Understanding Isoelectric Focusing for Mono vs Polydisperse Samples
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Evolution and Objectives
Isoelectric focusing (IEF) emerged in the 1960s as a powerful analytical technique for protein separation based on their isoelectric points (pI). The technology evolved from early zone electrophoresis methods, with Svensson and Vesterberg pioneering the development of carrier ampholytes that created stable pH gradients. This breakthrough transformed protein analysis by enabling high-resolution separation of complex mixtures.
The evolution of IEF technology has been marked by significant advancements in both methodology and instrumentation. Traditional IEF initially utilized polyacrylamide gels with carrier ampholytes, but faced challenges with gradient stability and reproducibility. The 1980s saw the introduction of immobilized pH gradient (IPG) technology by Bjellqvist and Righetti, creating covalently linked buffering groups that dramatically improved stability and reproducibility.
Further technological progression led to the development of capillary isoelectric focusing (cIEF) in the 1990s, which miniaturized the process and enabled integration with mass spectrometry. Recent innovations include microfluidic IEF platforms and digital microfluidic devices that allow for automation and higher throughput analysis with minimal sample consumption.
The distinction between monodisperse and polydisperse samples represents a critical focus in contemporary IEF research. Monodisperse samples contain molecules with uniform properties, while polydisperse samples contain heterogeneous populations with varying characteristics. Traditional IEF methodologies were primarily optimized for monodisperse protein samples, creating challenges when analyzing complex polydisperse mixtures like antibodies with post-translational modifications or nanoparticles with surface charge variations.
The primary objectives of current IEF technology development include enhancing resolution for complex polydisperse samples, improving quantitative analysis capabilities, and developing specialized methodologies for challenging sample types. Researchers aim to overcome limitations in analyzing samples with subtle charge differences or those prone to precipitation at their isoelectric points.
Future technological trajectories point toward integration with advanced detection systems, development of novel ampholytes for extreme pH ranges, and computational tools for data interpretation. Machine learning algorithms are increasingly being applied to extract meaningful patterns from complex IEF data, particularly for polydisperse samples where traditional analysis methods may be insufficient.
The ultimate goal is to establish IEF as a robust analytical platform capable of high-resolution characterization of both monodisperse and polydisperse samples across diverse applications including biopharmaceutical quality control, proteomics research, and nanomaterial characterization.
The evolution of IEF technology has been marked by significant advancements in both methodology and instrumentation. Traditional IEF initially utilized polyacrylamide gels with carrier ampholytes, but faced challenges with gradient stability and reproducibility. The 1980s saw the introduction of immobilized pH gradient (IPG) technology by Bjellqvist and Righetti, creating covalently linked buffering groups that dramatically improved stability and reproducibility.
Further technological progression led to the development of capillary isoelectric focusing (cIEF) in the 1990s, which miniaturized the process and enabled integration with mass spectrometry. Recent innovations include microfluidic IEF platforms and digital microfluidic devices that allow for automation and higher throughput analysis with minimal sample consumption.
The distinction between monodisperse and polydisperse samples represents a critical focus in contemporary IEF research. Monodisperse samples contain molecules with uniform properties, while polydisperse samples contain heterogeneous populations with varying characteristics. Traditional IEF methodologies were primarily optimized for monodisperse protein samples, creating challenges when analyzing complex polydisperse mixtures like antibodies with post-translational modifications or nanoparticles with surface charge variations.
The primary objectives of current IEF technology development include enhancing resolution for complex polydisperse samples, improving quantitative analysis capabilities, and developing specialized methodologies for challenging sample types. Researchers aim to overcome limitations in analyzing samples with subtle charge differences or those prone to precipitation at their isoelectric points.
Future technological trajectories point toward integration with advanced detection systems, development of novel ampholytes for extreme pH ranges, and computational tools for data interpretation. Machine learning algorithms are increasingly being applied to extract meaningful patterns from complex IEF data, particularly for polydisperse samples where traditional analysis methods may be insufficient.
The ultimate goal is to establish IEF as a robust analytical platform capable of high-resolution characterization of both monodisperse and polydisperse samples across diverse applications including biopharmaceutical quality control, proteomics research, and nanomaterial characterization.
Market Applications of Isoelectric Focusing
Isoelectric focusing (IEF) has established itself as a cornerstone technology across multiple industries, with applications spanning from pharmaceutical development to food science. The market for IEF technologies continues to expand as industries recognize its value in protein characterization and separation processes.
In the pharmaceutical and biopharmaceutical sectors, IEF serves as a critical quality control tool for therapeutic protein manufacturing. The ability to distinguish between monodisperse and polydisperse samples enables manufacturers to ensure batch consistency and product purity. This application has become increasingly important with the rise of biosimilars and biobetters, where precise characterization of charge variants is essential for regulatory approval.
Clinical diagnostics represents another significant market application, where IEF techniques are employed in the detection of specific protein markers associated with various diseases. The technology's high resolution makes it particularly valuable for identifying subtle changes in protein profiles that may indicate pathological conditions. Hospitals and diagnostic laboratories utilize IEF for conditions ranging from multiple sclerosis to hemoglobinopathies.
The food and beverage industry has adopted IEF for protein characterization in various products, particularly dairy and plant-based alternatives. Manufacturers use this technique to analyze protein composition, which directly impacts functional properties such as emulsification, foaming, and gelation. This application has grown significantly with the increasing consumer demand for plant-based proteins and novel food formulations.
Academic and research institutions constitute a stable market segment for IEF technologies, where the technique is employed in fundamental protein research and proteomics studies. The ability to separate proteins based on their isoelectric points provides researchers with valuable insights into protein structure and function, supporting advancements in fields such as structural biology and drug discovery.
Environmental monitoring represents an emerging application area, where IEF is used to analyze protein biomarkers in environmental samples. This application helps in assessing ecosystem health and detecting contaminants that may affect protein structures in organisms.
The forensic science field has also begun incorporating IEF techniques for protein-based identification methods, complementing traditional DNA analysis in cases where genetic material may be degraded or limited.
As technology advances, miniaturized and automated IEF systems are opening new market opportunities in point-of-care diagnostics and personalized medicine, where rapid protein characterization can inform treatment decisions and monitor therapeutic responses in real-time.
In the pharmaceutical and biopharmaceutical sectors, IEF serves as a critical quality control tool for therapeutic protein manufacturing. The ability to distinguish between monodisperse and polydisperse samples enables manufacturers to ensure batch consistency and product purity. This application has become increasingly important with the rise of biosimilars and biobetters, where precise characterization of charge variants is essential for regulatory approval.
Clinical diagnostics represents another significant market application, where IEF techniques are employed in the detection of specific protein markers associated with various diseases. The technology's high resolution makes it particularly valuable for identifying subtle changes in protein profiles that may indicate pathological conditions. Hospitals and diagnostic laboratories utilize IEF for conditions ranging from multiple sclerosis to hemoglobinopathies.
The food and beverage industry has adopted IEF for protein characterization in various products, particularly dairy and plant-based alternatives. Manufacturers use this technique to analyze protein composition, which directly impacts functional properties such as emulsification, foaming, and gelation. This application has grown significantly with the increasing consumer demand for plant-based proteins and novel food formulations.
Academic and research institutions constitute a stable market segment for IEF technologies, where the technique is employed in fundamental protein research and proteomics studies. The ability to separate proteins based on their isoelectric points provides researchers with valuable insights into protein structure and function, supporting advancements in fields such as structural biology and drug discovery.
Environmental monitoring represents an emerging application area, where IEF is used to analyze protein biomarkers in environmental samples. This application helps in assessing ecosystem health and detecting contaminants that may affect protein structures in organisms.
The forensic science field has also begun incorporating IEF techniques for protein-based identification methods, complementing traditional DNA analysis in cases where genetic material may be degraded or limited.
As technology advances, miniaturized and automated IEF systems are opening new market opportunities in point-of-care diagnostics and personalized medicine, where rapid protein characterization can inform treatment decisions and monitor therapeutic responses in real-time.
Current IEF Techniques and Limitations
Isoelectric focusing (IEF) represents a powerful electrophoretic technique for separating amphoteric molecules, particularly proteins, based on their isoelectric points (pI). Current IEF methodologies can be broadly categorized into several formats: gel-based IEF, capillary IEF (cIEF), and more recently, microchip-based IEF systems. Each format offers distinct advantages while presenting specific limitations when analyzing monodisperse versus polydisperse samples.
Gel-based IEF remains the most traditional approach, utilizing polyacrylamide or agarose gels containing carrier ampholytes to establish pH gradients. While this method provides excellent resolution for monodisperse samples with distinct pI values, it struggles with polydisperse samples due to band broadening effects and limited dynamic range. Additionally, gel-based techniques suffer from extended analysis times (often 4-24 hours), poor reproducibility between runs, and challenges in automation.
Capillary IEF has emerged as a significant advancement, offering higher resolution, faster analysis times, and improved quantification capabilities. The confined environment of capillaries enables better heat dissipation, allowing for higher electric field strengths and consequently shorter separation times (typically 10-30 minutes). For monodisperse samples, cIEF delivers exceptional resolution, often distinguishing proteins differing by as little as 0.01 pH units.
However, when analyzing polydisperse samples, cIEF encounters several limitations. The narrow capillary diameter restricts sample loading capacity, potentially missing low-abundance components in heterogeneous mixtures. Furthermore, protein-wall interactions can cause peak broadening and distortion, particularly problematic for polydisperse samples with varying surface properties.
Microchip-based IEF systems represent the cutting edge of current technology, offering miniaturized platforms with integrated functionalities. These systems excel in rapid analysis (often under 5 minutes) and minimal sample consumption (nanoliter volumes). While promising for both mono and polydisperse samples, microchip IEF still faces challenges in reproducibility and standardization across different laboratories.
A significant limitation across all current IEF techniques lies in the carrier ampholytes used to establish pH gradients. These commercial mixtures often exhibit batch-to-batch variability, affecting reproducibility particularly for polydisperse samples where subtle pH differences can significantly impact separation profiles. Immobilized pH gradient (IPG) technology has partially addressed this issue but introduces new challenges in terms of sample loading and recovery.
Detection sensitivity represents another critical limitation, especially for polydisperse samples containing components at varying concentrations. While UV absorption remains the standard detection method, it lacks sensitivity for minor components. Fluorescence and mass spectrometry coupling have improved detection capabilities but introduce additional complexity and potential bias toward certain molecular species.
Gel-based IEF remains the most traditional approach, utilizing polyacrylamide or agarose gels containing carrier ampholytes to establish pH gradients. While this method provides excellent resolution for monodisperse samples with distinct pI values, it struggles with polydisperse samples due to band broadening effects and limited dynamic range. Additionally, gel-based techniques suffer from extended analysis times (often 4-24 hours), poor reproducibility between runs, and challenges in automation.
Capillary IEF has emerged as a significant advancement, offering higher resolution, faster analysis times, and improved quantification capabilities. The confined environment of capillaries enables better heat dissipation, allowing for higher electric field strengths and consequently shorter separation times (typically 10-30 minutes). For monodisperse samples, cIEF delivers exceptional resolution, often distinguishing proteins differing by as little as 0.01 pH units.
However, when analyzing polydisperse samples, cIEF encounters several limitations. The narrow capillary diameter restricts sample loading capacity, potentially missing low-abundance components in heterogeneous mixtures. Furthermore, protein-wall interactions can cause peak broadening and distortion, particularly problematic for polydisperse samples with varying surface properties.
Microchip-based IEF systems represent the cutting edge of current technology, offering miniaturized platforms with integrated functionalities. These systems excel in rapid analysis (often under 5 minutes) and minimal sample consumption (nanoliter volumes). While promising for both mono and polydisperse samples, microchip IEF still faces challenges in reproducibility and standardization across different laboratories.
A significant limitation across all current IEF techniques lies in the carrier ampholytes used to establish pH gradients. These commercial mixtures often exhibit batch-to-batch variability, affecting reproducibility particularly for polydisperse samples where subtle pH differences can significantly impact separation profiles. Immobilized pH gradient (IPG) technology has partially addressed this issue but introduces new challenges in terms of sample loading and recovery.
Detection sensitivity represents another critical limitation, especially for polydisperse samples containing components at varying concentrations. While UV absorption remains the standard detection method, it lacks sensitivity for minor components. Fluorescence and mass spectrometry coupling have improved detection capabilities but introduce additional complexity and potential bias toward certain molecular species.
Methodologies for Mono vs Polydisperse Separation
01 Gel composition and preparation for isoelectric focusing
Various gel compositions and preparation methods are used for isoelectric focusing to improve sample separation. These include specialized polyacrylamide gels, ampholyte-containing gels, and immobilized pH gradient gels. The composition of these gels affects resolution, reproducibility, and separation efficiency of proteins and other biomolecules based on their isoelectric points. Optimized gel formulations can enhance the detection of minor components in complex biological samples.- Gel composition and preparation for isoelectric focusing: Various gel compositions and preparation methods are used for isoelectric focusing to enhance sample separation. These include specialized polyacrylamide gel formulations, ampholyte-containing gels, and immobilized pH gradient gels. The composition of these gels affects resolution, separation efficiency, and reproducibility of results. Innovations in gel preparation techniques have led to improved stability and performance in isoelectric focusing applications.
- Apparatus and devices for isoelectric focusing: Specialized equipment and devices have been developed for isoelectric focusing sample separation. These include electrophoresis chambers, microfluidic devices, and automated systems that control parameters such as temperature, voltage, and buffer conditions. Advanced apparatus designs incorporate features for improved heat dissipation, sample loading, and detection capabilities, resulting in more efficient and reproducible separations.
- Sample preparation techniques for isoelectric focusing: Proper sample preparation is crucial for successful isoelectric focusing separation. Techniques include protein solubilization methods, removal of interfering substances, concentration adjustments, and addition of carrier ampholytes. Pretreatment steps such as dialysis, precipitation, or fractionation may be employed to improve resolution and prevent protein aggregation during the focusing process.
- Detection and analysis methods for separated samples: After isoelectric focusing separation, various detection and analysis methods are employed to visualize and quantify the separated components. These include staining techniques, immunodetection, mass spectrometry, and imaging systems. Advanced detection methods incorporate fluorescent markers, chemiluminescent reagents, and digital imaging technology to enhance sensitivity and provide quantitative analysis of the separated samples.
- Applications and innovations in isoelectric focusing: Isoelectric focusing has diverse applications in protein characterization, biomarker discovery, quality control, and diagnostics. Recent innovations include miniaturized systems, integration with other separation techniques, and specialized protocols for challenging samples. Advancements in this field have enabled the analysis of complex biological mixtures, post-translational modifications, and protein variants with improved resolution and sensitivity.
02 Equipment and apparatus for isoelectric focusing
Specialized equipment and apparatus have been developed for isoelectric focusing sample separation. These include electrophoresis chambers, microfluidic devices, and automated systems that control temperature, voltage, and other parameters during separation. Advanced equipment can improve resolution, reduce analysis time, and allow for high-throughput processing of multiple samples simultaneously. Some systems integrate sample preparation, separation, and detection into a single platform.Expand Specific Solutions03 Sample preparation techniques for isoelectric focusing
Effective sample preparation is crucial for successful isoelectric focusing separation. Techniques include protein extraction, purification, concentration, and denaturation methods that maintain the native charge properties of the molecules. Removal of interfering substances such as salts, detergents, and nucleic acids improves resolution and prevents artifacts. Specialized buffers and additives can enhance solubility and prevent protein aggregation during the focusing process.Expand Specific Solutions04 Detection and analysis methods after isoelectric focusing
Various detection and analysis methods are employed after isoelectric focusing separation to visualize and quantify the separated components. These include staining techniques (such as Coomassie blue, silver staining, or fluorescent dyes), immunoblotting, mass spectrometry, and image analysis software. Some methods allow for the recovery of separated proteins for further analysis while maintaining their biological activity. Advanced detection systems can provide high sensitivity for low-abundance proteins.Expand Specific Solutions05 Two-dimensional separation combining isoelectric focusing with other techniques
Two-dimensional separation methods combine isoelectric focusing with other separation techniques to achieve higher resolution and better characterization of complex samples. Common approaches include coupling isoelectric focusing with SDS-PAGE, size exclusion chromatography, or capillary electrophoresis. These combined methods separate proteins based on multiple physicochemical properties, significantly increasing the number of distinguishable components in complex biological samples and enabling more comprehensive proteome analysis.Expand Specific Solutions
Leading Companies and Research Institutions
Isoelectric Focusing (IEF) for mono vs polydisperse samples is currently in a growth phase, with the global market expanding due to increasing applications in proteomics and biopharmaceutical analysis. The technology has reached moderate maturity, with established players like Bio-Rad Laboratories, Agilent Technologies, and ProteinSimple offering commercial platforms. Academic institutions including MIT, Northeastern University, and Sichuan University are advancing fundamental research, while pharmaceutical companies such as Roche Diagnostics, Bristol Myers Squibb, and Regeneron are implementing IEF for quality control of biologics. The technology shows divergent maturity levels: highly developed for monodisperse protein analysis but still evolving for complex polydisperse samples, with recent innovations focusing on microfluidic approaches and integration with mass spectrometry for enhanced resolution and throughput.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced isoelectric focusing (IEF) systems specifically designed to differentiate between mono and polydisperse protein samples. Their technology utilizes immobilized pH gradient (IPG) strips with high-resolution capabilities that can separate proteins differing by as little as 0.01 pH units. For monodisperse samples, Bio-Rad's system employs narrow-range IPG strips that maximize resolution within a specific pH range, allowing for detailed characterization of subtle charge variants. For polydisperse samples, they've implemented a two-dimensional approach combining IEF with orthogonal separation techniques to resolve complex mixtures. Their ProteOn™ XPR36 system incorporates real-time analysis capabilities that can monitor the focusing process dynamically, providing insights into the behavior of both mono and polydisperse samples during separation. Bio-Rad has also developed specialized software algorithms that can distinguish between true isoelectric points and artifacts caused by protein-protein interactions or sample matrix effects.
Strengths: Exceptional resolution capabilities with their narrow-range IPG strips; comprehensive integration of hardware and analysis software; established reputation in protein separation technologies. Weaknesses: Their systems typically require significant laboratory infrastructure and technical expertise; higher cost compared to simpler electrophoresis methods; some of their advanced features may be excessive for routine applications.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. has developed the BD Accuri™ C6 Plus system with specialized isoelectric focusing capabilities for distinguishing between mono and polydisperse protein samples. Their approach integrates flow cytometry principles with isoelectric focusing to enable single-particle analysis of complex biological samples. For monodisperse samples, BD's technology employs fluorescently labeled pI markers that allow precise determination of isoelectric points with minimal sample preparation. Their system can process polydisperse samples through a patented microfluidic channel design that prevents protein aggregation during the focusing process, maintaining the integrity of individual components. BD has also implemented machine learning algorithms that can identify subtle patterns in electrophoretic mobility data, enabling automated classification of sample heterogeneity. Their technology incorporates real-time pH monitoring throughout the separation channel, ensuring accurate correlation between detected signals and actual isoelectric points. This is particularly valuable for analyzing therapeutic proteins where charge variants can significantly impact efficacy and safety profiles.
Strengths: Integration with flow cytometry enables single-particle analysis capabilities; high throughput processing suitable for clinical applications; excellent sensitivity for detecting minor components in complex mixtures. Weaknesses: Complex instrumentation requires specialized maintenance; higher initial investment compared to conventional electrophoresis systems; limited compatibility with certain buffer systems commonly used in protein analysis.
Key Patents and Innovations in IEF
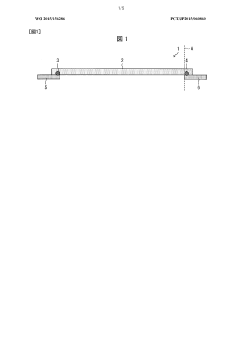
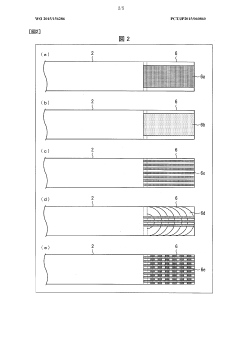
Isoelectric focusing apparatus and isoelectric focusing method
PatentWO2015156286A1
Innovation
- An isoelectric focusing instrument with a gel having a pH gradient and electrodes in contact with a solvent retention system that holds a solvent to prevent ionic contaminants from accumulating on the electrodes, using a solvent retention portion to diffuse contaminants into the solvent and maintain clear electrophoresis results.
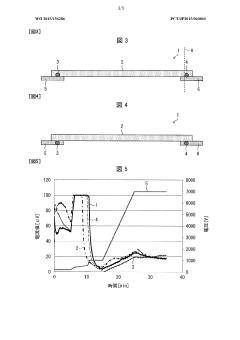
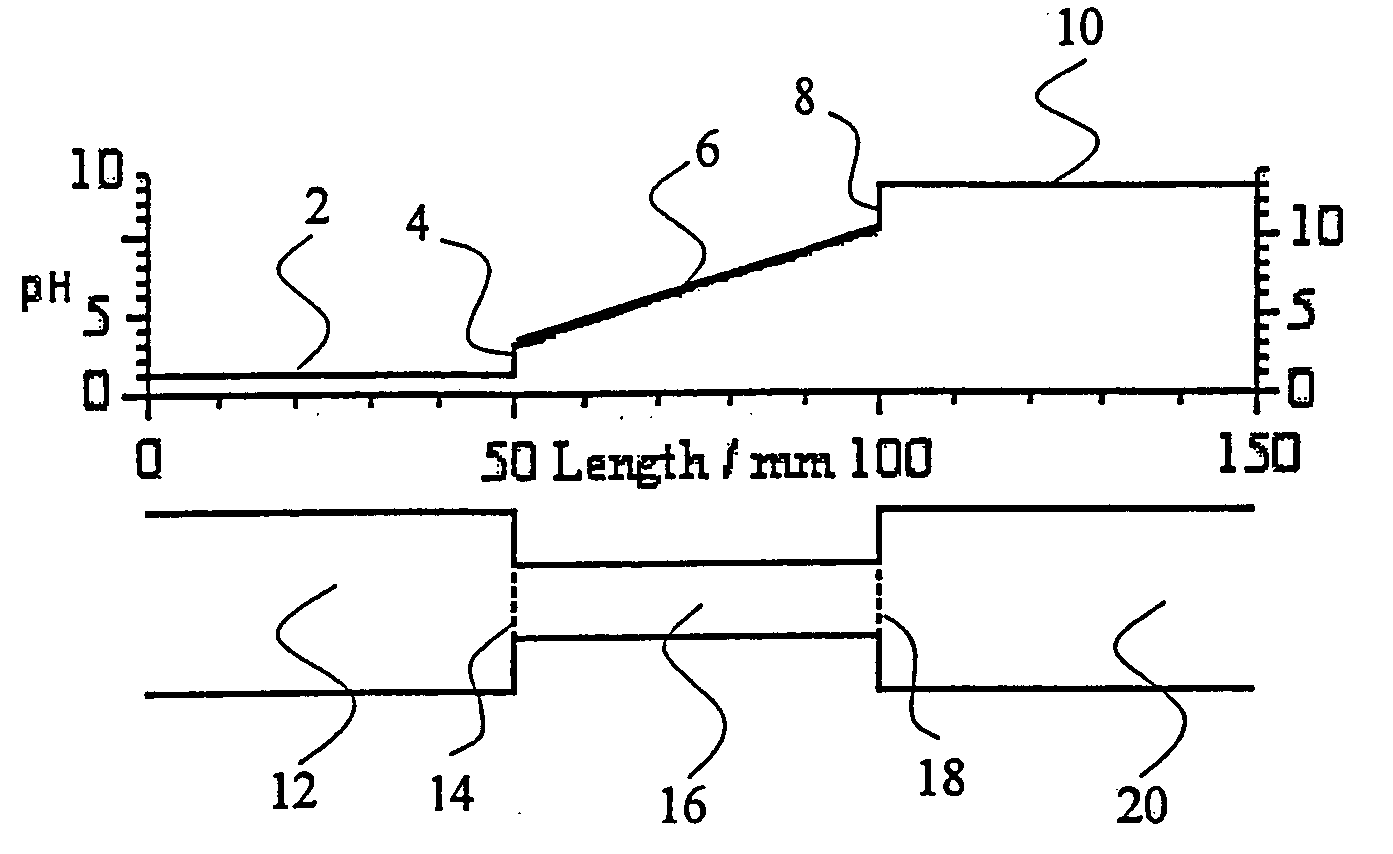
Method and apparatus to improve the concentration detection sensitivity in isoelectric focusing systems
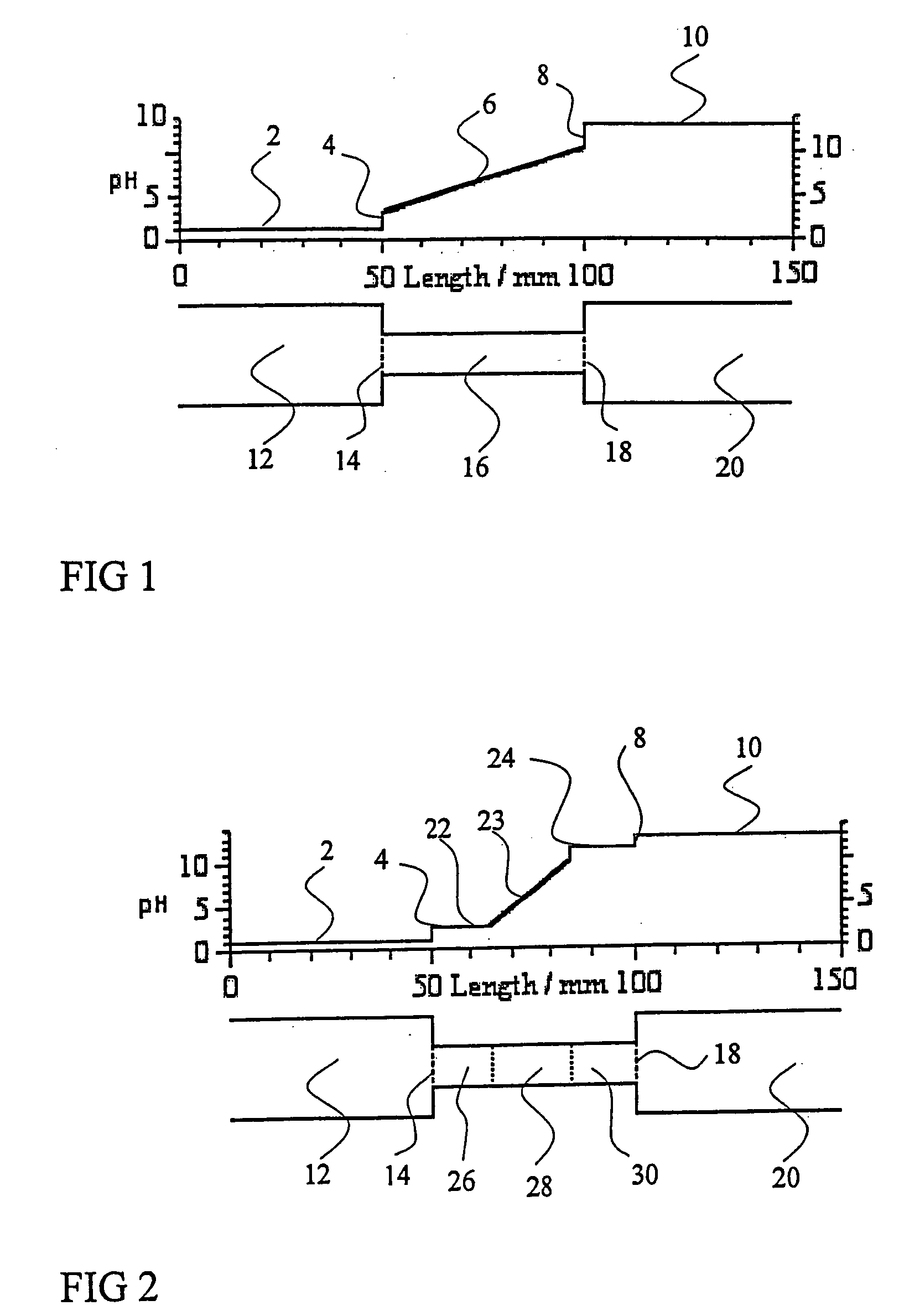
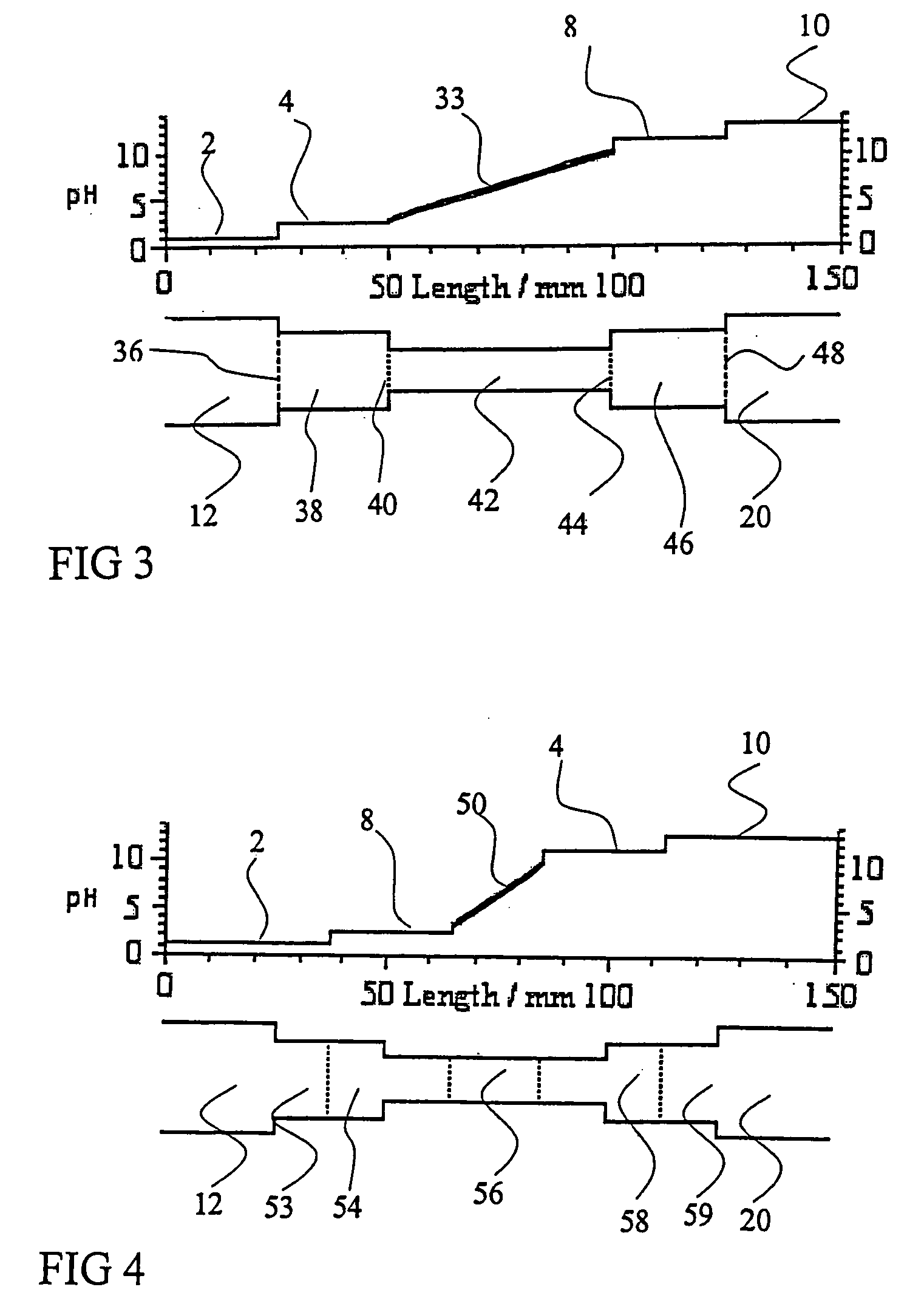
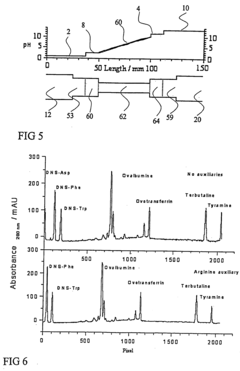
PatentInactiveUS20050161332A1
Innovation
- The addition of an auxiliary compartment to the separation capillary, combined with suitable auxiliary agents such as strong or weak electrolytes or ampholytic substances, increases the sample holding volume and forces ampholytic components into the detection area, improving concentration detection limits and correcting salt-induced pH gradient shifts.
Validation and Quality Control Protocols
Validation and quality control protocols are essential components in isoelectric focusing (IEF) experiments, particularly when comparing monodisperse and polydisperse samples. These protocols ensure the reliability, reproducibility, and accuracy of the analytical results obtained through IEF techniques.
For monodisperse samples, validation begins with purity assessment using complementary techniques such as size exclusion chromatography (SEC) or mass spectrometry to confirm sample homogeneity before IEF analysis. This step is critical as it establishes a baseline for subsequent IEF pattern interpretation. Conversely, polydisperse samples require characterization of heterogeneity parameters, including size distribution and charge variants, to properly contextualize IEF results.
Standard operating procedures (SOPs) must be established with specific parameters tailored to each sample type. For monodisperse samples, these include narrower pH gradients and higher resolution settings, while polydisperse samples benefit from wider pH ranges and optimized separation conditions. Regular calibration of pH gradients using well-characterized pI markers ensures accurate pI determination across experimental runs.
Quality control measures should incorporate system suitability tests performed at regular intervals. These tests utilize reference standards with known isoelectric points to verify system performance. For monodisperse samples, resolution parameters and peak symmetry are key quality indicators, whereas for polydisperse samples, the ability to resolve complex patterns and maintain consistent band distribution profiles becomes paramount.
Statistical analysis frameworks must be implemented to quantitatively assess IEF results. For monodisperse samples, this includes calculating the coefficient of variation for pI values across multiple runs, with acceptance criteria typically set at ≤1%. Polydisperse samples require more sophisticated statistical approaches, such as pattern recognition algorithms and distribution analysis, with established acceptance criteria for pattern reproducibility.
Documentation practices should include comprehensive records of all experimental conditions, including ampholyte composition, voltage gradients, and temperature control parameters. These records are particularly important when comparing results between mono and polydisperse samples, as subtle variations in conditions can significantly impact separation patterns.
Interlaboratory comparison studies provide an additional layer of validation, especially for novel applications or method transfers. These studies should include both monodisperse reference standards and well-characterized polydisperse samples to establish method robustness across different laboratory environments and instrument platforms.
For monodisperse samples, validation begins with purity assessment using complementary techniques such as size exclusion chromatography (SEC) or mass spectrometry to confirm sample homogeneity before IEF analysis. This step is critical as it establishes a baseline for subsequent IEF pattern interpretation. Conversely, polydisperse samples require characterization of heterogeneity parameters, including size distribution and charge variants, to properly contextualize IEF results.
Standard operating procedures (SOPs) must be established with specific parameters tailored to each sample type. For monodisperse samples, these include narrower pH gradients and higher resolution settings, while polydisperse samples benefit from wider pH ranges and optimized separation conditions. Regular calibration of pH gradients using well-characterized pI markers ensures accurate pI determination across experimental runs.
Quality control measures should incorporate system suitability tests performed at regular intervals. These tests utilize reference standards with known isoelectric points to verify system performance. For monodisperse samples, resolution parameters and peak symmetry are key quality indicators, whereas for polydisperse samples, the ability to resolve complex patterns and maintain consistent band distribution profiles becomes paramount.
Statistical analysis frameworks must be implemented to quantitatively assess IEF results. For monodisperse samples, this includes calculating the coefficient of variation for pI values across multiple runs, with acceptance criteria typically set at ≤1%. Polydisperse samples require more sophisticated statistical approaches, such as pattern recognition algorithms and distribution analysis, with established acceptance criteria for pattern reproducibility.
Documentation practices should include comprehensive records of all experimental conditions, including ampholyte composition, voltage gradients, and temperature control parameters. These records are particularly important when comparing results between mono and polydisperse samples, as subtle variations in conditions can significantly impact separation patterns.
Interlaboratory comparison studies provide an additional layer of validation, especially for novel applications or method transfers. These studies should include both monodisperse reference standards and well-characterized polydisperse samples to establish method robustness across different laboratory environments and instrument platforms.
Regulatory Considerations for IEF Applications
Isoelectric focusing (IEF) applications are subject to various regulatory frameworks depending on their intended use, particularly in clinical diagnostics, pharmaceutical development, and food safety. The U.S. Food and Drug Administration (FDA) regulates IEF technologies used in medical devices and diagnostic applications under the Medical Device Regulations (21 CFR Part 820). For IEF applications in pharmaceutical development, compliance with Good Manufacturing Practices (GMPs) is mandatory, especially when characterizing monoclonal antibodies and other biopharmaceuticals.
The European Medicines Agency (EMA) has established specific guidelines for the use of IEF in quality control of biological medicinal products. These guidelines emphasize validation requirements that differ significantly between monodisperse samples (like purified proteins) and polydisperse samples (such as complex biological mixtures). For monodisperse samples, regulatory bodies typically require demonstration of method specificity, accuracy, and precision, while polydisperse samples demand additional validation parameters including robustness across sample heterogeneity.
ISO standards, particularly ISO 13485 for medical devices, provide frameworks for quality management systems that manufacturers must implement when using IEF for analytical purposes. The International Conference on Harmonisation (ICH) guidelines, specifically ICH Q6B, address the specifications for biotechnological/biological products and include considerations for IEF as an identity test, with different acceptance criteria for mono versus polydisperse samples.
Regulatory submissions involving IEF data must address method validation according to ICH Q2(R1) guidelines. For monodisperse samples, validation focuses on demonstrating reproducibility of the isoelectric point (pI) determination, while polydisperse samples require validation of the entire pI distribution pattern. The FDA's Guidance for Industry on Analytical Procedures and Methods Validation for Drugs and Biologics provides specific recommendations for electrophoretic methods including IEF.
Laboratory accreditation standards such as ISO/IEC 17025 impose additional requirements on facilities performing IEF analysis, including proficiency testing and measurement uncertainty calculations that vary based on sample complexity. Recent regulatory trends indicate increasing scrutiny of IEF method transfer between laboratories, particularly for complex polydisperse samples where inter-laboratory reproducibility presents greater challenges.
Regulatory bodies are increasingly requiring risk assessments for analytical methods, with higher scrutiny for polydisperse sample analysis due to inherent variability. Documentation requirements also differ substantially, with polydisperse sample analysis typically requiring more comprehensive records of method development, optimization parameters, and acceptance criteria justification compared to the relatively straightforward requirements for monodisperse samples.
The European Medicines Agency (EMA) has established specific guidelines for the use of IEF in quality control of biological medicinal products. These guidelines emphasize validation requirements that differ significantly between monodisperse samples (like purified proteins) and polydisperse samples (such as complex biological mixtures). For monodisperse samples, regulatory bodies typically require demonstration of method specificity, accuracy, and precision, while polydisperse samples demand additional validation parameters including robustness across sample heterogeneity.
ISO standards, particularly ISO 13485 for medical devices, provide frameworks for quality management systems that manufacturers must implement when using IEF for analytical purposes. The International Conference on Harmonisation (ICH) guidelines, specifically ICH Q6B, address the specifications for biotechnological/biological products and include considerations for IEF as an identity test, with different acceptance criteria for mono versus polydisperse samples.
Regulatory submissions involving IEF data must address method validation according to ICH Q2(R1) guidelines. For monodisperse samples, validation focuses on demonstrating reproducibility of the isoelectric point (pI) determination, while polydisperse samples require validation of the entire pI distribution pattern. The FDA's Guidance for Industry on Analytical Procedures and Methods Validation for Drugs and Biologics provides specific recommendations for electrophoretic methods including IEF.
Laboratory accreditation standards such as ISO/IEC 17025 impose additional requirements on facilities performing IEF analysis, including proficiency testing and measurement uncertainty calculations that vary based on sample complexity. Recent regulatory trends indicate increasing scrutiny of IEF method transfer between laboratories, particularly for complex polydisperse samples where inter-laboratory reproducibility presents greater challenges.
Regulatory bodies are increasingly requiring risk assessments for analytical methods, with higher scrutiny for polydisperse sample analysis due to inherent variability. Documentation requirements also differ substantially, with polydisperse sample analysis typically requiring more comprehensive records of method development, optimization parameters, and acceptance criteria justification compared to the relatively straightforward requirements for monodisperse samples.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!