Optimize Ampholyte Gradients for Better Isoelectric Focusing
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ampholyte Gradient Technology Evolution and Objectives
Isoelectric focusing (IEF) has evolved significantly since its introduction in the 1960s as a powerful analytical technique for protein separation. The development of carrier ampholytes represented a pivotal breakthrough, enabling the creation of stable pH gradients essential for effective protein separation based on their isoelectric points. Early ampholytes were limited in range and stability, resulting in suboptimal resolution and reproducibility challenges that hindered broader application of IEF techniques.
The 1970s and 1980s witnessed substantial improvements in ampholyte chemistry, with the introduction of synthetic carrier ampholytes that offered greater pH range specificity and improved gradient stability. These advancements facilitated the integration of IEF with other separation techniques, particularly two-dimensional gel electrophoresis, which became a cornerstone methodology in proteomics research.
The 1990s marked the emergence of immobilized pH gradient (IPG) technology, representing a paradigm shift in the field. IPG strips incorporated covalently bound buffering groups into polyacrylamide gels, addressing many limitations of carrier ampholytes by providing superior gradient stability and reproducibility. This innovation significantly enhanced the analytical capabilities of IEF, particularly for complex protein mixtures.
Recent technological evolution has focused on miniaturization and automation of IEF systems, with microfluidic platforms enabling high-throughput analysis with minimal sample requirements. Concurrently, computational modeling of ampholyte behavior has advanced our understanding of gradient formation dynamics, allowing for more precise optimization of separation parameters.
Despite these advancements, several challenges persist in ampholyte gradient technology. Current limitations include insufficient resolution for closely related protein isoforms, gradient drift during extended separations, and challenges in reproducibility across different experimental setups. These issues are particularly problematic in applications requiring high-precision analysis, such as biomarker discovery and quality control in biopharmaceutical production.
The primary objectives for optimizing ampholyte gradients include enhancing gradient stability over extended separation times, improving resolution particularly in narrow pH ranges, and developing ampholyte formulations tailored for specific protein classes. Additional goals encompass reducing carrier ampholyte interference with downstream analytical techniques, minimizing batch-to-batch variability, and creating environmentally sustainable ampholyte production methods.
Future technological trajectories point toward the development of "smart" ampholytes with stimuli-responsive properties, hybrid systems combining carrier ampholytes with immobilized pH gradients, and novel buffer chemistries capable of maintaining stable gradients under challenging separation conditions. These innovations aim to address the growing demands for higher resolution, reproducibility, and analytical flexibility in proteomics research and biopharmaceutical applications.
The 1970s and 1980s witnessed substantial improvements in ampholyte chemistry, with the introduction of synthetic carrier ampholytes that offered greater pH range specificity and improved gradient stability. These advancements facilitated the integration of IEF with other separation techniques, particularly two-dimensional gel electrophoresis, which became a cornerstone methodology in proteomics research.
The 1990s marked the emergence of immobilized pH gradient (IPG) technology, representing a paradigm shift in the field. IPG strips incorporated covalently bound buffering groups into polyacrylamide gels, addressing many limitations of carrier ampholytes by providing superior gradient stability and reproducibility. This innovation significantly enhanced the analytical capabilities of IEF, particularly for complex protein mixtures.
Recent technological evolution has focused on miniaturization and automation of IEF systems, with microfluidic platforms enabling high-throughput analysis with minimal sample requirements. Concurrently, computational modeling of ampholyte behavior has advanced our understanding of gradient formation dynamics, allowing for more precise optimization of separation parameters.
Despite these advancements, several challenges persist in ampholyte gradient technology. Current limitations include insufficient resolution for closely related protein isoforms, gradient drift during extended separations, and challenges in reproducibility across different experimental setups. These issues are particularly problematic in applications requiring high-precision analysis, such as biomarker discovery and quality control in biopharmaceutical production.
The primary objectives for optimizing ampholyte gradients include enhancing gradient stability over extended separation times, improving resolution particularly in narrow pH ranges, and developing ampholyte formulations tailored for specific protein classes. Additional goals encompass reducing carrier ampholyte interference with downstream analytical techniques, minimizing batch-to-batch variability, and creating environmentally sustainable ampholyte production methods.
Future technological trajectories point toward the development of "smart" ampholytes with stimuli-responsive properties, hybrid systems combining carrier ampholytes with immobilized pH gradients, and novel buffer chemistries capable of maintaining stable gradients under challenging separation conditions. These innovations aim to address the growing demands for higher resolution, reproducibility, and analytical flexibility in proteomics research and biopharmaceutical applications.
Market Analysis for Advanced Isoelectric Focusing Applications
The global market for isoelectric focusing (IEF) technologies has been experiencing steady growth, primarily driven by increasing applications in proteomics research, pharmaceutical development, and clinical diagnostics. The current market size for IEF equipment and consumables is estimated at $1.2 billion, with a compound annual growth rate of 6.8% projected through 2028.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for approximately 45% of the total market share. These industries utilize advanced IEF techniques for protein characterization, quality control of biopharmaceuticals, and development of biosimilars. The optimization of ampholyte gradients directly addresses their need for higher resolution protein separation and improved reproducibility.
Academic and research institutions constitute the second-largest market segment at 30%, where IEF technologies are extensively employed in fundamental proteomics research, biomarker discovery, and educational purposes. This segment shows particular interest in innovative approaches to gradient optimization that can enhance research outcomes while maintaining cost-effectiveness.
Clinical diagnostics represents a rapidly growing segment (18% market share) with increasing adoption of IEF for disease biomarker identification and personalized medicine applications. The remaining market share is distributed among food testing, environmental analysis, and other industrial applications.
Geographically, North America leads the market with 38% share, followed by Europe (32%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the highest growth rate at 8.5% annually, driven by expanding research infrastructure in China, Japan, and South Korea, and increasing adoption of advanced protein analysis techniques.
Key market drivers include the growing emphasis on precision medicine, rising prevalence of protein-based therapeutics, and technological advancements in proteomics research. The demand for higher resolution protein separation with improved reproducibility directly aligns with efforts to optimize ampholyte gradients in IEF systems.
Market challenges include the high cost of advanced IEF equipment, technical complexity requiring specialized training, and competition from alternative protein separation technologies such as mass spectrometry. However, innovations in ampholyte gradient optimization present opportunities to address these challenges by improving performance while potentially reducing operational complexity.
Customer surveys indicate that researchers and laboratory professionals prioritize resolution, reproducibility, and ease of use when selecting IEF technologies. Optimized ampholyte gradients that deliver these benefits would likely command premium pricing and gain significant market traction, particularly in high-value applications such as monoclonal antibody characterization and biomarker validation.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for approximately 45% of the total market share. These industries utilize advanced IEF techniques for protein characterization, quality control of biopharmaceuticals, and development of biosimilars. The optimization of ampholyte gradients directly addresses their need for higher resolution protein separation and improved reproducibility.
Academic and research institutions constitute the second-largest market segment at 30%, where IEF technologies are extensively employed in fundamental proteomics research, biomarker discovery, and educational purposes. This segment shows particular interest in innovative approaches to gradient optimization that can enhance research outcomes while maintaining cost-effectiveness.
Clinical diagnostics represents a rapidly growing segment (18% market share) with increasing adoption of IEF for disease biomarker identification and personalized medicine applications. The remaining market share is distributed among food testing, environmental analysis, and other industrial applications.
Geographically, North America leads the market with 38% share, followed by Europe (32%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the highest growth rate at 8.5% annually, driven by expanding research infrastructure in China, Japan, and South Korea, and increasing adoption of advanced protein analysis techniques.
Key market drivers include the growing emphasis on precision medicine, rising prevalence of protein-based therapeutics, and technological advancements in proteomics research. The demand for higher resolution protein separation with improved reproducibility directly aligns with efforts to optimize ampholyte gradients in IEF systems.
Market challenges include the high cost of advanced IEF equipment, technical complexity requiring specialized training, and competition from alternative protein separation technologies such as mass spectrometry. However, innovations in ampholyte gradient optimization present opportunities to address these challenges by improving performance while potentially reducing operational complexity.
Customer surveys indicate that researchers and laboratory professionals prioritize resolution, reproducibility, and ease of use when selecting IEF technologies. Optimized ampholyte gradients that deliver these benefits would likely command premium pricing and gain significant market traction, particularly in high-value applications such as monoclonal antibody characterization and biomarker validation.
Current Challenges in Ampholyte Gradient Formation
Despite significant advancements in isoelectric focusing (IEF) technology, the formation of stable and reproducible ampholyte gradients remains a persistent challenge in the field. Current commercial carrier ampholytes exhibit batch-to-batch variability, which directly impacts the reproducibility of protein separation and identification. This inconsistency creates significant obstacles for researchers requiring precise and reliable results, particularly in clinical diagnostics and pharmaceutical quality control applications.
The spatial resolution of ampholyte gradients presents another major hurdle. Conventional carrier ampholytes often fail to establish uniform pH gradients across the entire separation medium, resulting in compressed or stretched regions that compromise separation efficiency. These irregularities lead to inconsistent protein migration and clustering of proteins with similar isoelectric points, ultimately reducing the analytical power of IEF techniques.
Temperature control during gradient formation represents a critical challenge that significantly affects gradient stability. Even minor temperature fluctuations can disrupt the established pH gradient, causing drift and distortion during the separation process. This thermal sensitivity necessitates sophisticated temperature regulation systems that add complexity and cost to IEF instrumentation.
The conductivity heterogeneity across the gradient poses additional complications. Regions with varying ionic strength create inconsistent electric field distribution, leading to irregular protein migration rates and distorted separation patterns. This phenomenon, known as "cathodic drift," causes progressive flattening of the pH gradient during extended separations, limiting the practical run time for high-resolution analyses.
Ampholyte-protein interactions further complicate gradient formation and stability. Carrier ampholytes can bind to target proteins, altering their apparent isoelectric points and migration behaviors. These interactions are often unpredictable and depend on the specific properties of both the ampholytes and the proteins being analyzed, making standardization difficult across different sample types.
The limited dynamic range of conventional ampholyte systems restricts their applicability for complex biological samples. Current formulations struggle to maintain stable gradients across wide pH ranges while simultaneously providing high resolution in specific regions of interest. This limitation forces researchers to compromise between range and resolution, often requiring multiple analyses with different ampholyte formulations.
Mobilization of established gradients for detection or collection purposes frequently disrupts the carefully formed pH distribution. Current methodologies for gradient immobilization are either inefficient or introduce additional variables that affect protein recovery and identification. This challenge is particularly significant for preparative applications where maintaining the integrity of separated proteins is essential.
The spatial resolution of ampholyte gradients presents another major hurdle. Conventional carrier ampholytes often fail to establish uniform pH gradients across the entire separation medium, resulting in compressed or stretched regions that compromise separation efficiency. These irregularities lead to inconsistent protein migration and clustering of proteins with similar isoelectric points, ultimately reducing the analytical power of IEF techniques.
Temperature control during gradient formation represents a critical challenge that significantly affects gradient stability. Even minor temperature fluctuations can disrupt the established pH gradient, causing drift and distortion during the separation process. This thermal sensitivity necessitates sophisticated temperature regulation systems that add complexity and cost to IEF instrumentation.
The conductivity heterogeneity across the gradient poses additional complications. Regions with varying ionic strength create inconsistent electric field distribution, leading to irregular protein migration rates and distorted separation patterns. This phenomenon, known as "cathodic drift," causes progressive flattening of the pH gradient during extended separations, limiting the practical run time for high-resolution analyses.
Ampholyte-protein interactions further complicate gradient formation and stability. Carrier ampholytes can bind to target proteins, altering their apparent isoelectric points and migration behaviors. These interactions are often unpredictable and depend on the specific properties of both the ampholytes and the proteins being analyzed, making standardization difficult across different sample types.
The limited dynamic range of conventional ampholyte systems restricts their applicability for complex biological samples. Current formulations struggle to maintain stable gradients across wide pH ranges while simultaneously providing high resolution in specific regions of interest. This limitation forces researchers to compromise between range and resolution, often requiring multiple analyses with different ampholyte formulations.
Mobilization of established gradients for detection or collection purposes frequently disrupts the carefully formed pH distribution. Current methodologies for gradient immobilization are either inefficient or introduce additional variables that affect protein recovery and identification. This challenge is particularly significant for preparative applications where maintaining the integrity of separated proteins is essential.
Contemporary Approaches to Ampholyte Gradient Optimization
01 Ampholyte composition for isoelectric focusing
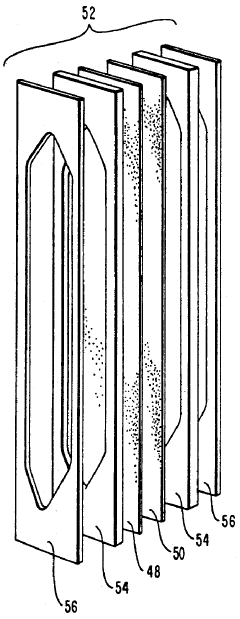
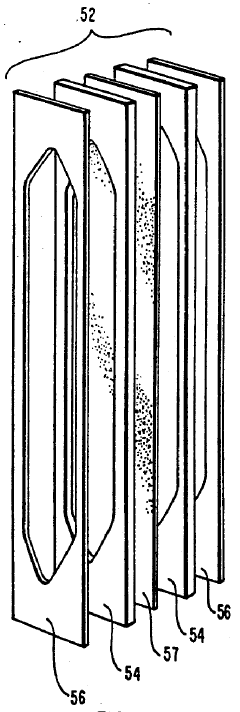
Specific ampholyte compositions are used to create pH gradients for isoelectric focusing. These compositions typically include carrier ampholytes with different isoelectric points that distribute themselves in an electric field to form a stable pH gradient. The ampholytes can be synthetic polymers containing both acidic and basic groups, allowing for precise control of the pH range and resolution of the separation.- Ampholyte composition for isoelectric focusing: Specific ampholyte compositions are used to create pH gradients for isoelectric focusing. These compositions typically include carrier ampholytes with different isoelectric points that distribute themselves in an electric field to form a stable pH gradient. The ampholytes are designed to have good buffering capacity, high conductivity, and solubility to ensure efficient separation of proteins and other biomolecules based on their isoelectric points.
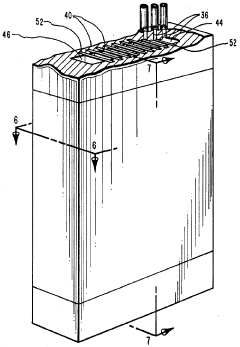
- Immobilized pH gradient techniques: Immobilized pH gradients (IPG) represent an advancement in isoelectric focusing where the pH gradient is created using immobilized buffers covalently linked to a gel matrix. This technique provides more stable gradients compared to carrier ampholytes and allows for better reproducibility and resolution. IPG strips can be prepared with various pH ranges and can be used for two-dimensional electrophoresis applications.
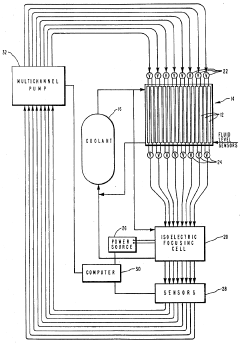
- Microfluidic and capillary-based isoelectric focusing systems: Miniaturized systems for isoelectric focusing utilize microfluidic channels or capillaries to perform separations with minimal sample volumes. These systems often incorporate innovative detection methods and can be integrated with other analytical techniques. The small dimensions allow for rapid analysis, reduced sample consumption, and potential for high-throughput applications in proteomics and diagnostics.
- Novel ampholyte formulations and synthesis methods: Innovations in the synthesis and formulation of ampholytes have led to improved performance in isoelectric focusing. These include the development of specialized ampholytes with narrow pH ranges, enhanced solubility, or specific properties tailored for particular applications. Novel synthetic routes and purification methods have been developed to produce ampholytes with better defined properties and reduced batch-to-batch variation.
- Detection and analysis methods for isoelectric focusing: Various detection techniques have been developed to visualize and analyze the results of isoelectric focusing separations. These include optical methods such as UV absorption, fluorescence, and imaging systems, as well as coupling with mass spectrometry or other analytical techniques. Advanced software and algorithms help in the interpretation of complex separation patterns and identification of separated components.
02 Immobilized pH gradient techniques
Immobilized pH gradients (IPG) represent an advancement in isoelectric focusing where the pH gradient is fixed in a gel matrix rather than formed by mobile carrier ampholytes. This technique uses acrylamide derivatives with buffering groups covalently linked to the gel matrix, providing more stable and reproducible pH gradients. IPG strips can be prepared with various pH ranges and are particularly useful for two-dimensional electrophoresis applications.Expand Specific Solutions03 Microfluidic and capillary isoelectric focusing systems
Miniaturized systems for isoelectric focusing utilize microfluidic channels or capillaries to perform high-resolution separations with minimal sample volumes. These systems often incorporate specialized detection methods and can be integrated with other analytical techniques. The reduced dimensions allow for faster analysis times, improved heat dissipation, and the possibility of parallel processing for high-throughput applications.Expand Specific Solutions04 Novel ampholyte formulations and synthesis methods
Innovative approaches to synthesizing and formulating ampholytes with specific properties have been developed to enhance separation performance. These include methods for creating ampholytes with narrower pI ranges, improved solubility, reduced conductivity, or specialized functionality. Some formulations incorporate non-traditional components such as dendrimers, nanoparticles, or custom-designed polymers to achieve specific separation characteristics or compatibility with downstream analysis.Expand Specific Solutions05 Detection and analysis methods for isoelectric focusing
Advanced detection techniques have been developed to visualize and quantify proteins separated by isoelectric focusing. These include whole-column imaging, fluorescent labeling strategies, and integration with mass spectrometry. Some methods allow for real-time monitoring of the focusing process, while others are optimized for specific applications such as clinical diagnostics or proteomics research. These detection methods enhance the sensitivity and information content obtained from isoelectric focusing separations.Expand Specific Solutions
Leading Companies in Electrophoresis and Separation Science
The isoelectric focusing (IEF) technology market is currently in a growth phase, with increasing applications in proteomics research and biopharmaceutical development. The global market size for electrophoresis techniques, including IEF, is estimated at approximately $2.5 billion, with steady annual growth. Leading players in ampholyte gradient optimization include Bio-Rad Laboratories and Becton, Dickinson & Co., who have established mature commercial platforms, while academic institutions like MIT and Xiamen University are advancing fundamental research. Life Technologies (now part of Thermo Fisher) and Tokyo Electron represent the technological maturity with industrial-scale applications. Research organizations such as PARC and Technion Research & Development Foundation are exploring next-generation approaches to improve resolution and reproducibility, indicating ongoing innovation potential in this specialized but critical separation technology.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. (BD) has developed sophisticated microfluidic platforms for optimizing ampholyte gradients in isoelectric focusing applications. Their approach leverages precision microchannels with controlled surface chemistry to create stable, reproducible pH gradients. BD's technology incorporates specialized electrode designs that generate uniform electric fields across separation channels, minimizing distortion in ampholyte distribution. They've developed proprietary ampholyte formulations with enhanced buffering capacity that maintain gradient stability even with high protein loads. Their systems utilize automated sample introduction mechanisms that prevent disruption of established gradients during loading, improving resolution and reproducibility. BD has also pioneered integrated detection systems that allow real-time monitoring of protein focusing without disturbing the separation process. Additionally, their technology includes thermal management systems that prevent gradient distortion due to Joule heating effects, enabling higher voltage operation and faster separation times while maintaining resolution integrity.
Strengths: Their microfluidic approach enables miniaturization and integration with other analytical techniques, reducing sample requirements and improving throughput. Weaknesses: Their specialized platforms may have limited compatibility with existing laboratory workflows, potentially requiring significant adaptation of established protocols.
Life Technologies Corp.
Technical Solution: Life Technologies (now part of Thermo Fisher Scientific) has developed innovative approaches to ampholyte gradient optimization for isoelectric focusing. Their ZOOM® IEF Fractionator system employs a modular design with interchangeable disks that create discrete pH chambers, allowing for customizable pH gradients with high resolution. This technology enables researchers to create step gradients or continuous gradients depending on application needs. Life Technologies has also developed proprietary ampholyte formulations with enhanced conductivity characteristics that reduce focusing times while maintaining separation integrity. Their approach incorporates specialized non-detergent sulfobetaine compounds that improve protein solubility at isoelectric points, minimizing precipitation issues common in traditional IEF methods. Additionally, they've pioneered automated gradient formation systems that utilize microprocessor-controlled mixing chambers to create highly reproducible ampholyte distributions. Their technology includes specialized membrane barriers that prevent gradient drift during extended focusing periods, significantly improving run-to-run consistency for complex proteomic analyses.
Strengths: Their modular approach offers exceptional flexibility for different sample types and research questions, with excellent integration into downstream mass spectrometry workflows. Weaknesses: The complexity of their systems requires significant initial investment in both equipment and training, and some of their proprietary consumables have limited shelf life, increasing ongoing operational costs.
Key Patents in Carrier Ampholyte Technology
Device for isoelectric focussing
PatentInactiveUS20050139470A1
Innovation
- An isoelectric focusing (IEF) module with an open channel, microfabricated on a planar substrate, allows for the exposure of the sample along its length, enabling easy access and analysis of proteins, coupled with a MALDI-MS module for efficient protein separation and identification, using a MALDI matrix to enhance ionization and reduce electroosmotic flow.
Apparatus and methods for isoelectric focusing of amphoteric substances incorporating ion selective membranes in electrode chambers
PatentInactiveUS5160594A
Innovation
- The use of bipolar membranes or a combination of anion and cation selective membranes to separate electrode compartments from the focusing cell compartments, preventing the passage of ions and maintaining a stable, narrow pH gradient.
Regulatory Standards for Analytical Separation Methods
Regulatory standards for analytical separation methods, particularly those involving isoelectric focusing (IEF) and ampholyte gradient optimization, are governed by multiple international bodies that ensure consistency, reliability, and safety across laboratory practices. The International Organization for Standardization (ISO) has established ISO 13485 and ISO 17025 standards that directly impact electrophoretic separation techniques, requiring validation protocols and quality management systems for laboratories implementing these methods.
The U.S. Food and Drug Administration (FDA) enforces stringent guidelines through 21 CFR Part 211 (Current Good Manufacturing Practice) and ICH Q2(R1) for analytical method validation, which specifically addresses the parameters necessary for optimized ampholyte gradients in IEF. These parameters include linearity, precision, specificity, and robustness—all critical for achieving reproducible pH gradients in analytical separations.
European regulatory frameworks, particularly those from the European Medicines Agency (EMA), provide additional guidance through EMA/CHMP/CVMP/QWP/70278/2012-Rev1, which outlines validation requirements for analytical procedures used in pharmaceutical analysis, including electrophoretic methods. These standards emphasize the importance of demonstrating that ampholyte gradient optimization procedures are fit for their intended purpose.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain specific monographs (USP <1058> and Ph. Eur. 2.2.54) detailing the requirements for electrophoretic separation techniques. These compendial methods establish acceptance criteria for resolution, reproducibility, and stability of pH gradients—factors directly influenced by ampholyte composition and distribution.
Regulatory compliance for optimized ampholyte gradients also necessitates adherence to Good Laboratory Practice (GLP) regulations, which govern the documentation of experimental procedures, instrument calibration, and data integrity. This is particularly relevant when developing novel approaches to gradient optimization, as comprehensive documentation of method development is required for regulatory acceptance.
Recent regulatory trends indicate a movement toward harmonization of analytical method standards across global markets, with initiatives like the International Council for Harmonisation (ICH) working to establish unified guidelines. This harmonization effort is particularly beneficial for researchers working on ampholyte gradient optimization, as it provides clearer pathways for method validation and acceptance across different regulatory jurisdictions.
Compliance with these regulatory standards requires laboratories to implement robust quality control measures, including system suitability tests that verify the performance of optimized ampholyte gradients before analytical runs. These tests typically evaluate parameters such as pH range accuracy, gradient stability over time, and resolution of standard proteins with known isoelectric points.
The U.S. Food and Drug Administration (FDA) enforces stringent guidelines through 21 CFR Part 211 (Current Good Manufacturing Practice) and ICH Q2(R1) for analytical method validation, which specifically addresses the parameters necessary for optimized ampholyte gradients in IEF. These parameters include linearity, precision, specificity, and robustness—all critical for achieving reproducible pH gradients in analytical separations.
European regulatory frameworks, particularly those from the European Medicines Agency (EMA), provide additional guidance through EMA/CHMP/CVMP/QWP/70278/2012-Rev1, which outlines validation requirements for analytical procedures used in pharmaceutical analysis, including electrophoretic methods. These standards emphasize the importance of demonstrating that ampholyte gradient optimization procedures are fit for their intended purpose.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain specific monographs (USP <1058> and Ph. Eur. 2.2.54) detailing the requirements for electrophoretic separation techniques. These compendial methods establish acceptance criteria for resolution, reproducibility, and stability of pH gradients—factors directly influenced by ampholyte composition and distribution.
Regulatory compliance for optimized ampholyte gradients also necessitates adherence to Good Laboratory Practice (GLP) regulations, which govern the documentation of experimental procedures, instrument calibration, and data integrity. This is particularly relevant when developing novel approaches to gradient optimization, as comprehensive documentation of method development is required for regulatory acceptance.
Recent regulatory trends indicate a movement toward harmonization of analytical method standards across global markets, with initiatives like the International Council for Harmonisation (ICH) working to establish unified guidelines. This harmonization effort is particularly beneficial for researchers working on ampholyte gradient optimization, as it provides clearer pathways for method validation and acceptance across different regulatory jurisdictions.
Compliance with these regulatory standards requires laboratories to implement robust quality control measures, including system suitability tests that verify the performance of optimized ampholyte gradients before analytical runs. These tests typically evaluate parameters such as pH range accuracy, gradient stability over time, and resolution of standard proteins with known isoelectric points.
Environmental Impact of Ampholyte Production and Disposal
The production and disposal of ampholytes used in isoelectric focusing (IEF) techniques present significant environmental concerns that warrant careful consideration. Conventional ampholyte synthesis typically involves chemical processes utilizing hazardous reagents such as epichlorohydrin, various amines, and strong acids. These manufacturing processes generate substantial chemical waste streams containing toxic compounds that require specialized treatment before disposal. Research indicates that for every kilogram of carrier ampholytes produced, approximately 15-20 liters of chemical waste may be generated, contributing to industrial pollution when improperly managed.
Environmental monitoring studies have detected ampholyte compounds in wastewater from research facilities and biopharmaceutical manufacturing plants. These compounds demonstrate variable biodegradability profiles, with some synthetic ampholytes showing persistence in aquatic environments. The environmental half-life of complex ampholyte mixtures can range from several weeks to months, potentially disrupting aquatic ecosystems through bioaccumulation in organisms and alteration of natural pH gradients in localized water systems.
Recent life cycle assessments of laboratory techniques have highlighted IEF as having a considerable environmental footprint, primarily due to the production and disposal of specialized chemicals like ampholytes. The carbon footprint associated with synthesizing high-purity ampholytes is estimated at 25-30 kg CO2 equivalent per liter of commercial ampholyte solution, reflecting the energy-intensive purification processes required to achieve the necessary purity for analytical applications.
Regulatory frameworks governing ampholyte disposal vary significantly across regions, creating inconsistent environmental protection standards. In the European Union, the REACH regulation classifies certain ampholytes as substances of environmental concern, requiring specific disposal protocols. In contrast, regulations in developing nations often lack specific provisions for these specialized chemicals, potentially leading to improper disposal practices.
Emerging green chemistry approaches are beginning to address these environmental challenges through the development of bio-based ampholytes derived from renewable resources. These alternatives utilize enzymatic synthesis pathways rather than traditional chemical routes, reducing hazardous waste generation by up to 70%. Additionally, recyclable ampholyte systems that can be recovered and reused across multiple IEF runs are showing promise in reducing the overall environmental impact of the technique.
The transition toward more sustainable ampholyte technologies faces economic barriers, as environmentally friendly alternatives currently command premium prices compared to conventional products. However, as regulatory pressures increase and analytical facilities adopt more comprehensive sustainability metrics, the incentive to develop and implement greener ampholyte technologies continues to grow across the biotechnology and pharmaceutical sectors.
Environmental monitoring studies have detected ampholyte compounds in wastewater from research facilities and biopharmaceutical manufacturing plants. These compounds demonstrate variable biodegradability profiles, with some synthetic ampholytes showing persistence in aquatic environments. The environmental half-life of complex ampholyte mixtures can range from several weeks to months, potentially disrupting aquatic ecosystems through bioaccumulation in organisms and alteration of natural pH gradients in localized water systems.
Recent life cycle assessments of laboratory techniques have highlighted IEF as having a considerable environmental footprint, primarily due to the production and disposal of specialized chemicals like ampholytes. The carbon footprint associated with synthesizing high-purity ampholytes is estimated at 25-30 kg CO2 equivalent per liter of commercial ampholyte solution, reflecting the energy-intensive purification processes required to achieve the necessary purity for analytical applications.
Regulatory frameworks governing ampholyte disposal vary significantly across regions, creating inconsistent environmental protection standards. In the European Union, the REACH regulation classifies certain ampholytes as substances of environmental concern, requiring specific disposal protocols. In contrast, regulations in developing nations often lack specific provisions for these specialized chemicals, potentially leading to improper disposal practices.
Emerging green chemistry approaches are beginning to address these environmental challenges through the development of bio-based ampholytes derived from renewable resources. These alternatives utilize enzymatic synthesis pathways rather than traditional chemical routes, reducing hazardous waste generation by up to 70%. Additionally, recyclable ampholyte systems that can be recovered and reused across multiple IEF runs are showing promise in reducing the overall environmental impact of the technique.
The transition toward more sustainable ampholyte technologies faces economic barriers, as environmentally friendly alternatives currently command premium prices compared to conventional products. However, as regulatory pressures increase and analytical facilities adopt more comprehensive sustainability metrics, the incentive to develop and implement greener ampholyte technologies continues to grow across the biotechnology and pharmaceutical sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!