Designing Isoelectric Focusing for Microfluidic System Integration
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic IEF Technology Background and Objectives
Isoelectric focusing (IEF) represents a powerful analytical technique that has evolved significantly since its introduction in the 1960s. This electrophoretic method separates amphoteric molecules, particularly proteins, based on their isoelectric points (pI) within a pH gradient. The integration of IEF with microfluidic systems marks a pivotal advancement in analytical chemistry and biomedical diagnostics, combining the high-resolution separation capabilities of IEF with the advantages of miniaturized platforms.
The historical trajectory of IEF technology began with conventional gel-based systems, progressing through capillary formats, and now advancing toward fully integrated microfluidic implementations. This evolution reflects broader trends in analytical instrumentation toward miniaturization, automation, and enhanced performance metrics. The microfluidic approach to IEF addresses limitations of traditional formats, including lengthy analysis times, substantial sample requirements, and limited portability.
Current technological developments in microfluidic IEF focus on creating stable pH gradients within microscale channels, optimizing electrode configurations for uniform electric field distribution, and developing surface treatments to minimize electroosmotic flow disruptions. These advancements aim to enhance separation resolution while maintaining the inherent benefits of microfluidic platforms, such as reduced reagent consumption and accelerated analysis times.
The primary objective of microfluidic IEF technology development is to establish robust, reproducible, and high-performance separation platforms suitable for integration into comprehensive lab-on-a-chip systems. This integration represents a critical step toward realizing portable, automated analytical devices capable of complex bioanalytical procedures with minimal user intervention. Such systems hold particular promise for point-of-care diagnostics, pharmaceutical development, and proteomics research.
Emerging trends in this field include the development of novel materials for pH gradient formation, implementation of advanced detection methodologies compatible with microscale dimensions, and exploration of multidimensional separation approaches that combine IEF with complementary techniques. Additionally, computational modeling increasingly informs design parameters, enabling optimization of channel geometries and operating conditions prior to physical implementation.
The convergence of microfluidic engineering principles with established electrophoretic theory presents unique opportunities for innovation. By addressing the technical challenges of miniaturization while preserving or enhancing separation performance, microfluidic IEF systems stand to revolutionize protein analysis workflows across multiple scientific and industrial sectors.
As the field progresses, key performance metrics guiding development include separation resolution, analysis speed, sample throughput, and system robustness. The ultimate technological goal remains the creation of fully integrated, automated analytical platforms that deliver laboratory-quality protein separations in compact, user-friendly formats suitable for deployment across diverse settings from clinical laboratories to field research environments.
The historical trajectory of IEF technology began with conventional gel-based systems, progressing through capillary formats, and now advancing toward fully integrated microfluidic implementations. This evolution reflects broader trends in analytical instrumentation toward miniaturization, automation, and enhanced performance metrics. The microfluidic approach to IEF addresses limitations of traditional formats, including lengthy analysis times, substantial sample requirements, and limited portability.
Current technological developments in microfluidic IEF focus on creating stable pH gradients within microscale channels, optimizing electrode configurations for uniform electric field distribution, and developing surface treatments to minimize electroosmotic flow disruptions. These advancements aim to enhance separation resolution while maintaining the inherent benefits of microfluidic platforms, such as reduced reagent consumption and accelerated analysis times.
The primary objective of microfluidic IEF technology development is to establish robust, reproducible, and high-performance separation platforms suitable for integration into comprehensive lab-on-a-chip systems. This integration represents a critical step toward realizing portable, automated analytical devices capable of complex bioanalytical procedures with minimal user intervention. Such systems hold particular promise for point-of-care diagnostics, pharmaceutical development, and proteomics research.
Emerging trends in this field include the development of novel materials for pH gradient formation, implementation of advanced detection methodologies compatible with microscale dimensions, and exploration of multidimensional separation approaches that combine IEF with complementary techniques. Additionally, computational modeling increasingly informs design parameters, enabling optimization of channel geometries and operating conditions prior to physical implementation.
The convergence of microfluidic engineering principles with established electrophoretic theory presents unique opportunities for innovation. By addressing the technical challenges of miniaturization while preserving or enhancing separation performance, microfluidic IEF systems stand to revolutionize protein analysis workflows across multiple scientific and industrial sectors.
As the field progresses, key performance metrics guiding development include separation resolution, analysis speed, sample throughput, and system robustness. The ultimate technological goal remains the creation of fully integrated, automated analytical platforms that deliver laboratory-quality protein separations in compact, user-friendly formats suitable for deployment across diverse settings from clinical laboratories to field research environments.
Market Analysis for Integrated Microfluidic Separation Systems
The global market for integrated microfluidic separation systems has experienced significant growth in recent years, driven by increasing demand for point-of-care diagnostics, personalized medicine, and advanced analytical tools. The market specifically for isoelectric focusing (IEF) within microfluidic platforms is projected to grow at a compound annual growth rate of 12.3% through 2028, reflecting the technology's expanding applications across multiple industries.
Healthcare and pharmaceutical sectors represent the largest market segments, accounting for approximately 65% of the total market share. The integration of IEF with microfluidic systems offers substantial benefits in these sectors, including reduced sample volumes, faster analysis times, and improved resolution for protein separation and characterization. This is particularly valuable for biomarker discovery, drug development, and clinical diagnostics.
Academic and research institutions constitute another significant market segment, driving innovation and new applications. The demand for more sophisticated analytical tools in proteomics and genomics research has created a steady market for advanced microfluidic separation technologies, with IEF being a critical component.
Regionally, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate due to increasing investment in healthcare infrastructure, expanding biotechnology sectors in China and India, and growing adoption of advanced analytical technologies.
Key market drivers include the rising prevalence of chronic diseases necessitating better diagnostic tools, increasing R&D investments in life sciences, and growing demand for miniaturized analytical systems. The trend toward decentralized testing and point-of-care diagnostics has further accelerated market growth for portable microfluidic systems incorporating IEF technology.
Market challenges include high development costs, technical complexities in system integration, and regulatory hurdles. The specialized nature of microfluidic IEF systems requires significant expertise and investment, creating barriers to entry for smaller companies. Additionally, standardization issues and compatibility concerns between different microfluidic platforms have slowed widespread adoption in some sectors.
Customer requirements are evolving toward more automated, user-friendly systems with improved reproducibility and reliability. End-users increasingly demand integrated solutions that combine multiple separation techniques within a single microfluidic platform, creating opportunities for comprehensive analytical systems that incorporate IEF alongside other separation modalities.
Healthcare and pharmaceutical sectors represent the largest market segments, accounting for approximately 65% of the total market share. The integration of IEF with microfluidic systems offers substantial benefits in these sectors, including reduced sample volumes, faster analysis times, and improved resolution for protein separation and characterization. This is particularly valuable for biomarker discovery, drug development, and clinical diagnostics.
Academic and research institutions constitute another significant market segment, driving innovation and new applications. The demand for more sophisticated analytical tools in proteomics and genomics research has created a steady market for advanced microfluidic separation technologies, with IEF being a critical component.
Regionally, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate due to increasing investment in healthcare infrastructure, expanding biotechnology sectors in China and India, and growing adoption of advanced analytical technologies.
Key market drivers include the rising prevalence of chronic diseases necessitating better diagnostic tools, increasing R&D investments in life sciences, and growing demand for miniaturized analytical systems. The trend toward decentralized testing and point-of-care diagnostics has further accelerated market growth for portable microfluidic systems incorporating IEF technology.
Market challenges include high development costs, technical complexities in system integration, and regulatory hurdles. The specialized nature of microfluidic IEF systems requires significant expertise and investment, creating barriers to entry for smaller companies. Additionally, standardization issues and compatibility concerns between different microfluidic platforms have slowed widespread adoption in some sectors.
Customer requirements are evolving toward more automated, user-friendly systems with improved reproducibility and reliability. End-users increasingly demand integrated solutions that combine multiple separation techniques within a single microfluidic platform, creating opportunities for comprehensive analytical systems that incorporate IEF alongside other separation modalities.
Current Challenges in Microfluidic IEF Implementation
Despite significant advancements in microfluidic technology, the integration of isoelectric focusing (IEF) into microfluidic systems presents several persistent challenges that impede widespread adoption. One fundamental obstacle is the miniaturization of traditional IEF components while maintaining separation efficiency. The reduced dimensions in microfluidic channels create surface-to-volume ratio issues that exacerbate protein adsorption to channel walls, leading to sample loss and reduced resolution.
Temperature control represents another critical challenge in microfluidic IEF implementation. Joule heating generated during electrophoresis can create temperature gradients across the separation channel, disrupting the pH gradient stability and causing band broadening. While conventional IEF systems can employ active cooling mechanisms, the integration of efficient heat dissipation systems in compact microfluidic devices remains problematic.
The establishment and maintenance of stable pH gradients within microfluidic channels pose significant technical difficulties. Carrier ampholyte-based gradients often suffer from drift and instability in miniaturized formats due to electroosmotic flow and electrolysis effects at the electrodes. Although immobilized pH gradient (IPG) approaches offer better stability, their fabrication within microchannels requires complex surface modification techniques that are difficult to standardize for mass production.
Integration of detection systems presents another substantial hurdle. While conventional IEF typically employs post-separation staining or immunoblotting, microfluidic IEF requires real-time, in-channel detection methods. Current optical detection approaches face sensitivity limitations due to the reduced optical path length in microchannels, while label-free detection methods often lack the required sensitivity for detecting low-abundance proteins.
Sample introduction and recovery mechanisms represent persistent engineering challenges. The precise loading of small sample volumes (typically nanoliters) without dispersion effects requires sophisticated microvalve systems. Similarly, the recovery of separated fractions for downstream analysis demands intricate channel designs and control systems that add complexity to device fabrication.
Fabrication reproducibility and scalability constitute significant barriers to commercial adoption. The production of microfluidic IEF devices with consistent channel dimensions, surface properties, and electrode positioning across multiple units remains challenging. Materials compatibility issues further complicate matters, as many polymers used in microfluidic fabrication exhibit poor resistance to extreme pH conditions or organic solvents often required in IEF protocols.
Lastly, system integration challenges persist when combining IEF with other analytical techniques in a single microfluidic platform. The interface between IEF and subsequent separation or detection modules often introduces band broadening and sample dilution, compromising the resolution achieved during the focusing step. These integration challenges highlight the need for holistic design approaches that consider the entire analytical workflow rather than optimizing individual components in isolation.
Temperature control represents another critical challenge in microfluidic IEF implementation. Joule heating generated during electrophoresis can create temperature gradients across the separation channel, disrupting the pH gradient stability and causing band broadening. While conventional IEF systems can employ active cooling mechanisms, the integration of efficient heat dissipation systems in compact microfluidic devices remains problematic.
The establishment and maintenance of stable pH gradients within microfluidic channels pose significant technical difficulties. Carrier ampholyte-based gradients often suffer from drift and instability in miniaturized formats due to electroosmotic flow and electrolysis effects at the electrodes. Although immobilized pH gradient (IPG) approaches offer better stability, their fabrication within microchannels requires complex surface modification techniques that are difficult to standardize for mass production.
Integration of detection systems presents another substantial hurdle. While conventional IEF typically employs post-separation staining or immunoblotting, microfluidic IEF requires real-time, in-channel detection methods. Current optical detection approaches face sensitivity limitations due to the reduced optical path length in microchannels, while label-free detection methods often lack the required sensitivity for detecting low-abundance proteins.
Sample introduction and recovery mechanisms represent persistent engineering challenges. The precise loading of small sample volumes (typically nanoliters) without dispersion effects requires sophisticated microvalve systems. Similarly, the recovery of separated fractions for downstream analysis demands intricate channel designs and control systems that add complexity to device fabrication.
Fabrication reproducibility and scalability constitute significant barriers to commercial adoption. The production of microfluidic IEF devices with consistent channel dimensions, surface properties, and electrode positioning across multiple units remains challenging. Materials compatibility issues further complicate matters, as many polymers used in microfluidic fabrication exhibit poor resistance to extreme pH conditions or organic solvents often required in IEF protocols.
Lastly, system integration challenges persist when combining IEF with other analytical techniques in a single microfluidic platform. The interface between IEF and subsequent separation or detection modules often introduces band broadening and sample dilution, compromising the resolution achieved during the focusing step. These integration challenges highlight the need for holistic design approaches that consider the entire analytical workflow rather than optimizing individual components in isolation.
Current Integration Approaches for IEF in Microfluidic Platforms
01 Isoelectric focusing apparatus design
Various apparatus designs have been developed for isoelectric focusing to improve separation efficiency and integration with other analytical techniques. These designs include specialized chambers, gel configurations, and electrode arrangements that enhance the resolution of protein separation based on their isoelectric points. Advanced apparatus designs also incorporate features for temperature control, sample loading, and detection systems to optimize the isoelectric focusing process.- Isoelectric focusing apparatus design: Various designs of apparatus for isoelectric focusing have been developed to improve separation efficiency and resolution. These designs include specialized chambers, electrode configurations, and cooling systems to maintain temperature control during the focusing process. The apparatus may incorporate features to handle different sample volumes and to facilitate the collection of separated proteins or other biomolecules after focusing is complete.
- Integration with other analytical techniques: Isoelectric focusing can be integrated with other analytical techniques to enhance protein characterization and separation. Common integrations include coupling with mass spectrometry, gel electrophoresis, or chromatography methods. These integrated systems allow for multidimensional analysis, providing more comprehensive information about complex biological samples and improving the detection and identification of proteins based on both their isoelectric points and other physicochemical properties.
- Microfluidic and miniaturized IEF systems: Miniaturized isoelectric focusing systems have been developed using microfluidic technology to reduce sample volume requirements and increase throughput. These systems integrate multiple functions on a single chip or device, allowing for rapid analysis with minimal reagent consumption. Microfluidic IEF systems often incorporate novel electrode arrangements, channel designs, and detection methods to achieve high-resolution separations in compact formats suitable for point-of-care diagnostics or high-throughput screening applications.
- Carrier ampholyte innovations: Advancements in carrier ampholyte formulations have improved the performance of isoelectric focusing. These innovations include the development of synthetic ampholytes with defined pH ranges, immobilized pH gradients, and specialized buffer systems that enhance stability and reproducibility. Novel ampholyte compositions can provide better resolution of proteins with similar isoelectric points and reduce issues such as cathodic drift or protein precipitation during the focusing process.
- Automated and high-throughput IEF systems: Automated systems for isoelectric focusing have been developed to increase throughput and reproducibility while reducing manual handling. These systems incorporate robotics, digital imaging, and software for data analysis to streamline the IEF workflow. High-throughput platforms may include parallel processing capabilities, automated sample loading and fraction collection, and integrated detection systems for real-time monitoring of the focusing process, making them suitable for proteomics research and clinical applications.
02 Integration with microfluidic systems
Isoelectric focusing has been successfully integrated with microfluidic platforms to enable miniaturized protein analysis. These integrated systems combine the high-resolution separation capabilities of isoelectric focusing with the advantages of microfluidics, including reduced sample volume, faster analysis times, and potential for automation. Microfluidic isoelectric focusing systems often incorporate specialized channels, electrodes, and detection methods optimized for small-scale separations.Expand Specific Solutions03 Multi-dimensional separation techniques
Isoelectric focusing has been integrated with other separation techniques to create powerful multi-dimensional analysis systems. These approaches typically combine isoelectric focusing with methods such as gel electrophoresis, chromatography, or mass spectrometry to achieve higher resolution protein separation based on multiple physicochemical properties. Such integrated multi-dimensional systems provide comprehensive protein characterization for complex biological samples.Expand Specific Solutions04 Detection and imaging systems
Advanced detection and imaging systems have been developed for integration with isoelectric focusing to enhance the visualization and quantification of separated proteins. These systems include various optical, fluorescence, and electrical detection methods that can be directly coupled with the isoelectric focusing process. Integrated detection systems enable real-time monitoring of the separation process and improve the sensitivity and accuracy of protein analysis.Expand Specific Solutions05 Automation and high-throughput applications
Isoelectric focusing has been integrated into automated and high-throughput systems for large-scale protein analysis. These integrated platforms incorporate robotics, automated sample handling, and computerized control systems to enable the processing of multiple samples simultaneously. Automation of isoelectric focusing increases reproducibility, reduces manual labor, and facilitates integration with laboratory information management systems for comprehensive data analysis.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidic IEF
The isoelectric focusing (IEF) microfluidic integration market is currently in a growth phase, with increasing adoption across biomedical research and diagnostics. The global market size for microfluidic technologies is expanding rapidly, projected to reach significant valuation as IEF applications gain traction. Technologically, the field shows moderate maturity with established players like Bio-Rad Laboratories and ProteinSimple leading commercial applications, while academic institutions including MIT, Harvard, and California Institute of Technology drive innovation. Pharmaceutical and healthcare companies such as Koninklijke Philips and Becton Dickinson are investing in integration capabilities, while specialized firms like Cellix and AcouSort focus on niche microfluidic solutions. The competitive landscape features collaboration between academic research centers and industry partners to overcome technical challenges in miniaturization, automation, and system integration.
Intabio LLC
Technical Solution: Intabio has developed a groundbreaking microfluidic platform specifically designed for isoelectric focusing of biotherapeutic proteins. Their Blaze™ system integrates IEF separation with mass spectrometry detection in a single automated workflow, representing a significant advancement in microfluidic IEF technology. The company's approach utilizes proprietary microchip designs featuring precisely etched channels with specialized surface modifications to minimize protein adsorption and electroosmotic flow disturbances. Intabio's technology incorporates innovative electrode configurations that generate stable electric fields across microchannels while minimizing bubble formation issues that typically plague microfluidic IEF systems. Their platform employs a unique transient pH gradient formation technique that allows rapid focusing of protein samples within minutes rather than hours required by conventional methods[4][7]. The system features integrated UV detection capabilities for real-time monitoring of protein focusing events, with sophisticated fluidic control systems enabling automated fraction collection of separated proteins for further analysis.
Strengths: Seamless integration with mass spectrometry provides comprehensive characterization of charge variants; rapid analysis times significantly improve throughput; automated operation reduces manual handling errors. Weaknesses: Relatively new technology with limited track record in diverse applications; specialized system design may limit flexibility for certain research applications; higher initial investment compared to traditional analytical methods.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced isoelectric focusing (IEF) technologies specifically designed for microfluidic integration. Their approach combines traditional IEF principles with miniaturized platforms, utilizing proprietary ampholyte mixtures that establish stable pH gradients within microchannels. The company's microfluidic IEF systems incorporate precision-etched channels with specialized surface treatments to minimize electroosmotic flow and protein adsorption issues. Bio-Rad's technology employs whole-channel imaging detection methods that allow real-time monitoring of protein focusing processes, enabling higher resolution separation of proteins with closely related isoelectric points. Their integrated systems feature temperature control mechanisms to prevent Joule heating effects that typically compromise separation efficiency in microfluidic IEF applications[1][3]. Bio-Rad has also pioneered the development of immobilized pH gradient (IPG) strips compatible with microfluidic platforms, enhancing reproducibility and stability during extended separation runs.
Strengths: Industry-leading expertise in electrophoresis technologies with established market presence; comprehensive integration solutions that address key technical challenges like Joule heating and protein adsorption; superior detection sensitivity. Weaknesses: Higher cost compared to academic solutions; proprietary systems may limit customization options for specialized research applications.
Key Patents and Innovations in Miniaturized IEF Systems
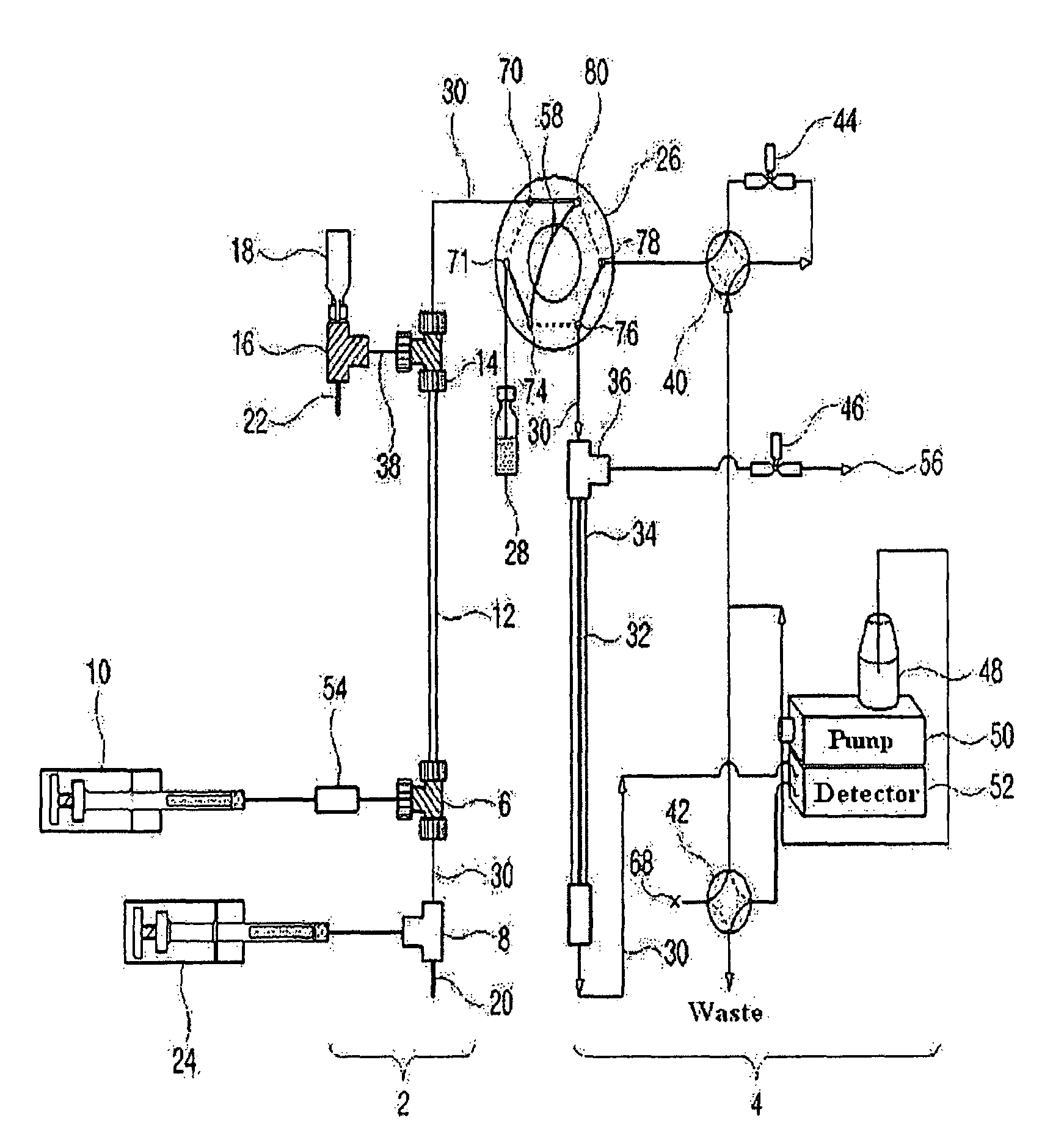
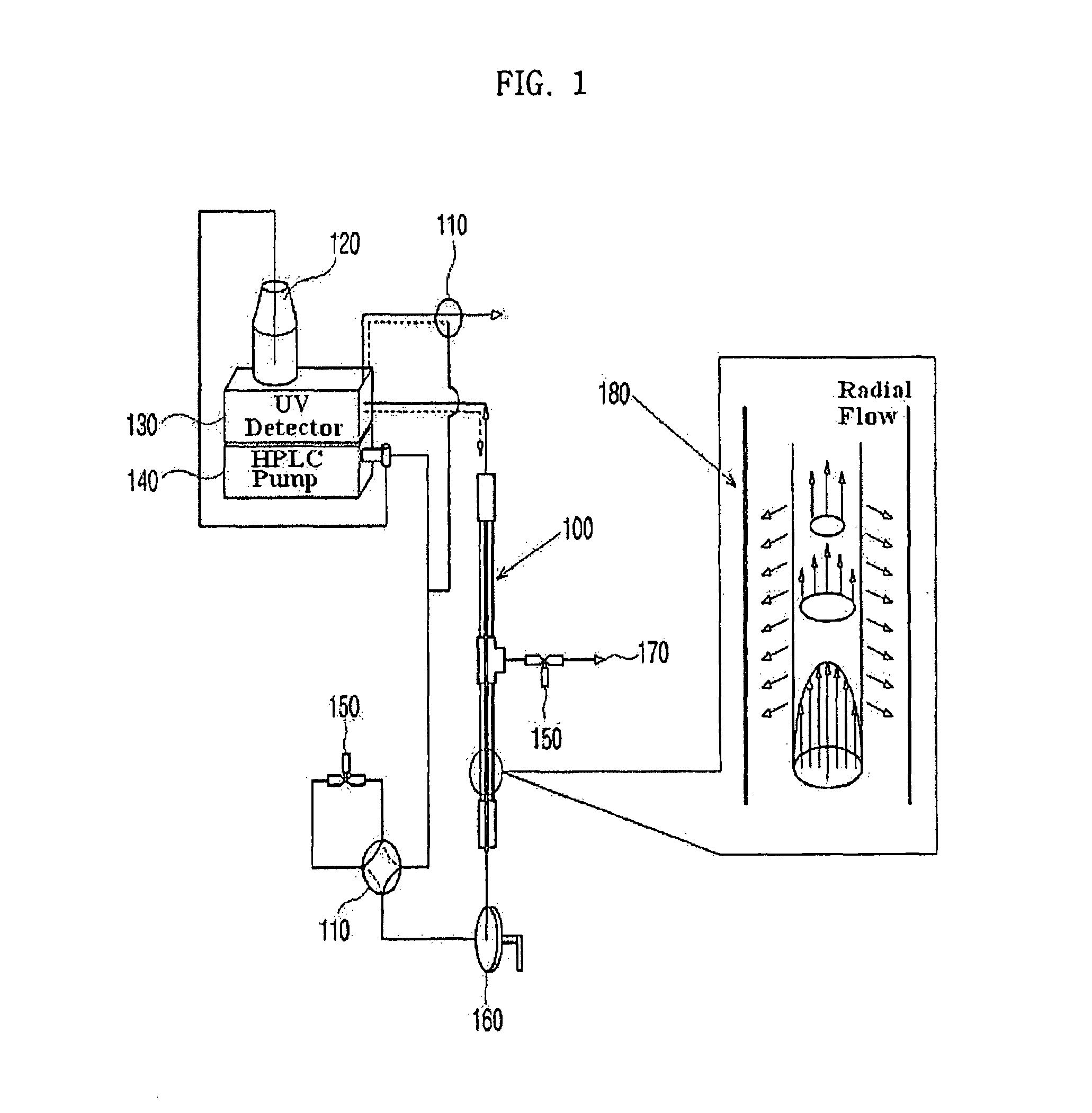
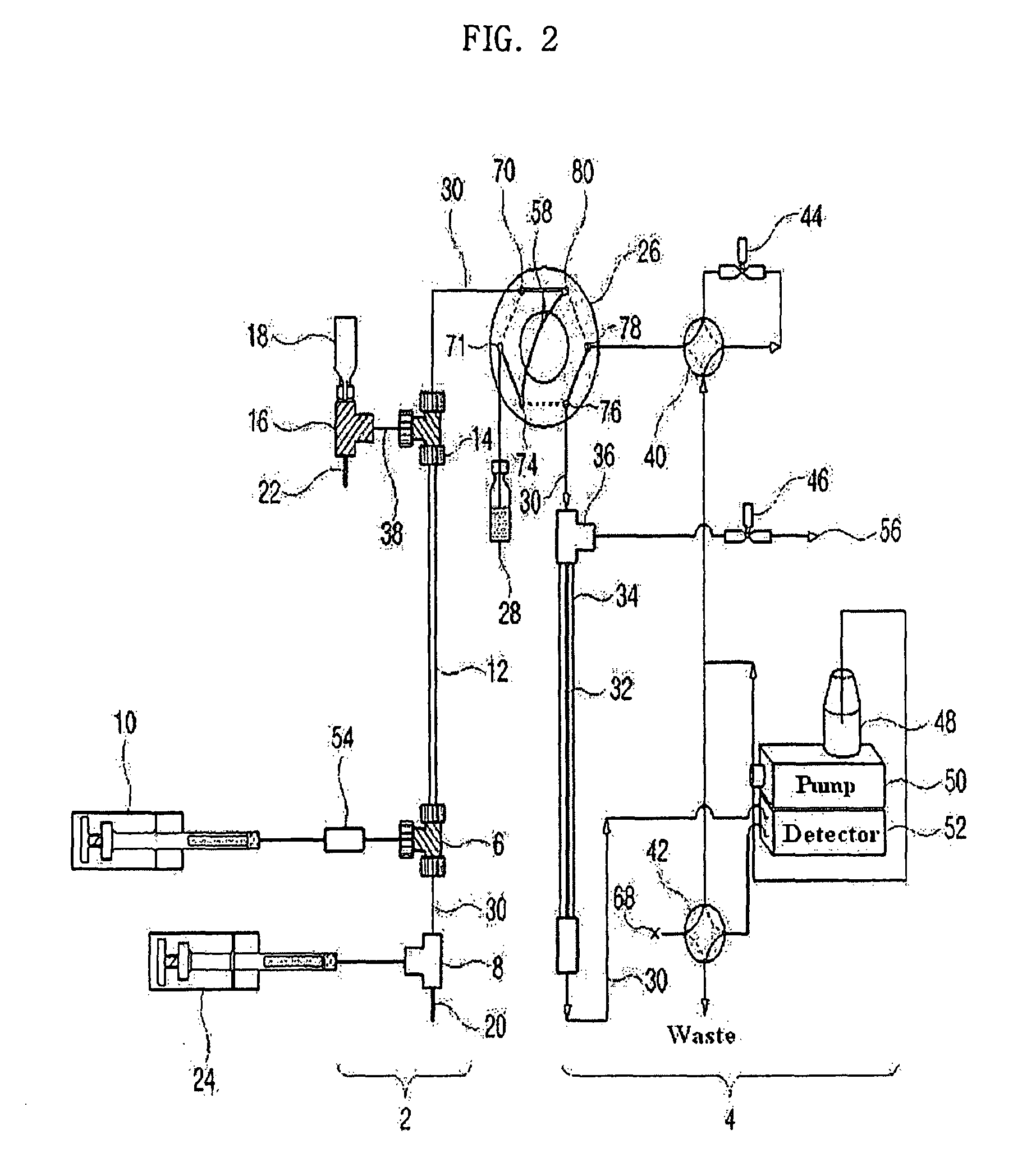
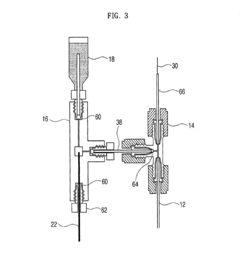
Apparatus for protein separation using capillary isoelectric focusing-hollow fiber flow field flow fractionation and method thereof
PatentInactiveUS8585884B2
Innovation
- A capillary isoelectric focusing-hollow fiber flow field flow fractionation apparatus that separates proteins based on isoelectric point (pI) and molecular weight in a two-dimensional, non-gel, and liquid phase manner, using a capillary isoelectric focusing unit connected to a hollow fiber flow field flow fractionation unit, allowing for automatic removal of ampholytes and avoiding protein denaturation.
An apparatus for protein separation using capillary isoelectric focusing-hollow fiber flow field flow fractionation and method thereof
PatentInactiveEP1987053A1
Innovation
- A capillary isoelectric focusing-hollow fiber flow field flow fractionation apparatus that separates proteins based on isoelectric point and molecular weight in a two-dimensional, non-gel, and liquid phase manner, using a combination of capillary isoelectric focusing and hollow fiber flow field flow fractionation units to automatically remove ampholytes and prevent protein denaturation.
Fabrication Techniques for Microfluidic IEF Devices
The fabrication of microfluidic IEF devices requires specialized techniques that balance precision, reproducibility, and cost-effectiveness. Traditional fabrication methods such as photolithography and soft lithography remain foundational in this field. Photolithography enables the creation of high-resolution channel patterns with feature sizes down to several micrometers, which is essential for maintaining precise pH gradients in IEF applications. Soft lithography, particularly using polydimethylsiloxane (PDMS), offers advantages in rapid prototyping and optical transparency, facilitating real-time visualization of protein focusing.
Recent advances have introduced alternative fabrication approaches that address specific challenges in microfluidic IEF. Laser ablation techniques provide direct writing capabilities for creating channels in polymer substrates without requiring cleanroom facilities, significantly reducing fabrication time and costs. This method has proven particularly valuable for iterative design optimization in research settings.
3D printing technologies have emerged as promising tools for microfluidic IEF device fabrication. Stereolithography (SLA) and digital light processing (DLP) can achieve resolutions suitable for microfluidic channels, while offering unprecedented geometric freedom. However, challenges remain in surface chemistry control and material biocompatibility when implementing these techniques for protein separation applications.
Surface modification represents a critical aspect of fabrication, as it directly impacts electroosmotic flow and protein adsorption. Techniques such as plasma treatment, chemical vapor deposition, and layer-by-layer assembly enable precise tailoring of surface properties. For IEF applications, hydrophilic coatings are typically applied to minimize protein adsorption and maintain separation efficiency.
Integration of electrodes presents unique fabrication challenges in microfluidic IEF. Techniques include sputtering, evaporation, and screen printing of conductive materials. Recent innovations have explored the use of liquid metal electrodes that can be injected into dedicated channels, offering improved durability and simplified fabrication processes.
Multilayer fabrication approaches have gained prominence for creating more complex IEF devices with integrated functionalities. Thermal bonding, plasma-assisted bonding, and adhesive bonding techniques enable the creation of three-dimensional architectures that incorporate sample preparation, separation, and detection components within a single device.
The selection of substrate materials significantly influences fabrication strategy and device performance. While glass offers excellent chemical resistance and optical properties, polymeric materials like PMMA, COC, and PC provide cost advantages and diverse fabrication options. Hybrid approaches combining different materials can leverage the strengths of each to optimize overall device performance.
Recent advances have introduced alternative fabrication approaches that address specific challenges in microfluidic IEF. Laser ablation techniques provide direct writing capabilities for creating channels in polymer substrates without requiring cleanroom facilities, significantly reducing fabrication time and costs. This method has proven particularly valuable for iterative design optimization in research settings.
3D printing technologies have emerged as promising tools for microfluidic IEF device fabrication. Stereolithography (SLA) and digital light processing (DLP) can achieve resolutions suitable for microfluidic channels, while offering unprecedented geometric freedom. However, challenges remain in surface chemistry control and material biocompatibility when implementing these techniques for protein separation applications.
Surface modification represents a critical aspect of fabrication, as it directly impacts electroosmotic flow and protein adsorption. Techniques such as plasma treatment, chemical vapor deposition, and layer-by-layer assembly enable precise tailoring of surface properties. For IEF applications, hydrophilic coatings are typically applied to minimize protein adsorption and maintain separation efficiency.
Integration of electrodes presents unique fabrication challenges in microfluidic IEF. Techniques include sputtering, evaporation, and screen printing of conductive materials. Recent innovations have explored the use of liquid metal electrodes that can be injected into dedicated channels, offering improved durability and simplified fabrication processes.
Multilayer fabrication approaches have gained prominence for creating more complex IEF devices with integrated functionalities. Thermal bonding, plasma-assisted bonding, and adhesive bonding techniques enable the creation of three-dimensional architectures that incorporate sample preparation, separation, and detection components within a single device.
The selection of substrate materials significantly influences fabrication strategy and device performance. While glass offers excellent chemical resistance and optical properties, polymeric materials like PMMA, COC, and PC provide cost advantages and diverse fabrication options. Hybrid approaches combining different materials can leverage the strengths of each to optimize overall device performance.
Regulatory Considerations for Lab-on-Chip Diagnostic Applications
The integration of isoelectric focusing (IEF) into microfluidic systems for diagnostic applications necessitates careful consideration of regulatory frameworks that govern medical devices and in vitro diagnostic products. In the United States, the Food and Drug Administration (FDA) classifies lab-on-chip diagnostic devices under the medical device regulatory pathway, with specific requirements depending on the intended use and risk classification.
For microfluidic IEF systems targeting clinical diagnostics, developers must navigate the premarket approval (PMA) or 510(k) clearance processes. The complexity of these integrated systems, combining multiple analytical functions including IEF separation, often places them in higher risk categories requiring more stringent validation protocols and clinical evidence.
European regulatory frameworks, particularly the In Vitro Diagnostic Regulation (IVDR), impose additional requirements for performance evaluation, technical documentation, and post-market surveillance. The IVDR's risk-based classification system may categorize microfluidic IEF devices in higher classes (B, C, or D) depending on their intended diagnostic purpose, especially when used for critical conditions or companion diagnostics.
Quality management systems compliant with ISO 13485 standards are essential for manufacturers developing microfluidic IEF systems. These standards ensure consistent design, development, production, and service processes that meet both regulatory requirements and customer needs. Additionally, IEC 62304 may apply when software components control critical IEF parameters such as electric field generation or pH gradient formation.
Analytical performance validation presents unique regulatory challenges for microfluidic IEF systems. Developers must demonstrate reproducibility, accuracy, and precision of the isoelectric point determination across different sample matrices and environmental conditions. The miniaturized nature of these systems requires specialized validation approaches that may not be fully addressed in existing regulatory guidelines.
Data privacy and security regulations, including HIPAA in the US and GDPR in Europe, become relevant when IEF microfluidic devices incorporate data storage or transmission capabilities. The trend toward connected diagnostic platforms necessitates compliance with cybersecurity requirements to protect patient information and ensure system integrity.
Regulatory pathways for novel technologies like microfluidic IEF systems often benefit from early engagement with regulatory bodies through pre-submission consultations or innovation pathways. These mechanisms can provide valuable guidance on validation requirements and help address regulatory uncertainties specific to this emerging technology domain.
For microfluidic IEF systems targeting clinical diagnostics, developers must navigate the premarket approval (PMA) or 510(k) clearance processes. The complexity of these integrated systems, combining multiple analytical functions including IEF separation, often places them in higher risk categories requiring more stringent validation protocols and clinical evidence.
European regulatory frameworks, particularly the In Vitro Diagnostic Regulation (IVDR), impose additional requirements for performance evaluation, technical documentation, and post-market surveillance. The IVDR's risk-based classification system may categorize microfluidic IEF devices in higher classes (B, C, or D) depending on their intended diagnostic purpose, especially when used for critical conditions or companion diagnostics.
Quality management systems compliant with ISO 13485 standards are essential for manufacturers developing microfluidic IEF systems. These standards ensure consistent design, development, production, and service processes that meet both regulatory requirements and customer needs. Additionally, IEC 62304 may apply when software components control critical IEF parameters such as electric field generation or pH gradient formation.
Analytical performance validation presents unique regulatory challenges for microfluidic IEF systems. Developers must demonstrate reproducibility, accuracy, and precision of the isoelectric point determination across different sample matrices and environmental conditions. The miniaturized nature of these systems requires specialized validation approaches that may not be fully addressed in existing regulatory guidelines.
Data privacy and security regulations, including HIPAA in the US and GDPR in Europe, become relevant when IEF microfluidic devices incorporate data storage or transmission capabilities. The trend toward connected diagnostic platforms necessitates compliance with cybersecurity requirements to protect patient information and ensure system integrity.
Regulatory pathways for novel technologies like microfluidic IEF systems often benefit from early engagement with regulatory bodies through pre-submission consultations or innovation pathways. These mechanisms can provide valuable guidance on validation requirements and help address regulatory uncertainties specific to this emerging technology domain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!