Is Isoelectric Focusing Compatible with Mass Spectrometry Analysis?
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF-MS Integration Background and Objectives
Isoelectric focusing (IEF) has evolved significantly since its introduction in the 1960s as a high-resolution protein separation technique. This analytical method separates proteins based on their isoelectric points (pI), the pH at which a protein carries no net electrical charge. The historical trajectory of IEF development shows continuous refinement from carrier ampholyte-based systems to immobilized pH gradient (IPG) technologies, which have dramatically improved reproducibility and resolution.
Mass spectrometry (MS) has simultaneously undergone revolutionary advancements, transforming from a specialized analytical tool to an indispensable platform for proteomics research. The development of soft ionization techniques like electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) in the late 1980s and early 1990s enabled the analysis of intact biomolecules, catalyzing the proteomics revolution.
The integration of these two powerful analytical techniques represents a significant opportunity to enhance proteome characterization capabilities. The primary objective of exploring IEF-MS compatibility is to develop robust methodologies that combine the high-resolution separating power of IEF with the exceptional sensitivity and specificity of MS analysis, creating a more comprehensive analytical platform for complex protein mixtures.
Current technological trends indicate growing interest in miniaturized and automated systems that can seamlessly connect separation techniques with MS analysis. The evolution toward microfluidic IEF platforms and direct coupling strategies demonstrates the field's movement toward more integrated analytical workflows.
The technical goals for IEF-MS integration include developing interfaces that preserve the separation achieved by IEF while ensuring efficient transfer to MS analysis, minimizing sample loss and contamination from ampholytes or other IEF components that may interfere with MS performance, and establishing reproducible protocols that maintain protein integrity throughout the analytical process.
Additionally, researchers aim to expand the dynamic range of protein detection, improve the identification of post-translational modifications, and enhance the analysis of low-abundance proteins in complex biological samples. These objectives align with the broader goals of proteomics research to achieve comprehensive protein characterization with high throughput and sensitivity.
Understanding the historical context, current technological landscape, and future objectives of IEF-MS integration provides essential groundwork for evaluating existing approaches and identifying promising directions for further development in this field.
Mass spectrometry (MS) has simultaneously undergone revolutionary advancements, transforming from a specialized analytical tool to an indispensable platform for proteomics research. The development of soft ionization techniques like electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) in the late 1980s and early 1990s enabled the analysis of intact biomolecules, catalyzing the proteomics revolution.
The integration of these two powerful analytical techniques represents a significant opportunity to enhance proteome characterization capabilities. The primary objective of exploring IEF-MS compatibility is to develop robust methodologies that combine the high-resolution separating power of IEF with the exceptional sensitivity and specificity of MS analysis, creating a more comprehensive analytical platform for complex protein mixtures.
Current technological trends indicate growing interest in miniaturized and automated systems that can seamlessly connect separation techniques with MS analysis. The evolution toward microfluidic IEF platforms and direct coupling strategies demonstrates the field's movement toward more integrated analytical workflows.
The technical goals for IEF-MS integration include developing interfaces that preserve the separation achieved by IEF while ensuring efficient transfer to MS analysis, minimizing sample loss and contamination from ampholytes or other IEF components that may interfere with MS performance, and establishing reproducible protocols that maintain protein integrity throughout the analytical process.
Additionally, researchers aim to expand the dynamic range of protein detection, improve the identification of post-translational modifications, and enhance the analysis of low-abundance proteins in complex biological samples. These objectives align with the broader goals of proteomics research to achieve comprehensive protein characterization with high throughput and sensitivity.
Understanding the historical context, current technological landscape, and future objectives of IEF-MS integration provides essential groundwork for evaluating existing approaches and identifying promising directions for further development in this field.
Market Analysis for IEF-MS Applications
The global market for Isoelectric Focusing-Mass Spectrometry (IEF-MS) integration is experiencing significant growth, driven by increasing demand for high-resolution protein analysis in pharmaceutical research, clinical diagnostics, and academic research. The combined market value for proteomics technologies incorporating IEF-MS capabilities reached approximately $4.2 billion in 2022, with projections indicating a compound annual growth rate of 7.8% through 2028.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 45% of the total IEF-MS applications market. This dominance stems from the critical role of protein characterization in drug development pipelines, particularly for biologics and personalized medicine approaches. The ability of integrated IEF-MS systems to provide detailed protein profiling with high throughput capabilities directly addresses industry needs for accelerated development timelines.
Clinical diagnostics represents the fastest-growing application segment, with adoption rates increasing by 12.3% annually. This growth is fueled by the expanding use of proteomics in disease biomarker discovery and validation. Healthcare providers are increasingly recognizing the value of IEF-MS in identifying protein-based indicators for early disease detection, treatment monitoring, and precision medicine applications.
Regionally, North America leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth trajectory, with China and India making substantial investments in proteomics research infrastructure and capabilities. These emerging markets are expected to significantly reshape the competitive landscape over the next five years.
Academic and research institutions constitute a substantial market segment, particularly for specialized IEF-MS instrumentation. This sector values the technology's ability to resolve complex protein mixtures and identify post-translational modifications with unprecedented precision. Government funding initiatives for proteomics research, particularly in cancer and neurodegenerative disease studies, continue to bolster this segment.
The market is characterized by increasing demand for integrated workflow solutions that combine IEF separation with direct MS analysis capabilities. End-users consistently prioritize systems offering reduced sample preparation requirements, higher throughput, improved reproducibility, and seamless data integration. Vendors capable of providing comprehensive solutions addressing these needs are gaining competitive advantage in this rapidly evolving market landscape.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 45% of the total IEF-MS applications market. This dominance stems from the critical role of protein characterization in drug development pipelines, particularly for biologics and personalized medicine approaches. The ability of integrated IEF-MS systems to provide detailed protein profiling with high throughput capabilities directly addresses industry needs for accelerated development timelines.
Clinical diagnostics represents the fastest-growing application segment, with adoption rates increasing by 12.3% annually. This growth is fueled by the expanding use of proteomics in disease biomarker discovery and validation. Healthcare providers are increasingly recognizing the value of IEF-MS in identifying protein-based indicators for early disease detection, treatment monitoring, and precision medicine applications.
Regionally, North America leads the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth trajectory, with China and India making substantial investments in proteomics research infrastructure and capabilities. These emerging markets are expected to significantly reshape the competitive landscape over the next five years.
Academic and research institutions constitute a substantial market segment, particularly for specialized IEF-MS instrumentation. This sector values the technology's ability to resolve complex protein mixtures and identify post-translational modifications with unprecedented precision. Government funding initiatives for proteomics research, particularly in cancer and neurodegenerative disease studies, continue to bolster this segment.
The market is characterized by increasing demand for integrated workflow solutions that combine IEF separation with direct MS analysis capabilities. End-users consistently prioritize systems offering reduced sample preparation requirements, higher throughput, improved reproducibility, and seamless data integration. Vendors capable of providing comprehensive solutions addressing these needs are gaining competitive advantage in this rapidly evolving market landscape.
Technical Challenges in IEF-MS Compatibility
The integration of Isoelectric Focusing (IEF) with Mass Spectrometry (MS) faces several significant technical challenges that have limited its widespread adoption. The fundamental incompatibility stems from the ampholytes used in IEF, which create substantial background noise in MS analysis. These carrier ampholytes, essential for establishing the pH gradient in IEF, ionize efficiently and produce overwhelming signals that can mask the analytes of interest, particularly when dealing with low-abundance proteins or peptides.
Sample recovery presents another major hurdle in the IEF-MS workflow. Proteins focused at their isoelectric points often become less soluble and may precipitate within the gel matrix or capillary. This precipitation phenomenon makes efficient extraction challenging, resulting in significant sample loss before MS analysis and reducing overall sensitivity. The recovery efficiency varies considerably depending on the physicochemical properties of the proteins, introducing bias in the analytical results.
Buffer incompatibility further complicates the IEF-MS interface. Traditional IEF buffers contain components like urea, thiourea, and detergents that are incompatible with electrospray ionization (ESI) sources commonly used in MS. These components can suppress ionization, form adducts, or cause contamination of the ion source, necessitating extensive sample clean-up procedures that add complexity and potential for sample loss.
The dynamic range limitations represent a significant challenge when coupling these techniques. While MS systems have improved their dynamic range capabilities, the combination with IEF introduces additional complexity. Proteins concentrated at specific pI values may create zones of extremely high concentration adjacent to regions with very low abundance species, exceeding the dynamic range capabilities of many MS instruments.
Reproducibility issues plague IEF-MS integration efforts. The establishment of stable pH gradients can vary between runs, affecting the positioning of proteins and subsequent MS identification. This variability is particularly problematic for quantitative proteomics applications where consistent performance is essential for reliable results.
Throughput limitations also restrict the practical application of IEF-MS workflows. The time required for IEF separation (often hours) followed by the necessary sample preparation steps for MS compatibility creates a bottleneck in high-throughput proteomics studies. This extended analysis time contrasts with the relatively rapid LC-MS approaches that have become standard in proteomics research.
Instrumentation complexity presents another barrier, as specialized equipment and interfaces are required to effectively couple IEF with MS. The development of integrated systems has been limited, and most current approaches involve multiple manual sample transfer steps that increase the risk of contamination and sample loss.
Sample recovery presents another major hurdle in the IEF-MS workflow. Proteins focused at their isoelectric points often become less soluble and may precipitate within the gel matrix or capillary. This precipitation phenomenon makes efficient extraction challenging, resulting in significant sample loss before MS analysis and reducing overall sensitivity. The recovery efficiency varies considerably depending on the physicochemical properties of the proteins, introducing bias in the analytical results.
Buffer incompatibility further complicates the IEF-MS interface. Traditional IEF buffers contain components like urea, thiourea, and detergents that are incompatible with electrospray ionization (ESI) sources commonly used in MS. These components can suppress ionization, form adducts, or cause contamination of the ion source, necessitating extensive sample clean-up procedures that add complexity and potential for sample loss.
The dynamic range limitations represent a significant challenge when coupling these techniques. While MS systems have improved their dynamic range capabilities, the combination with IEF introduces additional complexity. Proteins concentrated at specific pI values may create zones of extremely high concentration adjacent to regions with very low abundance species, exceeding the dynamic range capabilities of many MS instruments.
Reproducibility issues plague IEF-MS integration efforts. The establishment of stable pH gradients can vary between runs, affecting the positioning of proteins and subsequent MS identification. This variability is particularly problematic for quantitative proteomics applications where consistent performance is essential for reliable results.
Throughput limitations also restrict the practical application of IEF-MS workflows. The time required for IEF separation (often hours) followed by the necessary sample preparation steps for MS compatibility creates a bottleneck in high-throughput proteomics studies. This extended analysis time contrasts with the relatively rapid LC-MS approaches that have become standard in proteomics research.
Instrumentation complexity presents another barrier, as specialized equipment and interfaces are required to effectively couple IEF with MS. The development of integrated systems has been limited, and most current approaches involve multiple manual sample transfer steps that increase the risk of contamination and sample loss.
Current IEF-MS Interface Solutions
01 Buffer systems and pH gradients for isoelectric focusing
Specialized buffer systems are crucial for establishing stable pH gradients in isoelectric focusing (IEF). These systems typically include ampholytes or immobilized pH gradient (IPG) components that maintain consistent pH environments across the separation medium. Optimized buffer formulations enhance protein separation by ensuring proteins migrate precisely to their isoelectric points, improving resolution and reproducibility of results. The compatibility of these buffer systems with various sample types is essential for effective IEF applications.- Buffer systems and pH gradients for isoelectric focusing: Specialized buffer systems are essential for creating stable pH gradients in isoelectric focusing (IEF). These systems typically include ampholytes or immobilized pH gradient (IPG) components that establish and maintain the pH environment necessary for protein separation based on their isoelectric points. The compatibility of these buffer systems with sample components is critical for achieving high-resolution separation and preventing protein denaturation during the focusing process.
- Gel compositions and materials for IEF applications: Various gel compositions have been developed to enhance compatibility with different sample types in isoelectric focusing. These include polyacrylamide gels, agarose-based gels, and hybrid materials with specific modifications to improve protein separation while minimizing interactions that could interfere with focusing. The physical and chemical properties of these gel materials significantly impact sample compatibility, resolution, and the ability to recover proteins after separation.
- Detection systems compatible with IEF techniques: Detection methods used in conjunction with isoelectric focusing must be compatible with the separation conditions and not interfere with the focusing process. These include UV-visible detection, fluorescence-based systems, and various staining protocols designed to visualize separated proteins without disrupting the pH gradient or causing protein modifications. Advanced detection systems may incorporate real-time monitoring capabilities to track the focusing process as it occurs.
- Sample preparation techniques for improved IEF compatibility: Proper sample preparation is crucial for successful isoelectric focusing. This includes methods for removing contaminants that might interfere with the focusing process, such as salts, detergents, and other charged molecules. Techniques like dialysis, precipitation, and specialized cleanup kits help ensure that samples are compatible with IEF conditions. Additionally, sample loading methods and volumes must be optimized to prevent disruption of the pH gradient and ensure consistent results.
- Microfluidic and miniaturized IEF systems: Miniaturized and microfluidic isoelectric focusing systems offer advantages in terms of sample compatibility, reduced reagent consumption, and faster analysis times. These systems incorporate specialized channel designs, electrode configurations, and surface treatments to enhance compatibility with biological samples. Integration with other analytical techniques allows for comprehensive analysis of complex samples while maintaining the high resolution characteristic of isoelectric focusing.
02 Gel compositions and materials for IEF
Various gel compositions have been developed to enhance isoelectric focusing performance. These include polyacrylamide, agarose, and hybrid gel matrices with specific modifications to improve compatibility with different protein samples. The physical and chemical properties of these gels, such as pore size, hydrophilicity, and electrical conductivity, significantly impact protein migration and resolution. Advanced gel formulations may incorporate specialized additives to minimize protein aggregation and adsorption while maintaining compatibility with downstream analytical techniques.Expand Specific Solutions03 Sample preparation techniques for IEF compatibility
Effective sample preparation is critical for successful isoelectric focusing. This includes methods for removing interfering substances such as salts, detergents, and lipids that can disrupt pH gradients or cause protein precipitation. Specialized protocols have been developed for different sample types, including biological fluids, cell lysates, and tissue extracts. Compatibility enhancers such as chaotropic agents, reducing agents, and solubilizing additives help maintain protein solubility throughout the focusing process while preserving native charge characteristics.Expand Specific Solutions04 Microfluidic and miniaturized IEF systems
Miniaturized isoelectric focusing systems offer advantages in terms of sample volume requirements, analysis speed, and integration with other analytical techniques. These systems employ specialized microchannels, electrodes, and detection methods compatible with microscale separations. Innovations in surface treatments and channel geometries improve compatibility with diverse biomolecules while minimizing protein adsorption to surfaces. These platforms often incorporate novel materials and fabrication techniques to enhance separation efficiency while maintaining compatibility with biological samples.Expand Specific Solutions05 Detection and analysis methods compatible with IEF
Various detection methods have been developed to analyze proteins separated by isoelectric focusing. These include UV-visible spectroscopy, fluorescence detection, mass spectrometry, and immunological techniques. Each detection method requires specific compatibility considerations to ensure accurate protein identification and quantification. Staining protocols, imaging systems, and data analysis software have been optimized to work seamlessly with IEF separations, allowing for high-sensitivity detection while maintaining compatibility with the separation conditions used during focusing.Expand Specific Solutions
Leading Manufacturers and Research Groups
The isoelectric focusing (IEF) and mass spectrometry (MS) integration market is in a growth phase, with increasing adoption across proteomics and biopharmaceutical applications. The global market size is expanding rapidly due to rising demand for high-resolution protein characterization. Technologically, compatibility between IEF and MS has evolved from challenging to well-established, with companies like Thermo Fisher Scientific, Agilent Technologies, and Waters Corporation (via Micromass UK) leading innovation. These established players offer integrated solutions while emerging companies like Refeyn and Intabio are developing specialized technologies. Academic institutions including MIT and University of California collaborate with industry to advance methodologies, particularly for complex protein analysis. The technology continues to mature with improvements in sample preparation, instrumentation interfaces, and data analysis workflows.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed an integrated approach combining capillary isoelectric focusing (cIEF) with mass spectrometry (MS) through their OffGel fractionation technology. Their solution employs a specialized interface that allows for the collection of separated protein fractions from IEF gels which can then be directly analyzed by LC-MS. The system includes proprietary software for data integration and analysis that correlates the isoelectric point information with mass spectral data. Agilent's approach addresses the salt compatibility issues by incorporating a desalting step between the IEF separation and MS analysis, effectively removing ampholytes and other MS-interfering compounds. Their technology enables high-resolution protein characterization by combining the orthogonal separation mechanisms of pI-based fractionation with mass-based identification, particularly valuable for complex proteome analysis and post-translational modification studies.
Strengths: High throughput capability with automated fraction collection; excellent reproducibility; comprehensive software integration for data analysis. Weaknesses: The multi-step process introduces potential sample loss; requires specialized equipment and expertise; the desalting step adds complexity and time to the workflow.
Intabio LLC
Technical Solution: Intabio has developed the Blaze™ system, a revolutionary platform that integrates capillary isoelectric focusing with mass spectrometry for biopharmaceutical analysis. Their technology employs a microchip-based approach that performs IEF separation followed by an innovative "mobilization" step that transports focused proteins directly to ESI-MS detection. The system incorporates a proprietary interface that effectively removes ampholytes and other MS-interfering compounds before ionization. Intabio's platform features automated sample preparation and analysis, significantly reducing hands-on time compared to traditional methods. The technology is particularly optimized for charge variant analysis of therapeutic proteins, allowing simultaneous determination of isoelectric point and molecular mass in a single run. Their system includes specialized software for data interpretation that provides detailed characterization of post-translational modifications and other protein variants.
Strengths: Fully integrated workflow with minimal sample handling; rapid analysis (15 minutes per sample); excellent reproducibility; purpose-built for biopharmaceutical applications. Weaknesses: Limited flexibility for customization; primarily focused on protein therapeutics rather than general proteomics; relatively new technology with evolving capabilities.
Key Patents and Publications in IEF-MS Technology
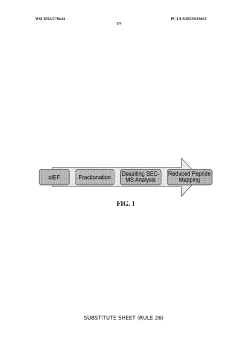
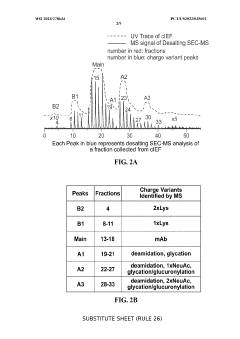
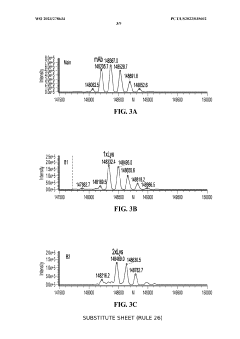
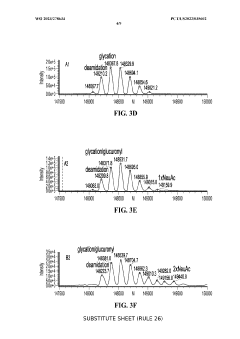
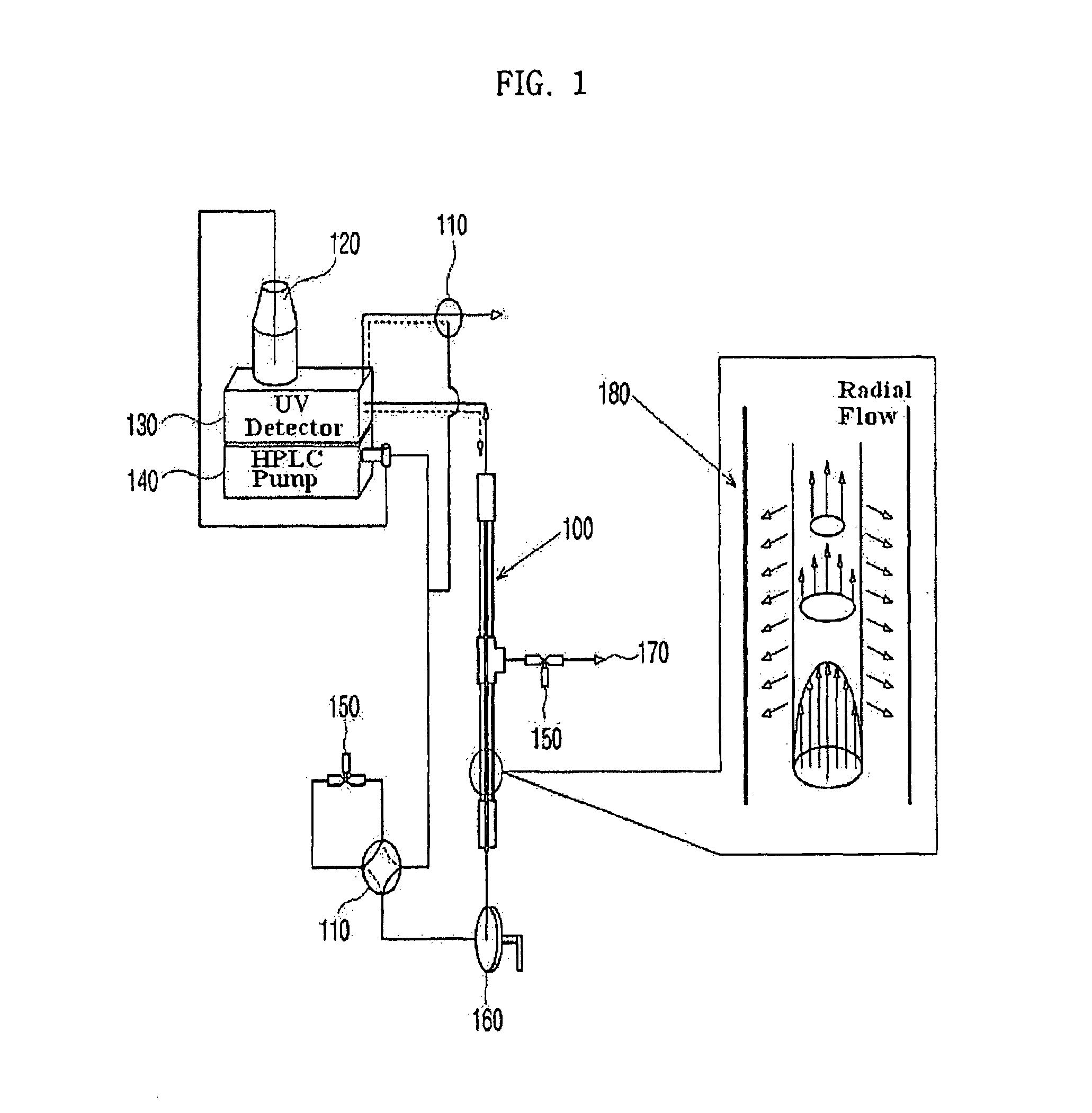
Coupling isoelectric focusing-based fractionation with mass spectrometry analysis
PatentWO2023278634A1
Innovation
- A method involving capillary isoelectric focusing with high-throughput fraction collection, followed by desalting size exclusion chromatography to modify the buffer for mass spectrometry compatibility, and subsequent mass spectrometry analysis for identifying site-specific protein modifications, allowing for high-resolution and high-throughput characterization of charge variants.
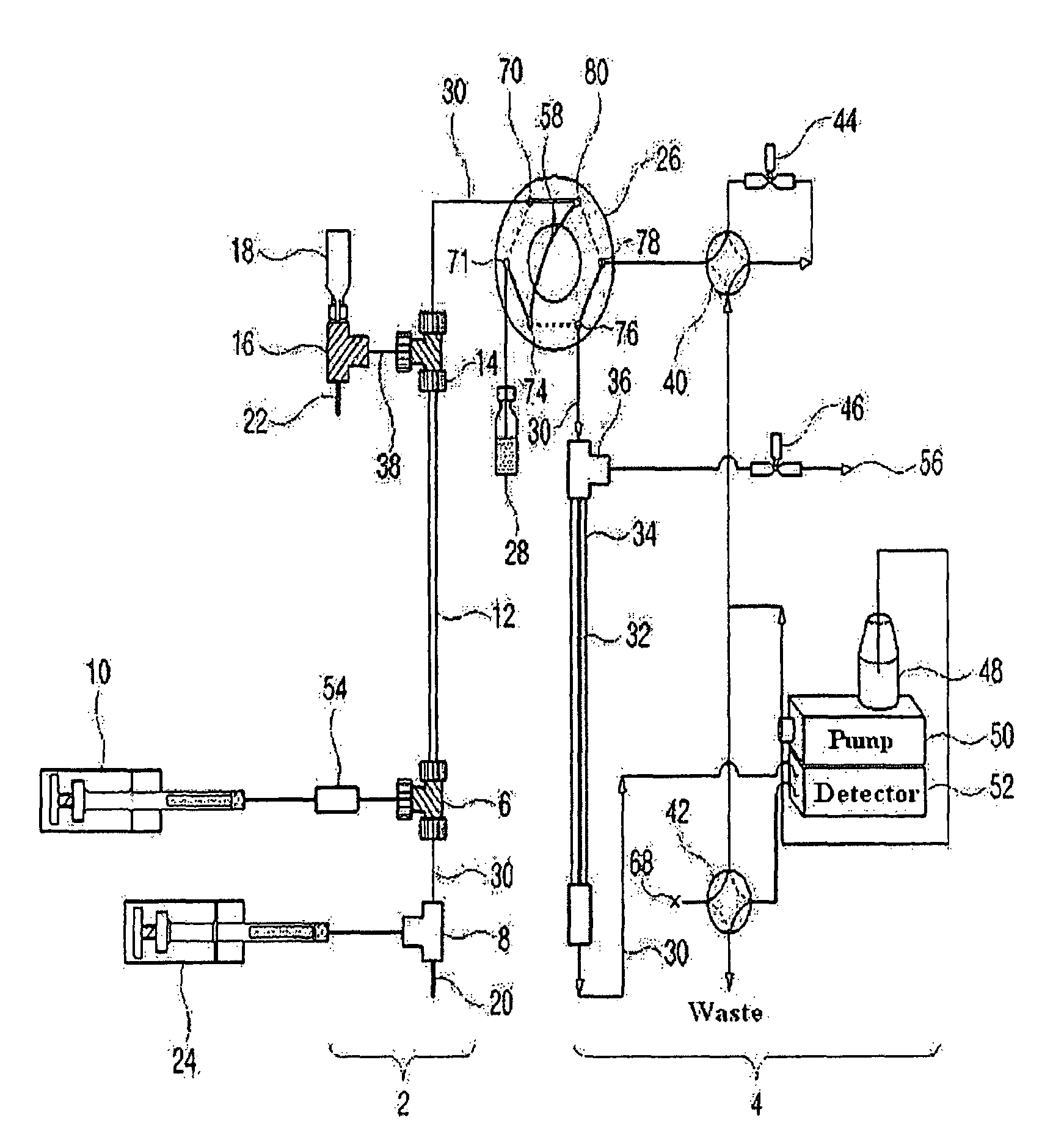
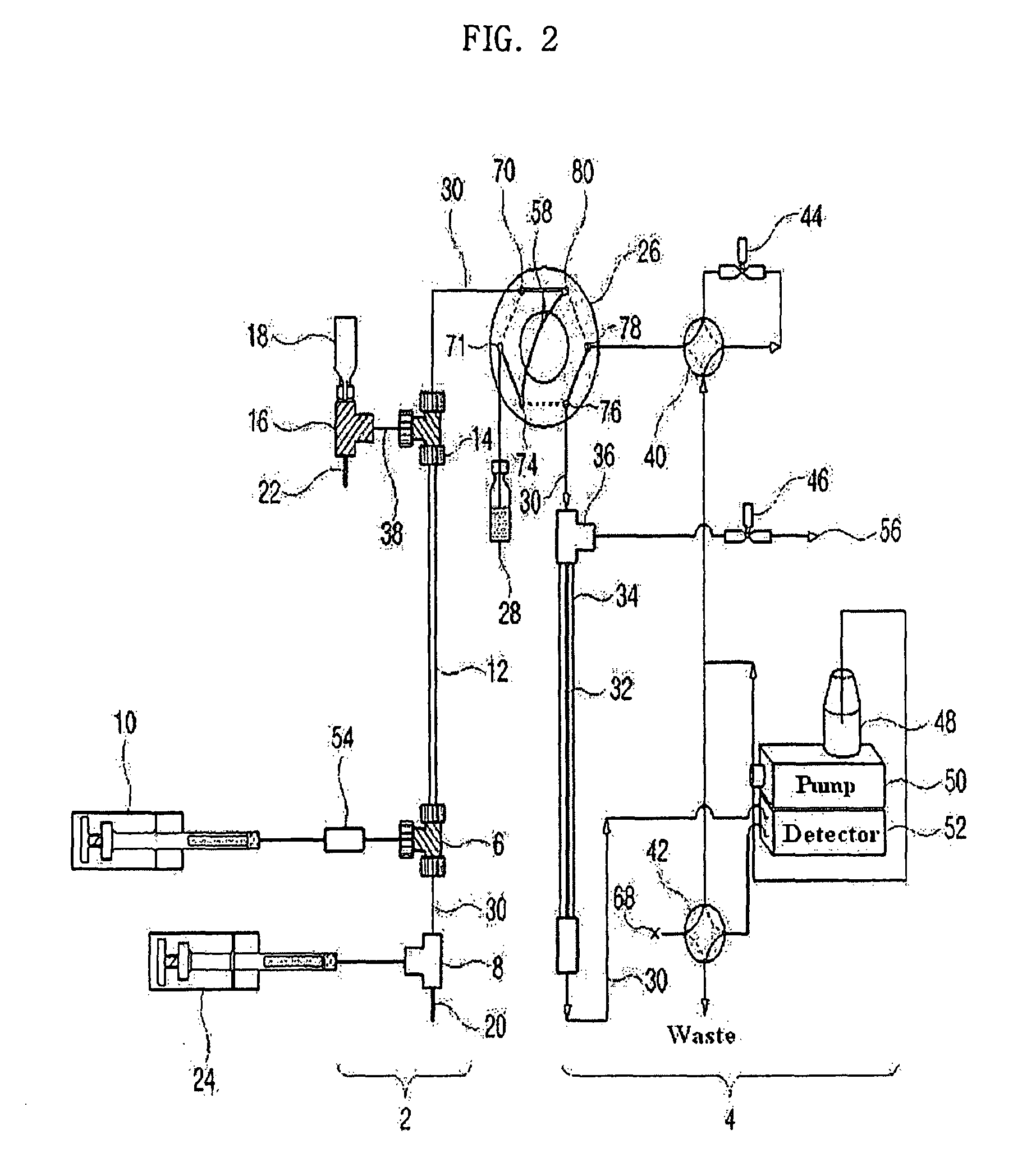
Apparatus for protein separation using capillary isoelectric focusing-hollow fiber flow field flow fractionation and method thereof
PatentInactiveUS8585884B2
Innovation
- A capillary isoelectric focusing-hollow fiber flow field flow fractionation apparatus that separates proteins based on isoelectric point (pI) and molecular weight in a two-dimensional, non-gel, and liquid phase manner, using a capillary isoelectric focusing unit connected to a hollow fiber flow field flow fractionation unit, allowing for automatic removal of ampholytes and avoiding protein denaturation.
Sample Preparation Optimization for IEF-MS
Optimizing sample preparation for Isoelectric Focusing coupled with Mass Spectrometry (IEF-MS) requires careful consideration of multiple factors to ensure compatibility between these two powerful analytical techniques. The primary challenge lies in reconciling the buffer systems and additives used in IEF with the stringent requirements of MS analysis.
Carrier ampholytes, essential for establishing pH gradients in IEF, can significantly suppress ionization in MS and contribute to background noise. Recent developments have focused on reducing ampholyte concentrations while maintaining effective pH gradient formation. Research indicates that using narrow-range ampholytes at concentrations below 0.5% can minimize interference while preserving separation efficiency.
Detergents present another critical consideration in IEF-MS sample preparation. While SDS and other ionic detergents are incompatible with MS due to ion suppression effects, MS-compatible alternatives such as acid-labile surfactants have shown promise. These surfactants maintain protein solubility during IEF but decompose under acidic conditions prior to MS analysis, significantly reducing interference.
Salt removal represents a crucial step in sample preparation workflows. High salt concentrations from biological samples can disrupt the electric field in IEF and cause ion suppression in MS. Implementation of desalting protocols using spin columns or dialysis membranes prior to IEF has demonstrated improved resolution in subsequent MS analysis, with studies showing up to 30% increase in peptide identification rates.
Protein extraction methods must be carefully selected to maintain compatibility across the workflow. Chaotropic agents like urea at concentrations below 4M have proven effective for protein solubilization while remaining compatible with both IEF and MS. However, prolonged exposure to urea can lead to carbamylation artifacts, necessitating time-controlled protocols.
Recent innovations include the development of immobilized pH gradient (IPG) strips specifically designed for MS compatibility, featuring reduced background contaminants and optimized ampholyte formulations. These specialized materials have shown a 40-60% improvement in protein identification rates compared to conventional IPG strips when coupled with MS analysis.
Temperature control during sample preparation and IEF separation has emerged as a critical parameter, with studies demonstrating that maintaining samples below 20°C throughout the workflow minimizes protein modifications that can complicate MS interpretation. Implementation of cooling systems during IEF runs has become standard practice in high-performance IEF-MS protocols.
Carrier ampholytes, essential for establishing pH gradients in IEF, can significantly suppress ionization in MS and contribute to background noise. Recent developments have focused on reducing ampholyte concentrations while maintaining effective pH gradient formation. Research indicates that using narrow-range ampholytes at concentrations below 0.5% can minimize interference while preserving separation efficiency.
Detergents present another critical consideration in IEF-MS sample preparation. While SDS and other ionic detergents are incompatible with MS due to ion suppression effects, MS-compatible alternatives such as acid-labile surfactants have shown promise. These surfactants maintain protein solubility during IEF but decompose under acidic conditions prior to MS analysis, significantly reducing interference.
Salt removal represents a crucial step in sample preparation workflows. High salt concentrations from biological samples can disrupt the electric field in IEF and cause ion suppression in MS. Implementation of desalting protocols using spin columns or dialysis membranes prior to IEF has demonstrated improved resolution in subsequent MS analysis, with studies showing up to 30% increase in peptide identification rates.
Protein extraction methods must be carefully selected to maintain compatibility across the workflow. Chaotropic agents like urea at concentrations below 4M have proven effective for protein solubilization while remaining compatible with both IEF and MS. However, prolonged exposure to urea can lead to carbamylation artifacts, necessitating time-controlled protocols.
Recent innovations include the development of immobilized pH gradient (IPG) strips specifically designed for MS compatibility, featuring reduced background contaminants and optimized ampholyte formulations. These specialized materials have shown a 40-60% improvement in protein identification rates compared to conventional IPG strips when coupled with MS analysis.
Temperature control during sample preparation and IEF separation has emerged as a critical parameter, with studies demonstrating that maintaining samples below 20°C throughout the workflow minimizes protein modifications that can complicate MS interpretation. Implementation of cooling systems during IEF runs has become standard practice in high-performance IEF-MS protocols.
Regulatory Considerations for IEF-MS Methods
The regulatory landscape for Isoelectric Focusing coupled with Mass Spectrometry (IEF-MS) methods encompasses multiple layers of oversight that researchers and manufacturers must navigate. In clinical diagnostics, IEF-MS methods fall under the purview of regulatory bodies such as the FDA in the United States, the EMA in Europe, and similar organizations worldwide. These agencies require extensive validation of analytical methods, particularly those used for protein characterization in biopharmaceutical development.
For IEF-MS applications in clinical settings, compliance with Clinical Laboratory Improvement Amendments (CLIA) standards is mandatory in the US, ensuring the accuracy, reliability, and timeliness of patient test results. Additionally, the College of American Pathologists (CAP) accreditation may be required for laboratories implementing these techniques for diagnostic purposes.
When IEF-MS is employed in pharmaceutical development, adherence to Good Manufacturing Practices (GMP) and International Conference on Harmonisation (ICH) guidelines becomes essential. Specifically, ICH Q6B guidelines address the characterization of biotechnological products, where IEF-MS can play a crucial role in establishing product specifications and monitoring batch-to-batch consistency.
Data integrity represents another critical regulatory consideration. Regulatory bodies increasingly scrutinize the robustness of data handling systems, requiring comprehensive audit trails and validation of software used in IEF-MS analysis. This is particularly relevant as the technique generates complex datasets that require sophisticated processing algorithms.
Method validation parameters for IEF-MS must be rigorously established, including specificity, accuracy, precision, detection limit, quantitation limit, linearity, and range. The validation approach should be tailored to the intended application, whether for identity testing, purity assessment, or quantitative determination of protein variants.
Environmental considerations also factor into regulatory compliance for IEF-MS methods. The disposal of chemical waste, particularly ampholytes and other reagents used in IEF, must comply with local environmental regulations. Furthermore, laboratories must implement appropriate safety measures for handling potentially hazardous materials used in sample preparation and analysis.
As IEF-MS technologies continue to evolve, regulatory frameworks are adapting to accommodate novel applications. Regulatory agencies are increasingly adopting risk-based approaches to method validation, focusing on critical quality attributes that impact product safety and efficacy. This evolution presents both challenges and opportunities for organizations implementing IEF-MS methods in regulated environments.
For IEF-MS applications in clinical settings, compliance with Clinical Laboratory Improvement Amendments (CLIA) standards is mandatory in the US, ensuring the accuracy, reliability, and timeliness of patient test results. Additionally, the College of American Pathologists (CAP) accreditation may be required for laboratories implementing these techniques for diagnostic purposes.
When IEF-MS is employed in pharmaceutical development, adherence to Good Manufacturing Practices (GMP) and International Conference on Harmonisation (ICH) guidelines becomes essential. Specifically, ICH Q6B guidelines address the characterization of biotechnological products, where IEF-MS can play a crucial role in establishing product specifications and monitoring batch-to-batch consistency.
Data integrity represents another critical regulatory consideration. Regulatory bodies increasingly scrutinize the robustness of data handling systems, requiring comprehensive audit trails and validation of software used in IEF-MS analysis. This is particularly relevant as the technique generates complex datasets that require sophisticated processing algorithms.
Method validation parameters for IEF-MS must be rigorously established, including specificity, accuracy, precision, detection limit, quantitation limit, linearity, and range. The validation approach should be tailored to the intended application, whether for identity testing, purity assessment, or quantitative determination of protein variants.
Environmental considerations also factor into regulatory compliance for IEF-MS methods. The disposal of chemical waste, particularly ampholytes and other reagents used in IEF, must comply with local environmental regulations. Furthermore, laboratories must implement appropriate safety measures for handling potentially hazardous materials used in sample preparation and analysis.
As IEF-MS technologies continue to evolve, regulatory frameworks are adapting to accommodate novel applications. Regulatory agencies are increasingly adopting risk-based approaches to method validation, focusing on critical quality attributes that impact product safety and efficacy. This evolution presents both challenges and opportunities for organizations implementing IEF-MS methods in regulated environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!