complex interactions that are difficult to control precisely during separation.
Temperature fluctuations represent another critical destabilizing factor. Even minor temperature gradients across the separation medium can cause localized changes in ampholyte behavior, leading to inconsistent pH values at supposedly identical positions. This temperature sensitivity is particularly problematic in high-voltage applications where Joule heating becomes significant, creating a feedback loop that further destabilizes the gradient.
Electroendosmosis effects contribute substantially to gradient instability, especially in gel-based systems. The movement of water molecules toward the cathode due to fixed charges in the separation matrix causes physical displacement of the gradient, compressing it in certain regions while expanding it in others. This mechanical distortion alters the effective resolution of the separation and introduces positional variability in protein focusing.
Sample-induced perturbations present additional complications. High-concentration protein samples can locally overwhelm the buffering capacity of ampholytes, creating "plateau effects" that distort the linearity of the gradient. Furthermore, proteins with strong buffering properties may themselves alter the local pH environment, creating micro-heterogeneities in the gradient that affect reproducibility.
Chemical instability of ampholytes during prolonged exposure to electric fields represents another significant challenge. Electrolysis at the electrodes generates acid at the anode and base at the cathode, which can progressively degrade ampholytes through oxidation or reduction reactions. This chemical degradation alters the buffering properties of the ampholytes, further contributing to gradient drift over time.
Recent research has highlighted the role of electrode reactions in gradient instability. The production of hydrogen and oxygen gases at the electrodes creates localized pH extremes that can penetrate the separation medium, particularly in systems with insufficient electrode buffers. These extreme pH zones can irreversibly alter ampholyte chemistry and disrupt the carefully established gradient.
Current pH Stabilization Methodologies
01 pH gradient stability in isoelectric focusing
Maintaining stable pH gradients is crucial for effective isoelectric focusing. Various techniques and additives are employed to prevent pH gradient drift and ensure reproducible results. These include the use of specialized carrier ampholytes, buffer systems, and temperature control mechanisms that work together to maintain the stability of the pH gradient during the separation process.- pH gradient stability in isoelectric focusing: Maintaining stable pH gradients is crucial for effective isoelectric focusing. Various techniques and compositions have been developed to enhance the stability of pH gradients during the separation process. These include the use of specialized carrier ampholytes, buffer systems, and additives that prevent gradient drift and ensure reproducible results. Stable pH gradients allow for more precise separation of proteins and other biomolecules based on their isoelectric points.
- Carrier ampholyte compositions for improved focusing: Specific carrier ampholyte compositions have been developed to improve the performance of isoelectric focusing. These compositions contain mixtures of amphoteric substances with different pKa values to create smooth and stable pH gradients. The formulations may include synthetic ampholytes, amino acids, or other zwitterionic compounds that maintain their buffering capacity throughout the focusing process, resulting in better resolution and separation of proteins.
- Gel formulations for enhanced pH stability: Specialized gel formulations have been developed to enhance pH stability during isoelectric focusing. These gels may incorporate immobilized pH gradients, cross-linked polymers, or specific additives that minimize electroendosmosis and prevent pH drift. The gel composition can significantly impact the resolution, reproducibility, and overall performance of the isoelectric focusing technique, particularly for applications requiring extended separation times.
- Instrumentation and apparatus for maintaining pH stability: Advanced instrumentation and apparatus designs have been created specifically to maintain pH stability during isoelectric focusing. These systems may include temperature control mechanisms, specialized electrode configurations, power supply management systems, and monitoring devices that detect and correct pH drift in real-time. Such equipment helps to ensure consistent and reliable separation results by maintaining stable conditions throughout the focusing process.
- Methods for monitoring and controlling pH stability: Various methods have been developed for monitoring and controlling pH stability during isoelectric focusing. These include real-time pH measurement techniques, mathematical modeling of pH gradient behavior, automated control systems, and specific protocols for sample preparation and running conditions. These methods help researchers optimize separation parameters, detect instabilities, and make adjustments to achieve more reliable and reproducible results in isoelectric focusing applications.
02 Carrier ampholyte formulations for improved stability
Specific formulations of carrier ampholytes can significantly enhance the stability of isoelectric focusing systems. These formulations include optimized mixtures of amphoteric compounds with different pI values that create smooth and stable pH gradients. The composition and concentration of these carrier ampholytes are carefully designed to minimize conductivity differences and prevent distortion of the pH gradient during the focusing process.Expand Specific Solutions03 Gel composition and additives for pH stabilization
The composition of the separation gel and various additives play important roles in stabilizing pH gradients during isoelectric focusing. Specialized gel formulations containing specific polymers, cross-linkers, and stabilizing agents help maintain the integrity of the pH gradient. Additionally, certain additives such as glycerol, urea, and non-ionic detergents can be incorporated to enhance pH stability and prevent protein precipitation at their isoelectric points.Expand Specific Solutions04 Equipment and apparatus design for pH stability
The design of isoelectric focusing equipment significantly impacts pH gradient stability. Advanced apparatus features include precise temperature control systems, optimized electrode configurations, and specialized cooling mechanisms that minimize Joule heating effects. Modern equipment may also incorporate automated monitoring systems that detect and compensate for pH drift during the separation process, ensuring more stable and reproducible results.Expand Specific Solutions05 Novel methods for enhancing pH gradient stability
Innovative approaches have been developed to improve pH stability in isoelectric focusing. These include immobilized pH gradient (IPG) techniques, where the pH gradient is covalently linked to the gel matrix, preventing drift. Other advancements include multi-compartment electrolyzers, sequential loading protocols, and pulsed field applications that help maintain stable pH gradients over extended separation times, particularly beneficial for complex protein mixtures and preparative applications.Expand Specific Solutions
Leading Manufacturers and Research Groups
The isoelectric focusing (IEF) pH stability market is currently in a growth phase, with increasing demand driven by proteomics research and diagnostic applications. The global market size is estimated at approximately $1.2 billion, expanding at 7-8% annually. Leading players include Bio-Rad Laboratories and Becton, Dickinson & Co., who have established mature commercial platforms, while academic institutions like Massachusetts Institute of Technology and Shanghai Jiao Tong University contribute significant research innovations. Technology maturity varies across approaches, with traditional carrier ampholyte systems well-established but facing stability challenges. Newer technologies from Life Technologies and JSR Corp. focus on immobilized pH gradients and novel polymer matrices that demonstrate superior pH stability. Research collaborations between companies like Koninklijke Philips and academic institutions are accelerating development of next-generation IEF technologies with enhanced reproducibility and resolution.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced ampholyte formulations specifically designed to maintain pH stability during isoelectric focusing (IEF). Their approach involves creating carrier ampholytes with enhanced buffering capacity and reduced conductivity variations across the pH gradient. The company's proprietary ampholyte mixtures contain optimized spacer ampholytes that help prevent pH drift during extended focusing times[1]. Bio-Rad's technology incorporates temperature control systems integrated with their IEF platforms, maintaining consistent temperature throughout the separation process to prevent thermal gradients that could disrupt pH stability[2]. Their ReadyStrip IPG strips feature a proprietary manufacturing process that creates highly uniform, covalently linked ampholytes within a homogeneous polyacrylamide gel matrix, significantly reducing batch-to-batch variation and improving reproducibility of pH gradients during extended focusing times[3]. Bio-Rad has also developed specialized rehydration and equilibration buffers containing optimized concentrations of detergents, reducing agents, and carrier ampholytes that work synergistically to maintain pH stability.
Strengths: Industry-leading expertise in electrophoresis technologies with decades of experience in IEF optimization. Their integrated systems approach addresses multiple factors affecting pH stability simultaneously. Weaknesses: Their proprietary solutions often require the use of Bio-Rad's complete workflow systems, potentially limiting flexibility for researchers using mixed-vendor equipment. Higher cost compared to generic alternatives.
Life Technologies Corp.
Technical Solution: Life Technologies has pioneered a comprehensive approach to pH stability in isoelectric focusing through their ZOOM IEF Fractionator system. This technology employs specialized membrane disks with precise pH values to create discrete pH compartments, effectively eliminating the continuous pH gradient that traditionally suffers from drift and instability[1]. Their innovation includes proprietary buffer formulations containing optimized concentrations of non-detergent sulfobetaines (NDSB) and glycerol that significantly reduce protein aggregation while enhancing pH stability during extended focusing times[2]. The company has developed a patented electrode design that minimizes electrolysis effects at the electrode-buffer interface, reducing pH fluctuations caused by water electrolysis and subsequent ion migration. Life Technologies' system incorporates real-time pH monitoring capabilities through integrated microelectrodes that provide continuous feedback on pH conditions throughout the separation process, allowing for automated adjustments to maintain stability[3]. Additionally, their approach includes specialized cooling systems that maintain uniform temperature distribution across the separation chamber, preventing thermal gradients that could disrupt pH stability.
Strengths: Their compartmentalized approach effectively addresses fundamental causes of pH instability in traditional IEF systems. The integrated monitoring and control systems provide superior reproducibility for complex samples. Weaknesses: The specialized equipment requirements create higher initial investment costs. The system has more complexity compared to traditional IEF methods, potentially requiring additional training and expertise.
Key Patents and Innovations in IEF Stability
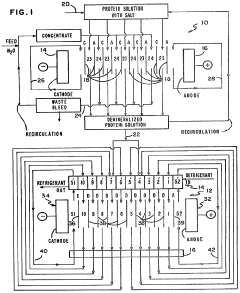
Separation of proteins using electrodialysis - isoelectric focusing combination
PatentInactiveUS4441978A
Innovation
- The integration of electrodialysis before isoelectric focusing allows for controlled salt removal, creating a demineralized protein solution that stabilizes the pH gradient and prevents protein precipitation, thereby enhancing the efficiency of the separation process.
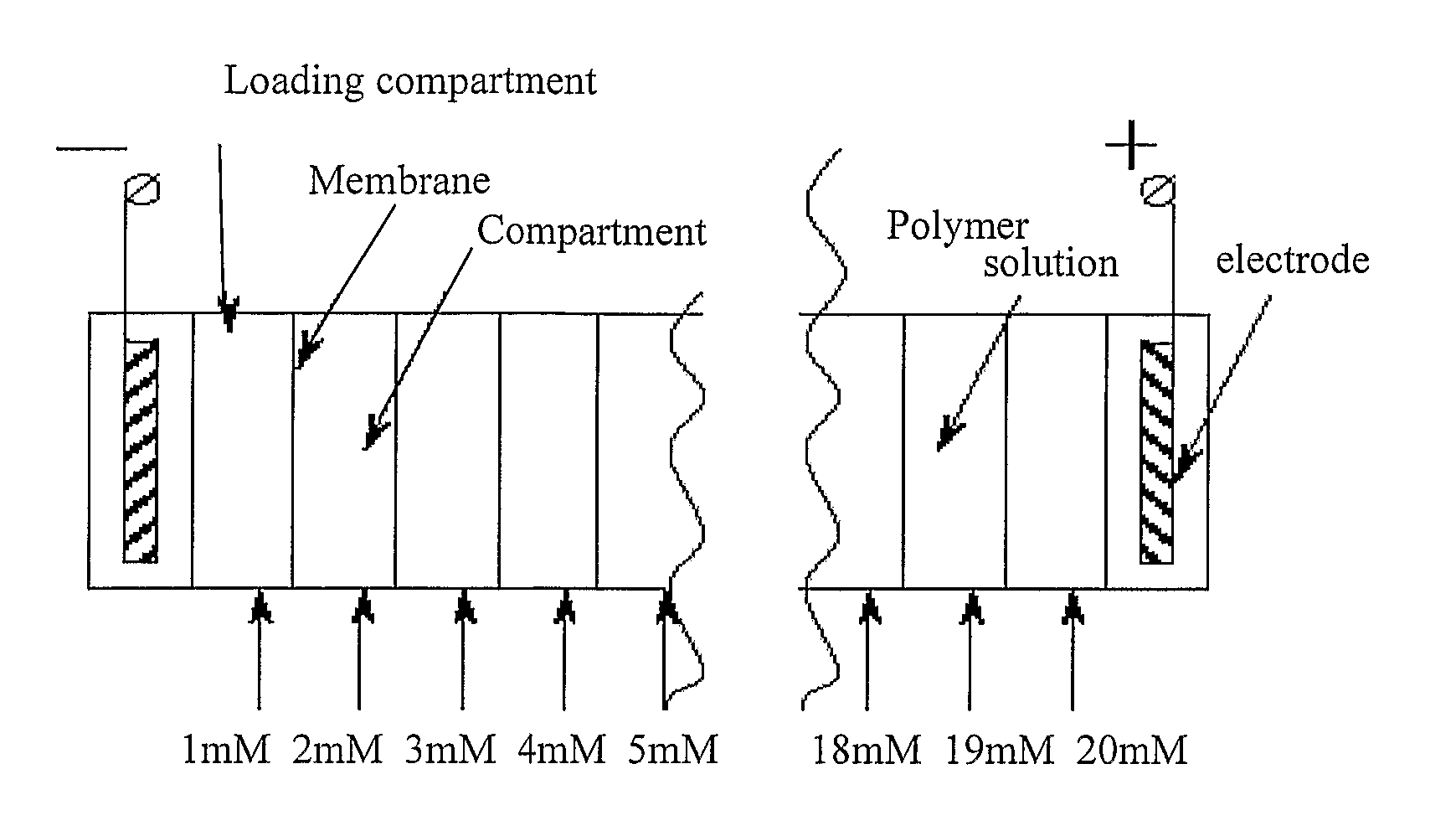
Electrophoretic Separation of Analytes by Molecular Mass
PatentInactiveUS20080314751A1
Innovation
- A novel method using a matrix modified with a charged separation agent, where analytes form a charged complex and are separated based on their total charge, allowing for separation by molecular weight in a low-friction medium with a charge density gradient, eliminating the need for gradient gels and improving mass determination accuracy.
Regulatory Considerations for IEF Applications
Isoelectric focusing (IEF) applications are subject to various regulatory frameworks depending on their intended use, particularly in clinical diagnostics, pharmaceutical development, and food safety testing. The U.S. Food and Drug Administration (FDA) maintains stringent requirements for IEF methods used in clinical diagnostics through the Clinical Laboratory Improvement Amendments (CLIA) and medical device regulations. These regulations mandate validation of pH stability parameters and documentation of method reproducibility to ensure consistent and reliable results.
In pharmaceutical applications, IEF techniques must comply with International Conference on Harmonisation (ICH) guidelines, specifically ICH Q2(R1) for analytical method validation. These guidelines emphasize the importance of demonstrating pH gradient stability over time as a critical quality attribute. Companies developing IEF-based methods for drug characterization must provide comprehensive validation data showing minimal pH drift during the focusing process.
The European Medicines Agency (EMA) has established additional requirements for capillary IEF methods used in biosimilar comparability studies, where pH stability directly impacts the ability to make accurate comparisons between reference products and biosimilars. These regulations specifically address the need for controlled pH environments and reproducible gradient formation.
For food safety applications, regulatory bodies such as the AOAC International have developed standardized IEF protocols that include specific requirements for pH gradient stability verification. These standards are particularly relevant for allergen detection and protein characterization in food products, where false results could have significant public health implications.
Regulatory compliance also extends to the reagents used in IEF applications. Ampholytes and other pH gradient-forming chemicals must meet purity specifications outlined in pharmacopeial standards such as the United States Pharmacopeia (USP) and European Pharmacopoeia (EP). These standards include limits on contaminants that could potentially disrupt pH stability during the focusing process.
Laboratory accreditation bodies, including ISO/IEC 17025 certification authorities, require documented evidence of pH stability monitoring as part of method validation for IEF techniques. This includes establishing acceptance criteria for pH drift and implementing regular system suitability tests to verify ongoing compliance with these criteria.
Recent regulatory trends indicate increasing scrutiny of IEF methods that utilize novel stabilizing agents or modified ampholyte formulations. Regulatory submissions now typically require comparative data demonstrating that these innovations maintain or improve pH stability without introducing new variables that could compromise analytical integrity or patient safety.
In pharmaceutical applications, IEF techniques must comply with International Conference on Harmonisation (ICH) guidelines, specifically ICH Q2(R1) for analytical method validation. These guidelines emphasize the importance of demonstrating pH gradient stability over time as a critical quality attribute. Companies developing IEF-based methods for drug characterization must provide comprehensive validation data showing minimal pH drift during the focusing process.
The European Medicines Agency (EMA) has established additional requirements for capillary IEF methods used in biosimilar comparability studies, where pH stability directly impacts the ability to make accurate comparisons between reference products and biosimilars. These regulations specifically address the need for controlled pH environments and reproducible gradient formation.
For food safety applications, regulatory bodies such as the AOAC International have developed standardized IEF protocols that include specific requirements for pH gradient stability verification. These standards are particularly relevant for allergen detection and protein characterization in food products, where false results could have significant public health implications.
Regulatory compliance also extends to the reagents used in IEF applications. Ampholytes and other pH gradient-forming chemicals must meet purity specifications outlined in pharmacopeial standards such as the United States Pharmacopeia (USP) and European Pharmacopoeia (EP). These standards include limits on contaminants that could potentially disrupt pH stability during the focusing process.
Laboratory accreditation bodies, including ISO/IEC 17025 certification authorities, require documented evidence of pH stability monitoring as part of method validation for IEF techniques. This includes establishing acceptance criteria for pH drift and implementing regular system suitability tests to verify ongoing compliance with these criteria.
Recent regulatory trends indicate increasing scrutiny of IEF methods that utilize novel stabilizing agents or modified ampholyte formulations. Regulatory submissions now typically require comparative data demonstrating that these innovations maintain or improve pH stability without introducing new variables that could compromise analytical integrity or patient safety.
Environmental Impact of IEF Reagents
The environmental impact of reagents used in isoelectric focusing (IEF) represents a significant concern as this analytical technique becomes more widely adopted in research, clinical diagnostics, and industrial applications. Carrier ampholytes, which are essential components in establishing pH gradients, often contain complex mixtures of polyamino-polycarboxylic acids that can persist in the environment after disposal. These compounds typically demonstrate low biodegradability and may accumulate in aquatic ecosystems, potentially disrupting natural pH balances and affecting sensitive aquatic organisms.
Immobilized pH gradient (IPG) strips, while offering improved pH stability, introduce environmental challenges through their manufacturing process and disposal. The acrylamide used in these strips is neurotoxic before polymerization, requiring careful handling and disposal protocols. Additionally, the chemical crosslinkers and catalysts employed in IPG production can contribute to environmental contamination if not properly managed.
Detergents and chaotropic agents commonly used in sample preparation for IEF, such as urea, thiourea, and CHAPS, present further environmental concerns. These compounds can increase biochemical oxygen demand in water systems and potentially disrupt microbial communities essential for natural water purification processes. Studies have shown that some of these reagents demonstrate persistence in conventional wastewater treatment systems.
Recent research has focused on developing more environmentally friendly alternatives for IEF applications. Bio-based ampholytes derived from sustainable sources show promise as replacements for traditional synthetic carrier ampholytes. These compounds offer comparable separation performance while demonstrating enhanced biodegradability profiles. Similarly, advances in green chemistry have led to the development of less toxic chaotropic agents and detergents with improved environmental compatibility.
Laboratory waste management practices significantly influence the environmental footprint of IEF procedures. Implementation of waste segregation protocols, neutralization procedures, and specialized disposal methods can substantially reduce environmental impact. Some institutions have successfully implemented recovery and recycling programs for certain IEF reagents, particularly expensive carrier ampholytes, which simultaneously reduces costs and environmental burden.
Regulatory frameworks governing laboratory waste disposal vary considerably across regions, with more stringent requirements typically found in Europe and North America. Compliance with these regulations not only mitigates environmental risks but also drives innovation toward greener alternatives. The development of standardized environmental impact assessments specifically for electrophoresis techniques would provide valuable guidance for researchers and industry practitioners seeking to minimize ecological footprints while maintaining analytical performance.
Immobilized pH gradient (IPG) strips, while offering improved pH stability, introduce environmental challenges through their manufacturing process and disposal. The acrylamide used in these strips is neurotoxic before polymerization, requiring careful handling and disposal protocols. Additionally, the chemical crosslinkers and catalysts employed in IPG production can contribute to environmental contamination if not properly managed.
Detergents and chaotropic agents commonly used in sample preparation for IEF, such as urea, thiourea, and CHAPS, present further environmental concerns. These compounds can increase biochemical oxygen demand in water systems and potentially disrupt microbial communities essential for natural water purification processes. Studies have shown that some of these reagents demonstrate persistence in conventional wastewater treatment systems.
Recent research has focused on developing more environmentally friendly alternatives for IEF applications. Bio-based ampholytes derived from sustainable sources show promise as replacements for traditional synthetic carrier ampholytes. These compounds offer comparable separation performance while demonstrating enhanced biodegradability profiles. Similarly, advances in green chemistry have led to the development of less toxic chaotropic agents and detergents with improved environmental compatibility.
Laboratory waste management practices significantly influence the environmental footprint of IEF procedures. Implementation of waste segregation protocols, neutralization procedures, and specialized disposal methods can substantially reduce environmental impact. Some institutions have successfully implemented recovery and recycling programs for certain IEF reagents, particularly expensive carrier ampholytes, which simultaneously reduces costs and environmental burden.
Regulatory frameworks governing laboratory waste disposal vary considerably across regions, with more stringent requirements typically found in Europe and North America. Compliance with these regulations not only mitigates environmental risks but also drives innovation toward greener alternatives. The development of standardized environmental impact assessments specifically for electrophoresis techniques would provide valuable guidance for researchers and industry practitioners seeking to minimize ecological footprints while maintaining analytical performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!