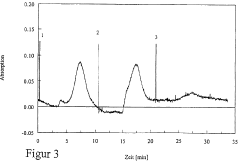
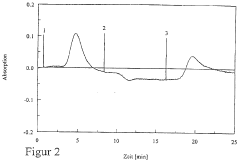
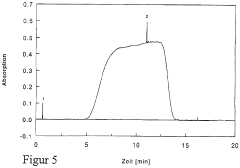
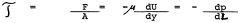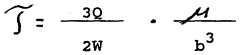Optimize Isoelectric Focusing Parameters for Protein Purification
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Evolution and Purification Goals
Isoelectric focusing (IEF) has evolved significantly since its introduction in the 1960s as a technique for protein separation based on differences in isoelectric points. Initially developed by Svensson and further refined by Vesterberg, this technique has transformed from a purely analytical method to a powerful preparative tool in protein purification workflows. The evolution of IEF technology has been marked by continuous improvements in resolution, reproducibility, and scalability, addressing the growing demands of biopharmaceutical and research applications.
Early IEF systems utilized carrier ampholytes to establish pH gradients, which often suffered from gradient instability and poor reproducibility. The introduction of immobilized pH gradient (IPG) technology in the 1980s represented a revolutionary advancement, providing more stable and reproducible pH gradients. This innovation significantly enhanced the separation capacity and resolution of IEF systems, enabling the purification of proteins with minimal differences in their isoelectric points.
The integration of IEF with other separation techniques, such as gel electrophoresis and chromatography, has further expanded its capabilities. Two-dimensional electrophoresis, combining IEF with SDS-PAGE, became a cornerstone of proteomics research. More recently, the development of capillary IEF and microfluidic IEF platforms has enabled high-throughput applications with minimal sample requirements, addressing the needs of modern bioanalytical laboratories.
The primary goal of optimizing IEF parameters for protein purification is to achieve maximum resolution and recovery while maintaining protein stability and activity. This involves careful consideration of multiple factors including pH gradient range, electric field strength, temperature control, and buffer composition. The optimization process aims to balance these parameters to effectively separate target proteins from contaminants while preserving their native structure and biological function.
Another critical objective is to enhance the scalability of IEF processes, facilitating the transition from analytical to preparative applications. This includes developing strategies for continuous-flow IEF systems and improving sample loading capacity without compromising resolution. The ultimate aim is to establish robust and reproducible IEF protocols that can be integrated into industrial-scale protein purification workflows.
Recent technological trends indicate a growing focus on automation and integration of IEF within comprehensive purification platforms. This includes the development of intelligent control systems that can dynamically adjust parameters during the focusing process, responding to real-time monitoring of protein behavior. Such advancements promise to further enhance the precision and efficiency of IEF-based protein purification, supporting the increasing demands for high-purity protein products in various applications.
Early IEF systems utilized carrier ampholytes to establish pH gradients, which often suffered from gradient instability and poor reproducibility. The introduction of immobilized pH gradient (IPG) technology in the 1980s represented a revolutionary advancement, providing more stable and reproducible pH gradients. This innovation significantly enhanced the separation capacity and resolution of IEF systems, enabling the purification of proteins with minimal differences in their isoelectric points.
The integration of IEF with other separation techniques, such as gel electrophoresis and chromatography, has further expanded its capabilities. Two-dimensional electrophoresis, combining IEF with SDS-PAGE, became a cornerstone of proteomics research. More recently, the development of capillary IEF and microfluidic IEF platforms has enabled high-throughput applications with minimal sample requirements, addressing the needs of modern bioanalytical laboratories.
The primary goal of optimizing IEF parameters for protein purification is to achieve maximum resolution and recovery while maintaining protein stability and activity. This involves careful consideration of multiple factors including pH gradient range, electric field strength, temperature control, and buffer composition. The optimization process aims to balance these parameters to effectively separate target proteins from contaminants while preserving their native structure and biological function.
Another critical objective is to enhance the scalability of IEF processes, facilitating the transition from analytical to preparative applications. This includes developing strategies for continuous-flow IEF systems and improving sample loading capacity without compromising resolution. The ultimate aim is to establish robust and reproducible IEF protocols that can be integrated into industrial-scale protein purification workflows.
Recent technological trends indicate a growing focus on automation and integration of IEF within comprehensive purification platforms. This includes the development of intelligent control systems that can dynamically adjust parameters during the focusing process, responding to real-time monitoring of protein behavior. Such advancements promise to further enhance the precision and efficiency of IEF-based protein purification, supporting the increasing demands for high-purity protein products in various applications.
Market Demand for Advanced Protein Purification
The global protein purification market has witnessed substantial growth in recent years, driven primarily by advancements in biopharmaceutical research and development. As of 2023, the market size for protein purification technologies exceeded $8 billion, with projections indicating a compound annual growth rate of 7.2% through 2030. This growth trajectory underscores the increasing demand for more efficient and precise purification methodologies, particularly isoelectric focusing (IEF) techniques.
Biopharmaceutical companies represent the largest segment of end-users, accounting for approximately 45% of the market share. These organizations are increasingly investing in advanced protein purification technologies to enhance the development of monoclonal antibodies, recombinant proteins, and other biological therapeutics. The precision offered by optimized IEF parameters directly translates to higher purity levels, which is critical for regulatory compliance and product efficacy.
Academic and research institutions constitute another significant market segment, representing about 30% of the demand. These entities require sophisticated protein purification capabilities for fundamental research in proteomics, structural biology, and biomarker discovery. The ability to precisely separate proteins based on their isoelectric points enables researchers to isolate specific protein variants and isoforms with unprecedented accuracy.
Geographically, North America dominates the market with a 40% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is experiencing the fastest growth rate at 9.5% annually, primarily due to expanding biotechnology sectors in China, India, and South Korea. This regional diversification indicates a global recognition of the importance of advanced protein purification methodologies.
Industry surveys reveal that 78% of protein scientists and bioprocess engineers consider optimization of IEF parameters as "highly important" or "critical" to their work. The primary drivers behind this demand include the need for higher resolution separation (cited by 65% of respondents), improved reproducibility (58%), and reduced processing time (52%). Additionally, 47% of professionals indicated that current IEF technologies do not fully meet their requirements for complex protein mixtures.
The market is also witnessing a shift toward automated and integrated purification systems that incorporate optimized IEF parameters. This trend is particularly evident in contract manufacturing organizations (CMOs) and contract research organizations (CROs), where throughput and consistency are paramount. These organizations are willing to invest in premium solutions that offer demonstrable improvements in separation efficiency and product quality.
Biopharmaceutical companies represent the largest segment of end-users, accounting for approximately 45% of the market share. These organizations are increasingly investing in advanced protein purification technologies to enhance the development of monoclonal antibodies, recombinant proteins, and other biological therapeutics. The precision offered by optimized IEF parameters directly translates to higher purity levels, which is critical for regulatory compliance and product efficacy.
Academic and research institutions constitute another significant market segment, representing about 30% of the demand. These entities require sophisticated protein purification capabilities for fundamental research in proteomics, structural biology, and biomarker discovery. The ability to precisely separate proteins based on their isoelectric points enables researchers to isolate specific protein variants and isoforms with unprecedented accuracy.
Geographically, North America dominates the market with a 40% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is experiencing the fastest growth rate at 9.5% annually, primarily due to expanding biotechnology sectors in China, India, and South Korea. This regional diversification indicates a global recognition of the importance of advanced protein purification methodologies.
Industry surveys reveal that 78% of protein scientists and bioprocess engineers consider optimization of IEF parameters as "highly important" or "critical" to their work. The primary drivers behind this demand include the need for higher resolution separation (cited by 65% of respondents), improved reproducibility (58%), and reduced processing time (52%). Additionally, 47% of professionals indicated that current IEF technologies do not fully meet their requirements for complex protein mixtures.
The market is also witnessing a shift toward automated and integrated purification systems that incorporate optimized IEF parameters. This trend is particularly evident in contract manufacturing organizations (CMOs) and contract research organizations (CROs), where throughput and consistency are paramount. These organizations are willing to invest in premium solutions that offer demonstrable improvements in separation efficiency and product quality.
Current IEF Challenges and Technical Limitations
Despite significant advancements in isoelectric focusing (IEF) technology, several persistent challenges continue to limit its widespread application in protein purification processes. One of the primary technical limitations is the phenomenon of protein precipitation at or near the isoelectric point (pI). When proteins reach their pI during focusing, their net charge approaches zero, significantly reducing solubility and promoting aggregation, which can lead to substantial sample loss and reduced recovery rates.
Temperature control represents another critical challenge in IEF operations. The application of high voltage generates considerable Joule heating, which can denature sensitive proteins and create temperature gradients within the separation medium. These gradients disrupt the uniformity of the pH gradient and compromise resolution. Current cooling systems often struggle to maintain consistent temperatures throughout the separation chamber, particularly in preparative-scale applications.
pH gradient instability, known as "drift," continues to plague IEF processes. Over time, carrier ampholytes migrate toward the electrodes, causing distortion of the initially established pH gradient. This drift significantly impacts reproducibility and makes it difficult to maintain consistent separation conditions across multiple runs. Although immobilized pH gradient (IPG) technology has partially addressed this issue, it introduces other limitations related to loading capacity and flexibility.
Protein-ampholyte interactions present another significant technical hurdle. Carrier ampholytes can bind to proteins, altering their apparent pI values and migration behaviors. These interactions are often unpredictable and vary depending on the specific protein characteristics, making standardization challenging. Furthermore, residual ampholytes can interfere with downstream applications and analysis, necessitating additional purification steps.
Scaling challenges severely limit industrial adoption of IEF. While analytical-scale IEF is well-established, scaling to preparative or industrial levels introduces significant complications in maintaining uniform electric fields, heat dissipation, and pH gradient stability. Current large-format systems often suffer from reduced resolution and extended processing times, diminishing the technique's advantages.
Instrumentation limitations further constrain IEF applications. Many commercial systems lack real-time monitoring capabilities for critical parameters such as local pH, protein concentration, and temperature distribution. This absence of feedback mechanisms makes process optimization largely empirical and time-consuming, requiring extensive trial-and-error approaches.
Electrolysis effects at the electrodes generate extreme pH values and reactive species that can damage proteins near the electrode regions. Current electrode designs and buffer systems provide only partial mitigation of these detrimental effects, particularly during extended separation times required for complex protein mixtures.
Temperature control represents another critical challenge in IEF operations. The application of high voltage generates considerable Joule heating, which can denature sensitive proteins and create temperature gradients within the separation medium. These gradients disrupt the uniformity of the pH gradient and compromise resolution. Current cooling systems often struggle to maintain consistent temperatures throughout the separation chamber, particularly in preparative-scale applications.
pH gradient instability, known as "drift," continues to plague IEF processes. Over time, carrier ampholytes migrate toward the electrodes, causing distortion of the initially established pH gradient. This drift significantly impacts reproducibility and makes it difficult to maintain consistent separation conditions across multiple runs. Although immobilized pH gradient (IPG) technology has partially addressed this issue, it introduces other limitations related to loading capacity and flexibility.
Protein-ampholyte interactions present another significant technical hurdle. Carrier ampholytes can bind to proteins, altering their apparent pI values and migration behaviors. These interactions are often unpredictable and vary depending on the specific protein characteristics, making standardization challenging. Furthermore, residual ampholytes can interfere with downstream applications and analysis, necessitating additional purification steps.
Scaling challenges severely limit industrial adoption of IEF. While analytical-scale IEF is well-established, scaling to preparative or industrial levels introduces significant complications in maintaining uniform electric fields, heat dissipation, and pH gradient stability. Current large-format systems often suffer from reduced resolution and extended processing times, diminishing the technique's advantages.
Instrumentation limitations further constrain IEF applications. Many commercial systems lack real-time monitoring capabilities for critical parameters such as local pH, protein concentration, and temperature distribution. This absence of feedback mechanisms makes process optimization largely empirical and time-consuming, requiring extensive trial-and-error approaches.
Electrolysis effects at the electrodes generate extreme pH values and reactive species that can damage proteins near the electrode regions. Current electrode designs and buffer systems provide only partial mitigation of these detrimental effects, particularly during extended separation times required for complex protein mixtures.
Mainstream IEF Parameter Optimization Approaches
01 pH gradient formation and control
The establishment and control of pH gradients is fundamental to isoelectric focusing. This involves the use of carrier ampholytes or immobilized pH gradients to create a stable pH environment across the separation medium. Proper gradient formation ensures proteins migrate to their isoelectric points where they have no net charge. Parameters such as ampholyte concentration, gradient range, and stability over time significantly impact resolution and reproducibility of protein separation.- pH gradient optimization for isoelectric focusing: The pH gradient is a critical parameter in isoelectric focusing that determines the separation efficiency of proteins based on their isoelectric points. Optimization involves selecting appropriate ampholytes, buffer systems, and gradient ranges to achieve the desired resolution. Techniques for creating stable and reproducible pH gradients include immobilized pH gradients (IPG) and carrier ampholyte-based systems, which can be tailored to specific protein separation requirements.
- Electric field parameters and power settings: The electric field strength, voltage, and current are crucial parameters that affect the speed and resolution of isoelectric focusing. Proper control of these electrical parameters prevents sample heating and protein denaturation while ensuring efficient separation. Advanced systems incorporate programmable power supplies that allow for step-wise voltage increases and constant power modes to optimize focusing conditions while maintaining sample integrity.
- Sample preparation and loading techniques: Effective sample preparation is essential for successful isoelectric focusing, including protein solubilization, denaturation, and reduction of interfering substances. Loading techniques such as cup loading, rehydration loading, and paper bridge loading affect the resolution and reproducibility of the separation. The concentration, volume, and application method of the sample must be optimized based on the specific proteins being analyzed and the focusing system used.
- Gel composition and support media: The composition of the gel matrix significantly influences the resolution and separation efficiency in isoelectric focusing. Parameters such as acrylamide concentration, crosslinking density, and additives affect pore size and electroendosmosis. Various support media including polyacrylamide gels, agarose, and specialized membranes can be selected based on the molecular weight range of target proteins and the desired resolution.
- Detection and analysis systems: Advanced detection systems are essential for visualizing and quantifying proteins after isoelectric focusing. These include staining methods (Coomassie, silver, fluorescent dyes), imaging technologies, and integration with mass spectrometry for protein identification. Modern systems incorporate automated image analysis software that can determine isoelectric points, quantify protein abundance, and compare multiple samples for differential expression analysis.
02 Electric field parameters and power settings
The electric field applied during isoelectric focusing critically affects separation efficiency. Key parameters include voltage, current, and power settings, which must be optimized based on the sample and medium characteristics. Typically, a constant voltage is applied initially, followed by constant power to prevent overheating. The field strength influences the migration rate of proteins and the time required to reach equilibrium, while proper power management prevents sample degradation and improves resolution.Expand Specific Solutions03 Buffer system composition and optimization
The composition of buffer systems significantly impacts isoelectric focusing performance. This includes selection of appropriate carrier ampholytes, additives to reduce protein aggregation, and detergents to maintain protein solubility. Optimization involves adjusting ionic strength, selecting compatible salts, and incorporating stabilizing agents. The buffer system must maintain protein native states while allowing efficient separation based on isoelectric points without introducing artifacts or interference with downstream analysis.Expand Specific Solutions04 Sample preparation and loading techniques
Proper sample preparation is essential for successful isoelectric focusing. This includes protein solubilization, removal of interfering substances, and determination of optimal sample concentration and volume. Loading techniques vary depending on the focusing apparatus and can include direct application, cup loading, or rehydration loading. The method chosen affects protein entry into the gel, resolution, and potential streaking. Standardized protocols ensure reproducibility across experiments and minimize artifacts.Expand Specific Solutions05 Detection and imaging systems
Various detection methods are employed to visualize proteins after isoelectric focusing. These include staining techniques (Coomassie, silver, fluorescent dyes), immunodetection, and direct imaging systems. Parameters such as sensitivity, dynamic range, and compatibility with downstream processing must be considered. Advanced imaging systems can provide real-time monitoring of the focusing process, allowing for optimization of run conditions and determination of endpoint. Quantitative analysis of focused proteins requires calibration and standardization of detection methods.Expand Specific Solutions
Leading Companies in IEF Equipment and Reagents
The isoelectric focusing (IEF) protein purification market is currently in a growth phase, with increasing demand driven by biopharmaceutical development and proteomics research. The global market size for protein purification technologies is expanding at approximately 8-10% annually, reaching several billion dollars. Technologically, IEF parameters optimization represents a maturing field with established players like Bio-Rad Laboratories and ProteinSimple leading commercial applications through advanced instrumentation and reagents. Major pharmaceutical companies such as Novartis and Roche are investing in optimized IEF technologies for their protein therapeutics development pipelines. Academic institutions including Tsinghua University and Texas A&M are advancing fundamental research, while specialized biotechnology firms like Canton Biologics and Energenesis Biomedical are developing novel applications for enhanced protein separation efficiency and reproducibility.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced IEF systems incorporating multi-compartment electrolyzers with proprietary ampholyte mixtures that create stable pH gradients for enhanced protein separation. Their technology utilizes computer-controlled power supplies that implement precise voltage ramping protocols to minimize protein aggregation and improve resolution. Bio-Rad's approach includes specialized sample preparation methods involving chaotropic agents and detergents that maintain protein solubility throughout the focusing process. Their systems incorporate real-time monitoring of current and voltage parameters to automatically adjust focusing conditions based on sample characteristics. Additionally, Bio-Rad has pioneered the integration of IEF with downstream purification steps through automated fraction collection systems that maintain protein stability post-separation.
Strengths: Industry-leading expertise in ampholyte chemistry and pH gradient stability; comprehensive integration with downstream purification workflows; extensive validation across diverse protein classes. Weaknesses: Higher cost compared to basic systems; requires specialized training for optimal parameter selection; some proprietary consumables limit customization options.
ProteinSimple
Technical Solution: ProteinSimple has revolutionized IEF technology with their capillary-based systems that dramatically reduce sample volume requirements while increasing throughput. Their platform utilizes proprietary microfluidic channels coated with specialized polymers that minimize protein adsorption during focusing. The company has developed algorithms that dynamically adjust electric field strength based on real-time conductivity measurements, preventing overheating while maintaining separation efficiency. Their systems incorporate whole-column imaging detection that allows visualization of protein focusing in real-time, enabling immediate parameter optimization. ProteinSimple's technology includes automated pH gradient formation using precise mixtures of carrier ampholytes with optimized spacer compounds that enhance resolution in specific pH ranges critical for therapeutic protein purification.
Strengths: Miniaturized format reduces sample consumption and increases throughput; real-time visualization enables rapid parameter optimization; highly reproducible results with minimal operator intervention. Weaknesses: Limited scalability for preparative applications; higher initial investment compared to traditional systems; specialized consumables required for optimal performance.
Key Patents in IEF Parameter Control
A protein purification system based on isoelectric focusing and isotachophoresis
PatentWO1991010129A1
Innovation
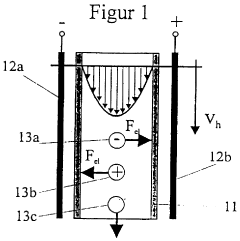
- A method and apparatus that utilize rapid recirculation of process fluid through a narrow channel in a continuous flow electrophoresis chamber, with external heat exchangers to manage Joule heat and minimize shear stress, allowing for stable fluid flow without solid supports, enabling efficient protein purification in free solution.
Process and device for isoelectric particle separation
PatentWO1998013689A1
Innovation
- The method involves controlling the pH of a guide liquid to separate particles based on their charge, using an external electric field to move charged particles to a collecting device where they are temporarily fixed, allowing for efficient separation and collection without additional additives, and enabling continuous processing.
Scale-up Strategies for Industrial Applications
Scaling up isoelectric focusing (IEF) from laboratory to industrial scale presents significant engineering challenges that require systematic approaches. The transition demands careful consideration of equipment design, process parameters, and economic factors. Industrial-scale IEF systems typically employ continuous flow or large-format gel-based setups that can handle volumes ranging from several liters to hundreds of liters, compared to milliliter-scale laboratory operations.
Key considerations for successful scale-up include maintaining pH gradient stability across larger dimensions. As the separation distance increases, gradient drift becomes more pronounced, necessitating robust buffer systems and precise temperature control mechanisms. Industrial applications often incorporate segmented cooling systems that maintain temperature uniformity within ±0.5°C across the entire separation chamber, preventing localized heating that can disrupt protein migration patterns.
Power management represents another critical factor in scale-up strategies. Industrial systems require sophisticated power distribution designs that deliver consistent electric field strength while preventing overheating. Modern approaches utilize pulsed field applications and segmented electrode arrays to maintain field homogeneity across larger separation distances, typically employing 10-20 kW power supplies with advanced surge protection.
Process intensification techniques have emerged as valuable strategies for industrial IEF applications. These include multi-stage cascading systems where proteins undergo sequential focusing steps, each with optimized parameters for specific pH ranges. This approach has demonstrated up to 40% higher throughput compared to single-stage operations while maintaining resolution quality.
Continuous monitoring and feedback control systems are essential components of industrial-scale IEF. Real-time conductivity mapping and pH sensors distributed throughout the separation chamber allow for dynamic adjustment of operating parameters. Advanced systems incorporate machine learning algorithms that predict and compensate for gradient drift based on historical performance data, reducing process variability by up to 60%.
Economic considerations must guide scale-up decisions, with capital equipment costs for industrial IEF systems ranging from $500,000 to several million dollars. Operating costs, including power consumption (typically 15-25 kWh per batch), buffer components, and maintenance, must be balanced against purification efficiency and product value. Hybrid approaches that combine IEF with complementary separation techniques often provide the most cost-effective solutions for industrial protein purification.
Key considerations for successful scale-up include maintaining pH gradient stability across larger dimensions. As the separation distance increases, gradient drift becomes more pronounced, necessitating robust buffer systems and precise temperature control mechanisms. Industrial applications often incorporate segmented cooling systems that maintain temperature uniformity within ±0.5°C across the entire separation chamber, preventing localized heating that can disrupt protein migration patterns.
Power management represents another critical factor in scale-up strategies. Industrial systems require sophisticated power distribution designs that deliver consistent electric field strength while preventing overheating. Modern approaches utilize pulsed field applications and segmented electrode arrays to maintain field homogeneity across larger separation distances, typically employing 10-20 kW power supplies with advanced surge protection.
Process intensification techniques have emerged as valuable strategies for industrial IEF applications. These include multi-stage cascading systems where proteins undergo sequential focusing steps, each with optimized parameters for specific pH ranges. This approach has demonstrated up to 40% higher throughput compared to single-stage operations while maintaining resolution quality.
Continuous monitoring and feedback control systems are essential components of industrial-scale IEF. Real-time conductivity mapping and pH sensors distributed throughout the separation chamber allow for dynamic adjustment of operating parameters. Advanced systems incorporate machine learning algorithms that predict and compensate for gradient drift based on historical performance data, reducing process variability by up to 60%.
Economic considerations must guide scale-up decisions, with capital equipment costs for industrial IEF systems ranging from $500,000 to several million dollars. Operating costs, including power consumption (typically 15-25 kWh per batch), buffer components, and maintenance, must be balanced against purification efficiency and product value. Hybrid approaches that combine IEF with complementary separation techniques often provide the most cost-effective solutions for industrial protein purification.
Environmental Impact of IEF Reagents
The environmental impact of reagents used in isoelectric focusing (IEF) represents a significant concern in the broader context of sustainable laboratory practices. Carrier ampholytes, which are essential components in IEF techniques, typically contain polyamino-polycarboxylic acid compounds that may persist in aquatic environments after disposal. Studies have shown that these compounds exhibit slow biodegradation rates, with half-lives ranging from 28 to 76 days depending on their molecular complexity and environmental conditions.
Waste streams from IEF processes often contain not only carrier ampholytes but also urea, thiourea, and detergents like CHAPS or Triton X-100, which can contribute to aquatic toxicity when improperly disposed. Recent environmental assessments indicate that conventional wastewater treatment facilities remove only 60-75% of these compounds, allowing the remainder to enter natural water systems.
The production of IEF reagents also carries a substantial carbon footprint. Manufacturing processes for high-purity ampholytes require multiple purification steps and consume significant energy resources. Life cycle assessments reveal that producing one kilogram of research-grade carrier ampholytes generates approximately 18-22 kg of CO2 equivalent emissions, comparable to driving an average passenger vehicle for 45-55 miles.
Alternative, more environmentally friendly IEF reagents are emerging in response to these concerns. Bio-derived ampholytes produced from renewable resources show promise, with early studies demonstrating comparable separation efficiency while reducing environmental impact by 30-40%. Additionally, immobilized pH gradient (IPG) technologies, which require fewer chemical additives, represent a greener alternative to carrier ampholyte-based methods.
Regulatory frameworks governing laboratory waste disposal are becoming increasingly stringent worldwide. The European Union's REACH regulations and the United States EPA guidelines now specifically address the disposal of electrophoresis reagents, mandating specialized treatment protocols for IEF waste streams. Laboratories failing to comply with these regulations face significant penalties, with fines ranging from $10,000 to $50,000 per violation in the United States.
Best practices for minimizing the environmental impact of IEF procedures include reagent recycling systems, which can recover and purify up to 70% of carrier ampholytes for reuse. Additionally, microfluidic IEF platforms reduce reagent consumption by up to 95% compared to traditional methods while maintaining comparable resolution. These innovations represent promising directions for developing more sustainable protein purification methodologies.
Waste streams from IEF processes often contain not only carrier ampholytes but also urea, thiourea, and detergents like CHAPS or Triton X-100, which can contribute to aquatic toxicity when improperly disposed. Recent environmental assessments indicate that conventional wastewater treatment facilities remove only 60-75% of these compounds, allowing the remainder to enter natural water systems.
The production of IEF reagents also carries a substantial carbon footprint. Manufacturing processes for high-purity ampholytes require multiple purification steps and consume significant energy resources. Life cycle assessments reveal that producing one kilogram of research-grade carrier ampholytes generates approximately 18-22 kg of CO2 equivalent emissions, comparable to driving an average passenger vehicle for 45-55 miles.
Alternative, more environmentally friendly IEF reagents are emerging in response to these concerns. Bio-derived ampholytes produced from renewable resources show promise, with early studies demonstrating comparable separation efficiency while reducing environmental impact by 30-40%. Additionally, immobilized pH gradient (IPG) technologies, which require fewer chemical additives, represent a greener alternative to carrier ampholyte-based methods.
Regulatory frameworks governing laboratory waste disposal are becoming increasingly stringent worldwide. The European Union's REACH regulations and the United States EPA guidelines now specifically address the disposal of electrophoresis reagents, mandating specialized treatment protocols for IEF waste streams. Laboratories failing to comply with these regulations face significant penalties, with fines ranging from $10,000 to $50,000 per violation in the United States.
Best practices for minimizing the environmental impact of IEF procedures include reagent recycling systems, which can recover and purify up to 70% of carrier ampholytes for reuse. Additionally, microfluidic IEF platforms reduce reagent consumption by up to 95% compared to traditional methods while maintaining comparable resolution. These innovations represent promising directions for developing more sustainable protein purification methodologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!