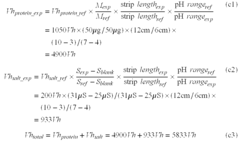Measure Protein Separation Efficiency in Isoelectric Focusing
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Protein Separation Background and Objectives
Isoelectric focusing (IEF) represents a cornerstone technique in protein separation and analysis, evolving significantly since its introduction in the 1960s. This high-resolution electrophoretic method separates proteins based on their isoelectric points (pI), the specific pH at which a protein carries no net electrical charge. The historical trajectory of IEF technology shows continuous refinement from traditional gel-based systems to contemporary capillary and microchip platforms, reflecting broader trends in analytical chemistry toward miniaturization and automation.
The fundamental principle underlying IEF involves establishing a stable pH gradient within a separation medium, allowing proteins to migrate under an electric field until reaching their respective pI positions. At these points, proteins become stationary due to charge neutralization, resulting in focused bands with exceptional resolution. This capability has positioned IEF as an indispensable tool in proteomics, biopharmaceutical development, and clinical diagnostics.
Recent technological advancements have significantly enhanced IEF capabilities, particularly through the integration of immobilized pH gradients (IPGs), which offer superior reproducibility compared to carrier ampholyte-based methods. Additionally, the coupling of IEF with mass spectrometry and other detection techniques has expanded its analytical power, enabling more comprehensive protein characterization and identification.
The primary objective of measuring protein separation efficiency in IEF is to quantitatively assess and optimize the resolving power of this technique across various experimental conditions. This involves evaluating key performance metrics such as resolution, peak capacity, separation time, and reproducibility. By establishing standardized measurement protocols, researchers aim to enhance the reliability and comparability of IEF data across different laboratories and applications.
Another critical goal is to develop innovative approaches for visualizing and quantifying protein distribution patterns within pH gradients. This includes exploring advanced imaging technologies, fluorescent labeling strategies, and computational algorithms for pattern recognition and data analysis. Such advancements would address current limitations in detecting low-abundance proteins and resolving complex protein mixtures.
The technological evolution of IEF continues to be driven by demands for higher throughput, greater sensitivity, and improved integration with downstream analytical processes. Current research focuses on developing microfluidic IEF platforms that offer advantages in terms of sample consumption, analysis speed, and automation potential. These developments align with broader trends toward point-of-care diagnostics and personalized medicine applications.
Understanding the theoretical foundations and practical limitations of IEF efficiency measurements is essential for advancing this technology toward its full potential in both research and clinical settings. This requires interdisciplinary collaboration among analytical chemists, bioengineers, and computational scientists to address persistent challenges in reproducibility, quantification, and data interpretation.
The fundamental principle underlying IEF involves establishing a stable pH gradient within a separation medium, allowing proteins to migrate under an electric field until reaching their respective pI positions. At these points, proteins become stationary due to charge neutralization, resulting in focused bands with exceptional resolution. This capability has positioned IEF as an indispensable tool in proteomics, biopharmaceutical development, and clinical diagnostics.
Recent technological advancements have significantly enhanced IEF capabilities, particularly through the integration of immobilized pH gradients (IPGs), which offer superior reproducibility compared to carrier ampholyte-based methods. Additionally, the coupling of IEF with mass spectrometry and other detection techniques has expanded its analytical power, enabling more comprehensive protein characterization and identification.
The primary objective of measuring protein separation efficiency in IEF is to quantitatively assess and optimize the resolving power of this technique across various experimental conditions. This involves evaluating key performance metrics such as resolution, peak capacity, separation time, and reproducibility. By establishing standardized measurement protocols, researchers aim to enhance the reliability and comparability of IEF data across different laboratories and applications.
Another critical goal is to develop innovative approaches for visualizing and quantifying protein distribution patterns within pH gradients. This includes exploring advanced imaging technologies, fluorescent labeling strategies, and computational algorithms for pattern recognition and data analysis. Such advancements would address current limitations in detecting low-abundance proteins and resolving complex protein mixtures.
The technological evolution of IEF continues to be driven by demands for higher throughput, greater sensitivity, and improved integration with downstream analytical processes. Current research focuses on developing microfluidic IEF platforms that offer advantages in terms of sample consumption, analysis speed, and automation potential. These developments align with broader trends toward point-of-care diagnostics and personalized medicine applications.
Understanding the theoretical foundations and practical limitations of IEF efficiency measurements is essential for advancing this technology toward its full potential in both research and clinical settings. This requires interdisciplinary collaboration among analytical chemists, bioengineers, and computational scientists to address persistent challenges in reproducibility, quantification, and data interpretation.
Market Analysis for Protein Separation Technologies
The protein separation technology market is experiencing robust growth, driven by increasing applications in proteomics research, pharmaceutical development, and diagnostic applications. The global market for protein separation and analysis technologies was valued at approximately $10.2 billion in 2022 and is projected to reach $16.5 billion by 2027, growing at a CAGR of 10.1%. Within this broader market, isoelectric focusing (IEF) technologies represent a significant segment due to their high resolution capabilities for protein separation.
Demand for advanced protein separation technologies is particularly strong in biopharmaceutical development, where the need for highly purified proteins for therapeutic applications continues to drive innovation. The rise of personalized medicine and biologics has further accelerated market growth, with monoclonal antibodies and recombinant proteins requiring sophisticated separation techniques for both research and production.
Academic and research institutions constitute another major market segment, with proteomics research expanding rapidly as scientists seek to understand protein function, interaction, and expression patterns. The demand for higher resolution, reproducibility, and efficiency in protein separation has led to continuous technological advancements in IEF methodologies and equipment.
Geographically, North America dominates the protein separation market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China and India, is experiencing the fastest growth due to increasing investments in biotechnology research and pharmaceutical manufacturing infrastructure.
Key market drivers include technological advancements in separation techniques, growing funding for proteomics research, increasing prevalence of chronic diseases necessitating protein-based diagnostics and therapeutics, and rising demand for personalized medicine approaches. The COVID-19 pandemic has further highlighted the importance of protein analysis technologies for vaccine and therapeutic development.
Market challenges include the high cost of advanced separation equipment, technical complexity requiring specialized training, and regulatory hurdles for clinical applications. Additionally, there is growing demand for miniaturized and automated systems that can provide higher throughput while maintaining separation efficiency.
Customer segments for IEF technologies include pharmaceutical and biotechnology companies (45%), academic and research institutions (30%), clinical diagnostic laboratories (15%), and contract research organizations (10%). Each segment has distinct requirements regarding throughput, resolution, automation, and cost-effectiveness.
The competitive landscape features established players like Thermo Fisher Scientific, Bio-Rad Laboratories, and Agilent Technologies alongside innovative startups developing novel separation technologies. Recent market trends indicate growing interest in integrated systems that combine multiple separation techniques, including IEF, to provide comprehensive protein characterization solutions.
Demand for advanced protein separation technologies is particularly strong in biopharmaceutical development, where the need for highly purified proteins for therapeutic applications continues to drive innovation. The rise of personalized medicine and biologics has further accelerated market growth, with monoclonal antibodies and recombinant proteins requiring sophisticated separation techniques for both research and production.
Academic and research institutions constitute another major market segment, with proteomics research expanding rapidly as scientists seek to understand protein function, interaction, and expression patterns. The demand for higher resolution, reproducibility, and efficiency in protein separation has led to continuous technological advancements in IEF methodologies and equipment.
Geographically, North America dominates the protein separation market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China and India, is experiencing the fastest growth due to increasing investments in biotechnology research and pharmaceutical manufacturing infrastructure.
Key market drivers include technological advancements in separation techniques, growing funding for proteomics research, increasing prevalence of chronic diseases necessitating protein-based diagnostics and therapeutics, and rising demand for personalized medicine approaches. The COVID-19 pandemic has further highlighted the importance of protein analysis technologies for vaccine and therapeutic development.
Market challenges include the high cost of advanced separation equipment, technical complexity requiring specialized training, and regulatory hurdles for clinical applications. Additionally, there is growing demand for miniaturized and automated systems that can provide higher throughput while maintaining separation efficiency.
Customer segments for IEF technologies include pharmaceutical and biotechnology companies (45%), academic and research institutions (30%), clinical diagnostic laboratories (15%), and contract research organizations (10%). Each segment has distinct requirements regarding throughput, resolution, automation, and cost-effectiveness.
The competitive landscape features established players like Thermo Fisher Scientific, Bio-Rad Laboratories, and Agilent Technologies alongside innovative startups developing novel separation technologies. Recent market trends indicate growing interest in integrated systems that combine multiple separation techniques, including IEF, to provide comprehensive protein characterization solutions.
Current Challenges in IEF Efficiency Measurement
Despite significant advancements in isoelectric focusing (IEF) technology, researchers and industry professionals continue to face substantial challenges in accurately measuring protein separation efficiency. The fundamental issue lies in the complex nature of protein mixtures and the multifaceted parameters that influence separation outcomes. Current methodologies often lack standardization, making comparative analyses between different laboratories and experimental setups problematic.
One of the primary challenges is the quantitative assessment of resolution in complex protein mixtures. Traditional approaches rely on measuring the distance between protein bands or peaks, but these methods become increasingly unreliable as sample complexity increases. When dealing with proteomes containing thousands of proteins, overlapping signals and background noise significantly impair accurate measurement of separation efficiency.
The dynamic range limitation presents another significant obstacle. High-abundance proteins often mask the detection of low-abundance species, leading to incomplete efficiency assessments. This becomes particularly problematic in biomarker discovery and clinical applications where proteins of interest may be present at extremely low concentrations relative to abundant proteins like albumin in serum samples.
Reproducibility remains a persistent challenge in IEF efficiency measurements. Environmental factors such as temperature fluctuations, pH gradient stability, and electric field homogeneity can dramatically affect separation outcomes. Even minor variations in these parameters can lead to significant differences in measured efficiency metrics, complicating cross-experimental comparisons and validation studies.
Current detection methods also impose limitations on efficiency measurements. While staining techniques like Coomassie Blue and silver staining are widely used, they exhibit variable sensitivity across different proteins and often have limited linear dynamic ranges. Fluorescence-based detection offers improved sensitivity but introduces its own biases related to labeling efficiency and potential alterations to protein properties.
The time-dependent nature of IEF presents additional complications for efficiency measurements. Protein focusing is not instantaneous, and determining the optimal endpoint for efficiency assessment remains subjective. Premature measurement may underestimate separation potential, while extended focusing times can lead to protein precipitation, band broadening due to diffusion, or cathodic drift, all of which distort efficiency metrics.
Integration of efficiency data across multiple dimensions represents another significant challenge. As IEF is often coupled with other separation techniques in multidimensional approaches, establishing unified metrics that accurately reflect overall separation performance becomes increasingly complex. Current computational tools struggle to integrate efficiency parameters across these different separation dimensions in a meaningful way.
One of the primary challenges is the quantitative assessment of resolution in complex protein mixtures. Traditional approaches rely on measuring the distance between protein bands or peaks, but these methods become increasingly unreliable as sample complexity increases. When dealing with proteomes containing thousands of proteins, overlapping signals and background noise significantly impair accurate measurement of separation efficiency.
The dynamic range limitation presents another significant obstacle. High-abundance proteins often mask the detection of low-abundance species, leading to incomplete efficiency assessments. This becomes particularly problematic in biomarker discovery and clinical applications where proteins of interest may be present at extremely low concentrations relative to abundant proteins like albumin in serum samples.
Reproducibility remains a persistent challenge in IEF efficiency measurements. Environmental factors such as temperature fluctuations, pH gradient stability, and electric field homogeneity can dramatically affect separation outcomes. Even minor variations in these parameters can lead to significant differences in measured efficiency metrics, complicating cross-experimental comparisons and validation studies.
Current detection methods also impose limitations on efficiency measurements. While staining techniques like Coomassie Blue and silver staining are widely used, they exhibit variable sensitivity across different proteins and often have limited linear dynamic ranges. Fluorescence-based detection offers improved sensitivity but introduces its own biases related to labeling efficiency and potential alterations to protein properties.
The time-dependent nature of IEF presents additional complications for efficiency measurements. Protein focusing is not instantaneous, and determining the optimal endpoint for efficiency assessment remains subjective. Premature measurement may underestimate separation potential, while extended focusing times can lead to protein precipitation, band broadening due to diffusion, or cathodic drift, all of which distort efficiency metrics.
Integration of efficiency data across multiple dimensions represents another significant challenge. As IEF is often coupled with other separation techniques in multidimensional approaches, establishing unified metrics that accurately reflect overall separation performance becomes increasingly complex. Current computational tools struggle to integrate efficiency parameters across these different separation dimensions in a meaningful way.
Established Methods for IEF Efficiency Quantification
01 Optimization of pH gradients for improved separation
The efficiency of isoelectric focusing separation can be enhanced by optimizing pH gradients. This involves careful selection and formulation of ampholytes or buffer systems to create stable, well-defined pH gradients across the separation medium. Optimized pH gradients allow proteins and other amphoteric molecules to migrate more precisely to their isoelectric points, resulting in sharper bands and improved resolution. Advanced techniques for controlling gradient formation and stability contribute significantly to separation efficiency.- Optimization of pH gradients for improved separation efficiency: The formation and stability of pH gradients are critical for effective isoelectric focusing. Techniques for optimizing pH gradients include using specialized ampholytes, controlling gradient formation, and maintaining gradient stability during the separation process. These optimizations help to achieve sharper protein bands, better resolution between closely related proteins, and improved reproducibility of separation results.
- Advanced electrode and buffer systems: Innovative electrode designs and buffer compositions can significantly enhance isoelectric focusing separation efficiency. These advancements include specialized electrode materials that reduce electrolysis effects, buffer systems that maintain stable pH conditions, and configurations that minimize protein precipitation at the electrodes. Such improvements lead to more consistent separations, reduced run times, and higher resolution of protein bands.
- Integration with other analytical techniques: Combining isoelectric focusing with complementary analytical methods creates powerful hybrid techniques that enhance overall separation efficiency. These integrations include coupling with mass spectrometry, gel electrophoresis, or chromatographic methods. Such combined approaches allow for multi-dimensional separations that can resolve complex protein mixtures with greater efficiency than single-dimension techniques alone.
- Microfluidic and miniaturized IEF systems: Miniaturization of isoelectric focusing systems using microfluidic platforms offers advantages for separation efficiency. These systems require smaller sample volumes, provide faster analysis times, and often achieve higher resolution separations. Innovations in chip design, channel geometry, and surface treatments have enabled more efficient protein separations while reducing sample consumption and improving throughput.
- Novel carrier ampholytes and additives: Development of new carrier ampholytes and separation additives has significantly improved isoelectric focusing performance. These innovations include synthetic ampholytes with narrower pH ranges, additives that prevent protein aggregation, and compounds that enhance protein solubility throughout the pH gradient. Such advancements result in higher resolution separations, reduced band broadening, and improved detection of low-abundance proteins.
02 Enhanced electrode and buffer systems
Specialized electrode designs and buffer systems play a crucial role in improving isoelectric focusing separation efficiency. Innovative electrode configurations can minimize electrolysis effects, reduce pH drift, and provide more uniform electric fields. Advanced buffer systems help maintain stable pH gradients throughout the separation process, preventing distortion of focusing patterns. These improvements lead to better reproducibility, increased resolution, and enhanced separation of closely related protein variants.Expand Specific Solutions03 Novel separation media and carrier ampholytes
The development of specialized separation media and carrier ampholytes has significantly improved isoelectric focusing efficiency. These include advanced gel formulations, membranes, and capillary systems with optimized pore structures that minimize electroosmotic flow and protein adsorption. Novel carrier ampholytes with enhanced conductivity properties and improved pH range coverage enable more precise focusing of target molecules. These materials contribute to higher resolution, reduced separation times, and improved detection sensitivity.Expand Specific Solutions04 Integration with detection and analysis systems
Integrating isoelectric focusing with advanced detection and analysis systems enhances overall separation efficiency. This includes coupling with mass spectrometry, fluorescence detection, or imaging technologies that enable real-time monitoring of the separation process. Such integrated systems allow for immediate analysis of separated components, reducing sample handling and potential loss. The combination of high-resolution separation with sensitive detection methods improves the identification and characterization of complex protein mixtures.Expand Specific Solutions05 Miniaturization and microfluidic approaches
Miniaturization and microfluidic technologies have revolutionized isoelectric focusing separation efficiency. These approaches utilize microscale channels and chambers to perform separations with minimal sample volumes and reduced analysis times. Microfluidic isoelectric focusing systems offer advantages including faster heat dissipation, more uniform electric fields, and the ability to integrate multiple analytical steps on a single platform. These innovations result in higher throughput, improved resolution, and enhanced reproducibility for complex biological samples.Expand Specific Solutions
Leading Companies in Protein Separation Industry
The isoelectric focusing protein separation technology market is currently in a growth phase, characterized by increasing demand for high-resolution protein analysis tools. The market size is expanding steadily, driven by applications in proteomics research, biopharmaceutical development, and clinical diagnostics. Technologically, the field shows moderate maturity with established players like Bio-Rad Laboratories and ProteinSimple offering commercial platforms, while academic institutions (MIT, University of California) continue to advance fundamental techniques. Research organizations like The Wistar Institute and Genome Institute of Singapore are developing novel applications, while pharmaceutical companies (Regeneron, Roche Diagnostics) are integrating these technologies into their R&D pipelines. Companies like WuXi AppTec and Life Technologies are expanding capabilities through improved reagents and automation, indicating a competitive landscape balanced between established technologies and ongoing innovation.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced isoelectric focusing (IEF) systems that incorporate multi-compartment electrolyzers with isoelectric membranes to improve protein separation efficiency. Their technology utilizes specialized immobilized pH gradient (IPG) strips with enhanced buffer capacity and optimized acrylamide matrices that minimize protein precipitation at their isoelectric points. The company has implemented digital imaging systems with fluorescent detection capabilities that can quantitatively measure separation efficiency through parameters such as peak capacity, resolution between adjacent protein bands, and recovery rates. Their ProteomeWorks platform integrates automated sample handling with sophisticated image analysis software that calculates separation metrics including delta-pI values between adjacent spots and the number of resolved protein spots per pH unit. Bio-Rad has also pioneered the use of orthogonal techniques combining IEF with subsequent chromatographic separations to enhance overall proteome coverage and quantification accuracy.
Strengths: Industry-leading IPG strip technology with superior reproducibility and resolution; comprehensive software solutions for quantitative analysis of separation efficiency; extensive experience in proteomics applications. Weaknesses: Higher cost compared to some competitors; systems may require significant technical expertise to optimize performance parameters.
Amersham Biosciences AB
Technical Solution: Amersham Biosciences (now part of Cytiva/GE Healthcare Life Sciences) has developed sophisticated approaches for measuring protein separation efficiency in isoelectric focusing through their Ettan IPGphor system. Their technology incorporates advanced imaging systems with differential in-gel electrophoresis (DIGE) capabilities that allow for multiplexed analysis of protein samples labeled with spectrally distinct fluorescent dyes. This enables direct comparison of separation efficiency across different experimental conditions within the same gel. Their image analysis software quantifies separation performance through metrics such as spot count, resolution between adjacent protein spots, and signal-to-noise ratios across the pH gradient. Amersham's technology also includes specialized algorithms for calculating focusing time to equilibrium and protein diffusion coefficients after power is turned off. Their systems incorporate internal calibration standards with known isoelectric points to normalize results across different experimental runs, enabling precise measurement of achieved resolution in terms of delta-pI values.
Strengths: Exceptional reproducibility through standardized protocols and equipment; comprehensive software for quantitative analysis; multiplexing capabilities through DIGE technology. Weaknesses: Relatively high cost of consumables; requires specialized training for optimal results; traditional gel-based approach has throughput limitations compared to newer microfluidic systems.
Key Technical Innovations in IEF Resolution Assessment
Method for increasing measurement precision of two-dimensional protein
PatentInactiveUS8262887B2
Innovation
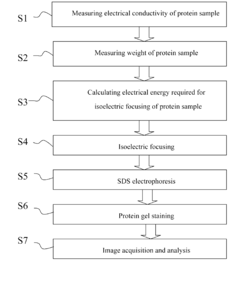
- Measuring the electrical conductivity of a protein sample to determine salt content and using a set of equations to calculate the total electrical energy required for protein and salt focusing, incorporating parameters like protein weight, pH-gradient gel strip length, and pH range.
Device for isoelectric focussing
PatentInactiveUS20050139470A1
Innovation
- An isoelectric focusing (IEF) module with an open channel, microfabricated on a planar substrate, allows for the exposure of the sample along its length, enabling easy access and analysis of proteins, coupled with a MALDI-MS module for efficient protein separation and identification, using a MALDI matrix to enhance ionization and reduce electroosmotic flow.
Validation Standards for IEF Performance
Establishing robust validation standards for Isoelectric Focusing (IEF) performance is critical for ensuring reproducibility and reliability in protein separation experiments. These standards must address multiple aspects of the IEF process, from sample preparation to result interpretation, providing researchers with clear benchmarks against which to measure their experimental outcomes.
The primary validation parameters for IEF performance include resolution, reproducibility, and accuracy of pI determination. Resolution can be quantitatively assessed using the delta pI method, which measures the minimum difference in isoelectric points that can be distinguished. Industry standards typically require a resolution of at least 0.05 pH units for high-performance IEF systems, though this may vary depending on the specific application and pH range.
Reproducibility standards encompass both intra-laboratory and inter-laboratory variability. For intra-laboratory validation, the coefficient of variation (CV) for protein band positions should not exceed 2% across multiple runs under identical conditions. Inter-laboratory standards are more lenient, typically accepting CV values up to 5% due to variations in equipment and environmental factors.
Standard reference materials (SRMs) play a crucial role in IEF validation. These include characterized protein mixtures with known isoelectric points that span the pH range of interest. Organizations such as the National Institute of Standards and Technology (NIST) and the European Commission's Joint Research Centre provide certified reference materials specifically designed for IEF validation. These SRMs should be regularly incorporated into experimental workflows to verify system performance.
Quality control procedures must be established and followed rigorously. These include regular calibration of pH gradients using colored pI markers, verification of power supply performance, and temperature monitoring during separation. Documentation of these quality control measures is essential for compliance with regulatory requirements in clinical and pharmaceutical applications.
Performance qualification protocols should include tests for linearity of the pH gradient, which can be assessed by plotting the positions of marker proteins against their known pI values. The R² value for this relationship should exceed 0.98 for a system to be considered properly validated. Additionally, detection sensitivity standards should be established, typically requiring visualization of protein bands containing as little as 10-50 ng of protein.
Validation standards must also address the stability of the focused protein bands, often quantified as the drift rate in mm/hour after the focusing process has reached equilibrium. Acceptable drift rates vary by application but generally should not exceed 0.5 mm/hour for analytical applications.
The primary validation parameters for IEF performance include resolution, reproducibility, and accuracy of pI determination. Resolution can be quantitatively assessed using the delta pI method, which measures the minimum difference in isoelectric points that can be distinguished. Industry standards typically require a resolution of at least 0.05 pH units for high-performance IEF systems, though this may vary depending on the specific application and pH range.
Reproducibility standards encompass both intra-laboratory and inter-laboratory variability. For intra-laboratory validation, the coefficient of variation (CV) for protein band positions should not exceed 2% across multiple runs under identical conditions. Inter-laboratory standards are more lenient, typically accepting CV values up to 5% due to variations in equipment and environmental factors.
Standard reference materials (SRMs) play a crucial role in IEF validation. These include characterized protein mixtures with known isoelectric points that span the pH range of interest. Organizations such as the National Institute of Standards and Technology (NIST) and the European Commission's Joint Research Centre provide certified reference materials specifically designed for IEF validation. These SRMs should be regularly incorporated into experimental workflows to verify system performance.
Quality control procedures must be established and followed rigorously. These include regular calibration of pH gradients using colored pI markers, verification of power supply performance, and temperature monitoring during separation. Documentation of these quality control measures is essential for compliance with regulatory requirements in clinical and pharmaceutical applications.
Performance qualification protocols should include tests for linearity of the pH gradient, which can be assessed by plotting the positions of marker proteins against their known pI values. The R² value for this relationship should exceed 0.98 for a system to be considered properly validated. Additionally, detection sensitivity standards should be established, typically requiring visualization of protein bands containing as little as 10-50 ng of protein.
Validation standards must also address the stability of the focused protein bands, often quantified as the drift rate in mm/hour after the focusing process has reached equilibrium. Acceptable drift rates vary by application but generally should not exceed 0.5 mm/hour for analytical applications.
Automation Trends in Protein Separation Analysis
The automation landscape in protein separation analysis has undergone significant transformation over the past decade, particularly in isoelectric focusing (IEF) applications. Traditional manual IEF processes required extensive hands-on time for sample preparation, gel loading, and result interpretation, creating bottlenecks in high-throughput protein research environments. Modern automated systems now integrate robotics, microfluidics, and advanced imaging technologies to streamline these workflows.
Recent developments include fully automated IEF platforms that handle multiple samples simultaneously while maintaining precise pH gradient control—a critical factor for accurate protein separation based on isoelectric points. These systems incorporate real-time monitoring capabilities that track protein migration patterns during the separation process, eliminating the need for post-run staining and manual inspection.
Machine learning algorithms have emerged as powerful tools for automated data analysis in IEF applications. These algorithms can identify subtle patterns in protein separation profiles that might be missed by human analysts, enabling more accurate quantification of separation efficiency. Integration of these AI-driven analytical tools with laboratory information management systems (LIMS) creates seamless data workflows from experiment to final report.
Miniaturization represents another significant automation trend, with microchip-based IEF systems reducing sample volume requirements from milliliters to microliters. These platforms incorporate automated sample loading mechanisms and high-resolution digital imaging systems that capture protein band formation with unprecedented clarity. The reduced scale also enables faster analysis times, with some systems completing separations in minutes rather than hours.
Cloud connectivity has transformed collaborative protein research by enabling remote monitoring and control of IEF experiments. Scientists can now initiate separation protocols, adjust parameters, and analyze results from any location, facilitating global research collaboration. These networked systems automatically archive experimental conditions and results, creating comprehensive digital records that enhance reproducibility.
Quality control automation has advanced through the implementation of internal calibration standards and automated performance verification routines. Modern IEF systems can self-diagnose issues such as electrode degradation or buffer depletion, automatically alerting operators before these factors compromise separation efficiency. This predictive maintenance approach minimizes downtime and ensures consistent analytical performance across multiple experiments.
Recent developments include fully automated IEF platforms that handle multiple samples simultaneously while maintaining precise pH gradient control—a critical factor for accurate protein separation based on isoelectric points. These systems incorporate real-time monitoring capabilities that track protein migration patterns during the separation process, eliminating the need for post-run staining and manual inspection.
Machine learning algorithms have emerged as powerful tools for automated data analysis in IEF applications. These algorithms can identify subtle patterns in protein separation profiles that might be missed by human analysts, enabling more accurate quantification of separation efficiency. Integration of these AI-driven analytical tools with laboratory information management systems (LIMS) creates seamless data workflows from experiment to final report.
Miniaturization represents another significant automation trend, with microchip-based IEF systems reducing sample volume requirements from milliliters to microliters. These platforms incorporate automated sample loading mechanisms and high-resolution digital imaging systems that capture protein band formation with unprecedented clarity. The reduced scale also enables faster analysis times, with some systems completing separations in minutes rather than hours.
Cloud connectivity has transformed collaborative protein research by enabling remote monitoring and control of IEF experiments. Scientists can now initiate separation protocols, adjust parameters, and analyze results from any location, facilitating global research collaboration. These networked systems automatically archive experimental conditions and results, creating comprehensive digital records that enhance reproducibility.
Quality control automation has advanced through the implementation of internal calibration standards and automated performance verification routines. Modern IEF systems can self-diagnose issues such as electrode degradation or buffer depletion, automatically alerting operators before these factors compromise separation efficiency. This predictive maintenance approach minimizes downtime and ensures consistent analytical performance across multiple experiments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!