Benchmark Isoelectric Focusing Performance for Protein Analysis
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Evolution and Objectives
Isoelectric focusing (IEF) emerged in the 1960s as a groundbreaking technique for protein separation based on their isoelectric points (pI). The pioneering work of Svensson and Vesterberg established the theoretical foundations and practical applications of this technology. Over subsequent decades, IEF evolved from conventional gel-based systems to more sophisticated capillary and microchip formats, significantly enhancing resolution and analytical capabilities.
The evolution of IEF technology has been characterized by continuous improvements in carrier ampholytes, gel matrices, and detection methods. Traditional polyacrylamide gels have been supplemented or replaced by immobilized pH gradient (IPG) strips, which offer superior reproducibility and resolution. This transition marked a critical advancement in the field, enabling more precise protein characterization and expanding the applicability of IEF across various research domains.
Recent technological developments have focused on miniaturization and automation, with microfluidic IEF systems representing the cutting edge of innovation. These platforms offer advantages including reduced sample consumption, faster analysis times, and potential for integration with other analytical techniques. The incorporation of advanced detection methods, such as fluorescence imaging and mass spectrometry coupling, has further expanded the analytical power of modern IEF systems.
Digital IEF represents another significant advancement, utilizing computational algorithms to enhance data analysis and interpretation. This approach enables more accurate quantification of protein isoforms and post-translational modifications, addressing longstanding challenges in proteomics research. The integration of artificial intelligence and machine learning algorithms is beginning to transform how IEF data is processed and interpreted.
The primary objectives of benchmarking IEF performance for protein analysis encompass several critical dimensions. First, establishing standardized protocols and reference materials to enable meaningful comparisons between different IEF platforms and methodologies. Second, defining key performance metrics including resolution, reproducibility, sensitivity, and dynamic range that collectively determine analytical quality. Third, developing validation strategies to assess the accuracy and reliability of IEF results across diverse protein samples.
Additional objectives include optimizing IEF conditions for challenging protein classes, such as membrane proteins, extremely basic or acidic proteins, and low-abundance species. There is also growing emphasis on enhancing the throughput capabilities of IEF systems to accommodate large-scale proteomics studies, while maintaining analytical rigor. Finally, improving the integration of IEF with complementary techniques, particularly mass spectrometry, remains a key goal for comprehensive protein characterization.
The evolution of IEF technology has been characterized by continuous improvements in carrier ampholytes, gel matrices, and detection methods. Traditional polyacrylamide gels have been supplemented or replaced by immobilized pH gradient (IPG) strips, which offer superior reproducibility and resolution. This transition marked a critical advancement in the field, enabling more precise protein characterization and expanding the applicability of IEF across various research domains.
Recent technological developments have focused on miniaturization and automation, with microfluidic IEF systems representing the cutting edge of innovation. These platforms offer advantages including reduced sample consumption, faster analysis times, and potential for integration with other analytical techniques. The incorporation of advanced detection methods, such as fluorescence imaging and mass spectrometry coupling, has further expanded the analytical power of modern IEF systems.
Digital IEF represents another significant advancement, utilizing computational algorithms to enhance data analysis and interpretation. This approach enables more accurate quantification of protein isoforms and post-translational modifications, addressing longstanding challenges in proteomics research. The integration of artificial intelligence and machine learning algorithms is beginning to transform how IEF data is processed and interpreted.
The primary objectives of benchmarking IEF performance for protein analysis encompass several critical dimensions. First, establishing standardized protocols and reference materials to enable meaningful comparisons between different IEF platforms and methodologies. Second, defining key performance metrics including resolution, reproducibility, sensitivity, and dynamic range that collectively determine analytical quality. Third, developing validation strategies to assess the accuracy and reliability of IEF results across diverse protein samples.
Additional objectives include optimizing IEF conditions for challenging protein classes, such as membrane proteins, extremely basic or acidic proteins, and low-abundance species. There is also growing emphasis on enhancing the throughput capabilities of IEF systems to accommodate large-scale proteomics studies, while maintaining analytical rigor. Finally, improving the integration of IEF with complementary techniques, particularly mass spectrometry, remains a key goal for comprehensive protein characterization.
Market Analysis for Protein Separation Technologies
The protein separation technologies market has witnessed substantial growth in recent years, driven by increasing demand for protein-based therapeutics and advancements in proteomics research. The global market for protein separation technologies was valued at approximately $11.5 billion in 2022 and is projected to reach $19.8 billion by 2028, growing at a CAGR of 9.5% during the forecast period.
Isoelectric focusing (IEF), a critical technique for protein analysis based on isoelectric points, represents a significant segment within this market. The IEF segment accounts for roughly 15% of the overall protein separation technologies market, with consistent growth observed due to its high resolution capabilities and increasing applications in biopharmaceutical quality control.
Geographically, North America dominates the protein separation market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate due to increasing investments in biotechnology research and expanding biopharmaceutical manufacturing capabilities.
By application segment, biopharmaceutical production represents the largest market share (45%), followed by academic research (25%), diagnostic applications (20%), and food analysis (10%). The demand for high-performance IEF systems is particularly strong in biopharmaceutical quality control, where precise protein characterization is essential for regulatory compliance.
Key market drivers include the growing biopharmaceutical pipeline, increasing focus on personalized medicine, technological advancements in separation techniques, and rising funding for proteomics research. The expanding biosimilars market has also created significant demand for analytical techniques that can verify protein similarity and purity.
Market restraints include the high cost of advanced separation systems, technical complexity requiring specialized training, and competition from alternative technologies such as mass spectrometry. Additionally, the COVID-19 pandemic initially disrupted supply chains but subsequently accelerated demand for protein analysis technologies for vaccine development and therapeutic research.
Customer needs analysis reveals growing demand for automated, high-throughput IEF systems with improved reproducibility and sensitivity. End-users increasingly seek integrated solutions that combine IEF with complementary techniques like mass spectrometry or chromatography, enabling comprehensive protein characterization workflows.
The competitive landscape features established players like Bio-Rad, Thermo Fisher Scientific, and Agilent Technologies alongside emerging companies developing novel microfluidic IEF platforms. Recent market trends indicate growing interest in miniaturized, chip-based IEF systems and AI-powered data analysis tools to enhance performance benchmarking capabilities.
Isoelectric focusing (IEF), a critical technique for protein analysis based on isoelectric points, represents a significant segment within this market. The IEF segment accounts for roughly 15% of the overall protein separation technologies market, with consistent growth observed due to its high resolution capabilities and increasing applications in biopharmaceutical quality control.
Geographically, North America dominates the protein separation market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate due to increasing investments in biotechnology research and expanding biopharmaceutical manufacturing capabilities.
By application segment, biopharmaceutical production represents the largest market share (45%), followed by academic research (25%), diagnostic applications (20%), and food analysis (10%). The demand for high-performance IEF systems is particularly strong in biopharmaceutical quality control, where precise protein characterization is essential for regulatory compliance.
Key market drivers include the growing biopharmaceutical pipeline, increasing focus on personalized medicine, technological advancements in separation techniques, and rising funding for proteomics research. The expanding biosimilars market has also created significant demand for analytical techniques that can verify protein similarity and purity.
Market restraints include the high cost of advanced separation systems, technical complexity requiring specialized training, and competition from alternative technologies such as mass spectrometry. Additionally, the COVID-19 pandemic initially disrupted supply chains but subsequently accelerated demand for protein analysis technologies for vaccine development and therapeutic research.
Customer needs analysis reveals growing demand for automated, high-throughput IEF systems with improved reproducibility and sensitivity. End-users increasingly seek integrated solutions that combine IEF with complementary techniques like mass spectrometry or chromatography, enabling comprehensive protein characterization workflows.
The competitive landscape features established players like Bio-Rad, Thermo Fisher Scientific, and Agilent Technologies alongside emerging companies developing novel microfluidic IEF platforms. Recent market trends indicate growing interest in miniaturized, chip-based IEF systems and AI-powered data analysis tools to enhance performance benchmarking capabilities.
Current IEF Challenges in Protein Analysis
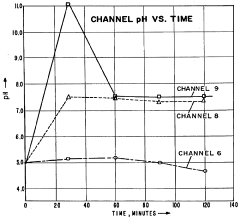
Despite significant advancements in isoelectric focusing (IEF) technology, several persistent challenges continue to limit its effectiveness for comprehensive protein analysis. One of the most significant obstacles remains the resolution of proteins with similar isoelectric points (pI), particularly in complex biological samples. Current IEF systems struggle to reliably separate proteins that differ by less than 0.05 pH units, creating overlapping bands that complicate accurate identification and quantification.
Sample preparation inconsistencies represent another major challenge, as protein solubility varies dramatically across the pH gradient. Hydrophobic proteins and membrane-bound proteins often precipitate at their isoelectric points, leading to poor recovery and underrepresentation in analytical results. This selective loss creates systematic biases in proteomic profiles and limits the technique's applicability for comprehensive proteome analysis.
Reproducibility issues continue to plague IEF applications, with run-to-run variations stemming from multiple sources including temperature fluctuations, ampholyte batch differences, and inconsistent electric field distribution. These variations make standardization difficult and complicate cross-laboratory comparisons, hindering the establishment of reliable benchmarks for protein characterization.
The dynamic range limitation presents a substantial barrier when analyzing biological samples with proteins spanning several orders of magnitude in abundance. High-abundance proteins often mask the detection of low-abundance species, which are frequently the most biologically significant in disease biomarker discovery and systems biology applications. Current IEF platforms typically achieve only 3-4 orders of magnitude in dynamic range, whereas biological samples often contain proteins spanning 6-10 orders of magnitude.
Post-translational modifications (PTMs) add another layer of complexity, as they can significantly alter protein pI values. The heterogeneity of PTMs creates multiple protein species from a single gene product, resulting in complex patterns that current IEF systems struggle to resolve consistently. This is particularly problematic for phosphorylated proteins, which can exhibit dramatic pI shifts depending on their modification state.
Integration with downstream analytical techniques remains suboptimal, with protein recovery from IEF gels or liquid fractions often being incomplete or selective. This creates bottlenecks in proteomic workflows and limits the throughput of IEF-based analyses. The transfer efficiency between IEF and subsequent techniques like mass spectrometry varies significantly between protein classes, introducing additional biases in multi-dimensional protein analysis platforms.
Automation and standardization deficiencies further complicate benchmarking efforts, as many IEF protocols still require significant manual intervention and expert interpretation. The lack of universally accepted performance metrics and reference standards makes objective comparison between different IEF platforms challenging, hindering technology development and clinical translation.
Sample preparation inconsistencies represent another major challenge, as protein solubility varies dramatically across the pH gradient. Hydrophobic proteins and membrane-bound proteins often precipitate at their isoelectric points, leading to poor recovery and underrepresentation in analytical results. This selective loss creates systematic biases in proteomic profiles and limits the technique's applicability for comprehensive proteome analysis.
Reproducibility issues continue to plague IEF applications, with run-to-run variations stemming from multiple sources including temperature fluctuations, ampholyte batch differences, and inconsistent electric field distribution. These variations make standardization difficult and complicate cross-laboratory comparisons, hindering the establishment of reliable benchmarks for protein characterization.
The dynamic range limitation presents a substantial barrier when analyzing biological samples with proteins spanning several orders of magnitude in abundance. High-abundance proteins often mask the detection of low-abundance species, which are frequently the most biologically significant in disease biomarker discovery and systems biology applications. Current IEF platforms typically achieve only 3-4 orders of magnitude in dynamic range, whereas biological samples often contain proteins spanning 6-10 orders of magnitude.
Post-translational modifications (PTMs) add another layer of complexity, as they can significantly alter protein pI values. The heterogeneity of PTMs creates multiple protein species from a single gene product, resulting in complex patterns that current IEF systems struggle to resolve consistently. This is particularly problematic for phosphorylated proteins, which can exhibit dramatic pI shifts depending on their modification state.
Integration with downstream analytical techniques remains suboptimal, with protein recovery from IEF gels or liquid fractions often being incomplete or selective. This creates bottlenecks in proteomic workflows and limits the throughput of IEF-based analyses. The transfer efficiency between IEF and subsequent techniques like mass spectrometry varies significantly between protein classes, introducing additional biases in multi-dimensional protein analysis platforms.
Automation and standardization deficiencies further complicate benchmarking efforts, as many IEF protocols still require significant manual intervention and expert interpretation. The lack of universally accepted performance metrics and reference standards makes objective comparison between different IEF platforms challenging, hindering technology development and clinical translation.
Benchmark Methodologies for IEF Performance
01 Gel composition and preparation for isoelectric focusing
Various gel compositions can be used for isoelectric focusing to improve separation performance. These include polyacrylamide gels, agarose gels, and composite gels with specific additives that enhance resolution and stability. The preparation methods involve controlling polymerization conditions, incorporating carrier ampholytes, and optimizing buffer systems to create pH gradients that remain stable during the focusing process.- Gel composition and preparation for isoelectric focusing: Various gel compositions have been developed to enhance isoelectric focusing performance. These include specialized polyacrylamide formulations, ampholyte mixtures, and gel matrices with optimized pore sizes. The preparation methods involve techniques to ensure uniform polymerization, consistent pH gradients, and minimal electroendosmosis. These gel compositions provide improved resolution, reduced band distortion, and enhanced separation of proteins based on their isoelectric points.
- Apparatus design for improved isoelectric focusing: Advanced apparatus designs have been developed to enhance isoelectric focusing performance. These include specialized cooling systems to prevent overheating, electrode configurations that ensure uniform electric fields, and innovative chamber designs that minimize pH gradient drift. Some apparatus incorporate automated sample loading and detection systems to improve reproducibility and efficiency. These design improvements lead to better resolution, increased throughput, and more reliable results in protein separation.
- Detection and analysis methods in isoelectric focusing: Advanced detection and analysis methods have been developed to enhance the performance of isoelectric focusing. These include fluorescent labeling techniques, real-time imaging systems, and computer-aided analysis of protein bands. Some methods incorporate mass spectrometry for protein identification after separation. These detection methods improve sensitivity, allow for quantitative analysis, and enable the identification of low-abundance proteins in complex mixtures.
- Buffer systems and additives for enhanced separation: Specialized buffer systems and additives have been developed to improve isoelectric focusing performance. These include carrier ampholytes with enhanced pH stability, detergents that prevent protein aggregation, and reagents that reduce electroendosmosis. Some formulations incorporate chaotropic agents to maintain protein solubility throughout the separation process. These buffer systems and additives lead to sharper protein bands, improved resolution of closely related proteins, and reduced background interference.
- Integration with other analytical techniques: Isoelectric focusing performance has been enhanced through integration with other analytical techniques. These include coupling with gel electrophoresis in two-dimensional separations, combination with chromatographic methods, and integration with mass spectrometry. Some approaches incorporate microfluidic platforms for miniaturized analysis. These integrated systems provide comprehensive protein characterization, improved throughput, and enhanced sensitivity for complex biological samples.
02 Equipment and apparatus design for improved IEF
Specialized equipment designs enhance isoelectric focusing performance through improved temperature control, uniform electric field generation, and integrated detection systems. These designs include microfluidic platforms, capillary electrophoresis systems, and multi-dimensional separation devices that minimize protein aggregation and improve resolution. Advanced cooling systems prevent thermal gradients that can distort focusing patterns.Expand Specific Solutions03 Detection and analysis methods for IEF results
Various detection techniques can be employed to analyze isoelectric focusing results with high sensitivity and accuracy. These include fluorescence detection, mass spectrometry coupling, imaging systems, and real-time monitoring technologies. Advanced software algorithms help in quantifying protein bands, determining isoelectric points, and comparing multiple samples for differential analysis.Expand Specific Solutions04 Carrier ampholytes and pH gradient formation
The selection and optimization of carrier ampholytes significantly impact isoelectric focusing performance. Synthetic and natural ampholytes with different pH ranges can be used to create stable pH gradients. Immobilized pH gradients (IPGs) offer advantages in reproducibility and resolution compared to conventional carrier ampholytes. The concentration and distribution of these compounds affect the linearity of the pH gradient and the resolution of separated proteins.Expand Specific Solutions05 Sample preparation and loading techniques
Proper sample preparation is crucial for successful isoelectric focusing. Techniques include protein solubilization methods, removal of interfering substances, and optimization of sample loading strategies. Prefractionation approaches, desalting procedures, and the use of specific additives can prevent protein precipitation at the isoelectric point and improve focusing of difficult samples like membrane proteins or highly basic proteins.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The isoelectric focusing (IEF) protein analysis market is currently in a growth phase, characterized by increasing demand for high-resolution protein separation techniques in biopharmaceutical development and proteomics research. The global market size for protein analysis technologies is expanding rapidly, projected to reach significant valuation as precision medicine and biologics development accelerate. Technologically, IEF benchmarking is evolving from traditional gel-based methods toward automated microfluidic platforms, with companies like Bio-Rad Laboratories, ProteinSimple, and Regeneron Pharmaceuticals leading innovation. Academic institutions including MIT, Peking University, and Shanghai Jiao Tong University contribute fundamental research, while pharmaceutical giants like Novartis leverage advanced IEF techniques for therapeutic protein characterization. The competitive landscape features established analytical instrument providers alongside specialized biotech firms developing next-generation IEF technologies with improved resolution, throughput, and reproducibility.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced isoelectric focusing (IEF) systems specifically designed for high-resolution protein analysis. Their Criterion™ and Mini-PROTEAN® IEF systems utilize immobilized pH gradient (IPG) strips that provide reproducible first-dimension separation for 2D electrophoresis. The company has implemented proprietary ReadyStrip™ IPG technology that offers precise pH gradients (ranging from 3-10, 3-6, 5-8, and 7-10) for optimal protein separation based on isoelectric points. Their benchmark methodology includes standardized protocols for sample preparation, focusing conditions, and quantitative analysis that have been validated across multiple protein types and concentrations. Bio-Rad's systems incorporate advanced power supplies with programmable voltage ramping to prevent protein precipitation during focusing, and their integrated cooling platforms maintain consistent temperatures to enhance reproducibility and prevent protein degradation during extended runs.
Strengths: Industry-leading reproducibility with coefficient of variation <5% across runs; comprehensive ecosystem of compatible reagents and analysis software; extensive validation data across diverse sample types. Weaknesses: Higher initial investment compared to basic systems; requires technical expertise for optimal results; proprietary consumables increase ongoing operational costs.
Intabio LLC
Technical Solution: Intabio (acquired by SCIEX in 2020) developed the Blaze™ system, an innovative platform that combines isoelectric focusing with mass spectrometry for rapid characterization of protein therapeutics. Their proprietary technology integrates capillary isoelectric focusing (cIEF), UV detection, and electrospray ionization in a single automated workflow. This approach enables simultaneous separation, quantification, and identification of protein charge variants in a single 15-minute run, dramatically reducing analysis time compared to conventional multi-step workflows that typically require days. The Blaze system achieves high-resolution separation of charge variants with differences as small as 0.05 pH units and provides direct mass identification of separated components. Intabio's benchmark methodology includes automated sample preparation, focusing, and direct MS integration, with specialized software for data analysis and reporting that meets biopharmaceutical quality control requirements.
Strengths: Unprecedented speed for combined charge variant separation and identification; direct integration with mass spectrometry eliminates sample transfer steps; minimal sample consumption (typically <50μg); comprehensive characterization of post-translational modifications. Weaknesses: Primarily optimized for monoclonal antibody analysis; high initial investment cost; requires expertise in both electrophoresis and mass spectrometry; limited throughput compared to plate-based methods.
Critical Patents and Innovations in IEF
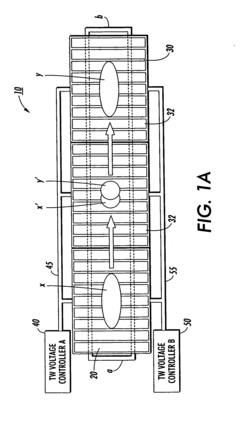
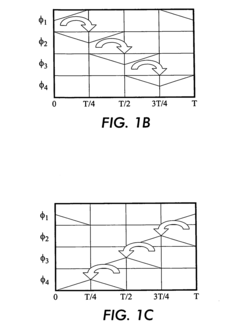
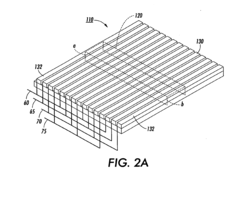
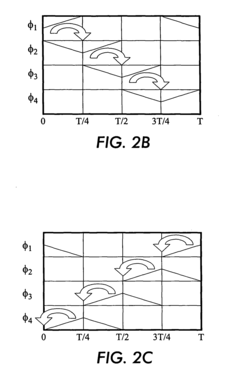
Isoelectric focusing (IEF) of proteins with sequential and oppositely directed traveling waves in gel electrophoresis
PatentInactiveEP1514875A1
Innovation
- The use of electrostatic traveling waves with opposite polarity to biomolecules, administered in sequential sweeps across electrode grids, reduces processing time, lowers operating voltages, and increases resolution by rapidly transporting biomolecules to their isoelectric points within an electrophoretic gel, employing a system with closely spaced parallel electrodes and multi-phase electrical signals.
Separation of proteins using electrodialysis - isoelectric focusing combination
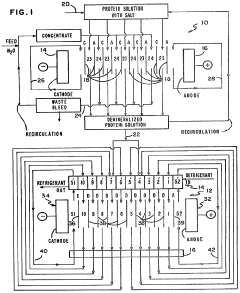
PatentInactiveUS4441978A
Innovation
- The integration of electrodialysis before isoelectric focusing allows for controlled salt removal, creating a demineralized protein solution that stabilizes the pH gradient and prevents protein precipitation, thereby enhancing the efficiency of the separation process.
Standardization Protocols for IEF Benchmarking
Standardization of isoelectric focusing (IEF) benchmarking protocols is essential for ensuring reproducibility and comparability across different laboratories and experimental setups. The establishment of robust standardization protocols begins with the definition of reference materials that serve as controls for system performance. These materials should include a set of well-characterized proteins with known isoelectric points (pIs) spanning the pH range of interest, typically 3-10 for broad-range analyses and narrower ranges for specialized applications.
Protocol standardization must address sample preparation procedures, including protein extraction, purification, and solubilization methods. Standardized buffer compositions, detergent concentrations, and reducing agent levels are critical parameters that significantly impact IEF performance. The inclusion of detailed procedures for sample handling, storage conditions, and freeze-thaw cycle limitations helps minimize pre-analytical variability.
Equipment calibration represents another crucial aspect of IEF standardization. Protocols should specify procedures for pH gradient verification using calibration markers and methods for assessing the linearity and stability of the gradient over time. Power supply settings, including voltage ramping protocols and maximum current limitations, must be clearly defined to ensure consistent field application across different instruments.
Temperature control during IEF separation is particularly important, as even minor temperature fluctuations can dramatically affect protein migration and focusing. Standardized protocols should mandate specific cooling systems and temperature monitoring requirements, with acceptable ranges clearly defined.
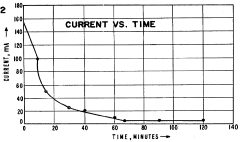
Run time standardization presents unique challenges due to variations in sample complexity. Protocols should establish endpoint determination criteria rather than fixed time points, such as current stabilization below a defined threshold or the cessation of visible protein movement in real-time monitoring systems.
Post-run processing standardization is equally important, encompassing gel fixation, staining procedures, and image acquisition parameters. For digital analysis, protocols must specify software settings, background correction methods, and spot detection algorithms to ensure consistent data interpretation.
Validation metrics form the cornerstone of effective benchmarking, including resolution measurements (typically defined as the minimum pI difference that can be distinguished), reproducibility assessments (coefficient of variation for pI determination), and sensitivity thresholds (minimum detectable protein concentration). These metrics should be determined using the reference materials under standardized conditions.
Documentation requirements complete the standardization framework, mandating detailed recording of all experimental parameters, environmental conditions, and any deviations from standard protocols. This comprehensive approach to standardization enables meaningful comparison of IEF performance across different laboratories and experimental platforms.
Protocol standardization must address sample preparation procedures, including protein extraction, purification, and solubilization methods. Standardized buffer compositions, detergent concentrations, and reducing agent levels are critical parameters that significantly impact IEF performance. The inclusion of detailed procedures for sample handling, storage conditions, and freeze-thaw cycle limitations helps minimize pre-analytical variability.
Equipment calibration represents another crucial aspect of IEF standardization. Protocols should specify procedures for pH gradient verification using calibration markers and methods for assessing the linearity and stability of the gradient over time. Power supply settings, including voltage ramping protocols and maximum current limitations, must be clearly defined to ensure consistent field application across different instruments.
Temperature control during IEF separation is particularly important, as even minor temperature fluctuations can dramatically affect protein migration and focusing. Standardized protocols should mandate specific cooling systems and temperature monitoring requirements, with acceptable ranges clearly defined.
Run time standardization presents unique challenges due to variations in sample complexity. Protocols should establish endpoint determination criteria rather than fixed time points, such as current stabilization below a defined threshold or the cessation of visible protein movement in real-time monitoring systems.
Post-run processing standardization is equally important, encompassing gel fixation, staining procedures, and image acquisition parameters. For digital analysis, protocols must specify software settings, background correction methods, and spot detection algorithms to ensure consistent data interpretation.
Validation metrics form the cornerstone of effective benchmarking, including resolution measurements (typically defined as the minimum pI difference that can be distinguished), reproducibility assessments (coefficient of variation for pI determination), and sensitivity thresholds (minimum detectable protein concentration). These metrics should be determined using the reference materials under standardized conditions.
Documentation requirements complete the standardization framework, mandating detailed recording of all experimental parameters, environmental conditions, and any deviations from standard protocols. This comprehensive approach to standardization enables meaningful comparison of IEF performance across different laboratories and experimental platforms.
Regulatory Compliance for Protein Analysis Methods
Regulatory compliance represents a critical framework governing protein analysis methodologies, particularly for isoelectric focusing (IEF) benchmarking procedures. The regulatory landscape for protein analysis encompasses multiple layers of oversight from international, national, and industry-specific bodies that establish standards ensuring analytical reliability, reproducibility, and safety.
The FDA's guidance documents, specifically 21 CFR Part 211 for pharmaceutical manufacturing and 21 CFR Part 820 for medical devices, outline requirements for validation of analytical methods including IEF when used in regulated product development. These regulations mandate thorough documentation of method performance characteristics, including specificity, accuracy, precision, and robustness—all essential parameters in IEF benchmark development.
International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1) on Validation of Analytical Procedures, provide specific frameworks for validating protein analysis methods. For IEF benchmarking, these guidelines necessitate demonstration of resolution capability, pH gradient stability, and reproducibility across multiple analyses—parameters that directly impact regulatory acceptance of protein characterization data.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain specific monographs addressing electrophoretic techniques for protein analysis. USP chapter <1056> on Biotechnology-Derived Articles and Ph. Eur. chapter 2.2.54 on Isoelectric Focusing establish performance criteria that benchmark IEF methods must satisfy for compliance, including system suitability tests and acceptance criteria.
ISO standards, particularly ISO 17025 for testing laboratories, impose additional requirements on the validation and verification of analytical methods. Laboratories performing IEF benchmarking must demonstrate technical competence through proficiency testing, method validation, and quality control procedures that ensure consistent performance across different operators and equipment.
Emerging regulations in biopharmaceutical development, such as those addressing biosimilars and advanced therapy medicinal products (ATMPs), have introduced more stringent requirements for protein characterization. These regulations specifically address higher-order structure analysis, where IEF plays a crucial role in establishing product comparability and batch-to-batch consistency.
Compliance with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) introduces additional layers of requirements for IEF benchmarking, including instrument qualification, software validation, and data integrity measures. These practices ensure that benchmark data generated through IEF maintains its scientific validity throughout the product lifecycle and can withstand regulatory scrutiny during inspections and submissions.
The FDA's guidance documents, specifically 21 CFR Part 211 for pharmaceutical manufacturing and 21 CFR Part 820 for medical devices, outline requirements for validation of analytical methods including IEF when used in regulated product development. These regulations mandate thorough documentation of method performance characteristics, including specificity, accuracy, precision, and robustness—all essential parameters in IEF benchmark development.
International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1) on Validation of Analytical Procedures, provide specific frameworks for validating protein analysis methods. For IEF benchmarking, these guidelines necessitate demonstration of resolution capability, pH gradient stability, and reproducibility across multiple analyses—parameters that directly impact regulatory acceptance of protein characterization data.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain specific monographs addressing electrophoretic techniques for protein analysis. USP chapter <1056> on Biotechnology-Derived Articles and Ph. Eur. chapter 2.2.54 on Isoelectric Focusing establish performance criteria that benchmark IEF methods must satisfy for compliance, including system suitability tests and acceptance criteria.
ISO standards, particularly ISO 17025 for testing laboratories, impose additional requirements on the validation and verification of analytical methods. Laboratories performing IEF benchmarking must demonstrate technical competence through proficiency testing, method validation, and quality control procedures that ensure consistent performance across different operators and equipment.
Emerging regulations in biopharmaceutical development, such as those addressing biosimilars and advanced therapy medicinal products (ATMPs), have introduced more stringent requirements for protein characterization. These regulations specifically address higher-order structure analysis, where IEF plays a crucial role in establishing product comparability and batch-to-batch consistency.
Compliance with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) introduces additional layers of requirements for IEF benchmarking, including instrument qualification, software validation, and data integrity measures. These practices ensure that benchmark data generated through IEF maintains its scientific validity throughout the product lifecycle and can withstand regulatory scrutiny during inspections and submissions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!