Is Isoelectric Focusing Effective for Complex Protein Samples?
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Background and Objectives
Isoelectric focusing (IEF) emerged in the 1960s as a groundbreaking technique for protein separation based on differences in isoelectric points (pI). This analytical method has evolved significantly over the past six decades, transitioning from conventional gel-based approaches to sophisticated capillary and microchip formats. The historical development of IEF reflects broader trends in proteomics research, where increasing demands for higher resolution, greater sensitivity, and improved reproducibility have driven continuous technological innovation.
The fundamental principle of IEF relies on establishing a pH gradient within a medium and applying an electric field, causing proteins to migrate until reaching their pI, where they carry no net charge and focusing occurs. This elegant mechanism provides exceptional resolving power compared to other electrophoretic techniques, making it particularly valuable for distinguishing protein isoforms and post-translational modifications that might otherwise remain undetected.
Recent technological advancements have expanded IEF capabilities through integration with other analytical platforms. Two-dimensional electrophoresis (2-DE), combining IEF with SDS-PAGE, has become a cornerstone methodology in proteomics research. Further innovations include immobilized pH gradient (IPG) strips, which have largely replaced carrier ampholytes due to their superior stability and reproducibility. Additionally, the development of ultra-narrow pH range gradients has enabled unprecedented resolution for targeted protein analysis.
The current trajectory of IEF technology is moving toward miniaturization, automation, and integration with mass spectrometry. Microfluidic IEF systems offer advantages of reduced sample consumption, faster analysis times, and potential for high-throughput applications. Meanwhile, the coupling of IEF with advanced MS techniques has created powerful workflows for comprehensive proteome characterization, addressing the growing complexity of biological samples in modern research.
The primary objective of contemporary IEF development is to overcome persistent challenges in analyzing complex protein mixtures. These challenges include resolving proteins with extreme pI values, minimizing protein precipitation at their pI, reducing sample loss during transfer between analytical dimensions, and improving detection sensitivity for low-abundance proteins. Additionally, there is significant interest in developing standardized protocols that enhance reproducibility across laboratories and enable reliable quantitative analysis.
For complex protein samples specifically, the technical goals include achieving higher loading capacity without compromising resolution, developing specialized pH gradients for challenging sample types, and creating integrated workflows that maintain protein solubility throughout the analytical process. The ultimate aim is to establish IEF as a robust, high-resolution technique capable of comprehensive proteome coverage, particularly for samples containing proteins with diverse physicochemical properties and wide dynamic range of concentrations.
The fundamental principle of IEF relies on establishing a pH gradient within a medium and applying an electric field, causing proteins to migrate until reaching their pI, where they carry no net charge and focusing occurs. This elegant mechanism provides exceptional resolving power compared to other electrophoretic techniques, making it particularly valuable for distinguishing protein isoforms and post-translational modifications that might otherwise remain undetected.
Recent technological advancements have expanded IEF capabilities through integration with other analytical platforms. Two-dimensional electrophoresis (2-DE), combining IEF with SDS-PAGE, has become a cornerstone methodology in proteomics research. Further innovations include immobilized pH gradient (IPG) strips, which have largely replaced carrier ampholytes due to their superior stability and reproducibility. Additionally, the development of ultra-narrow pH range gradients has enabled unprecedented resolution for targeted protein analysis.
The current trajectory of IEF technology is moving toward miniaturization, automation, and integration with mass spectrometry. Microfluidic IEF systems offer advantages of reduced sample consumption, faster analysis times, and potential for high-throughput applications. Meanwhile, the coupling of IEF with advanced MS techniques has created powerful workflows for comprehensive proteome characterization, addressing the growing complexity of biological samples in modern research.
The primary objective of contemporary IEF development is to overcome persistent challenges in analyzing complex protein mixtures. These challenges include resolving proteins with extreme pI values, minimizing protein precipitation at their pI, reducing sample loss during transfer between analytical dimensions, and improving detection sensitivity for low-abundance proteins. Additionally, there is significant interest in developing standardized protocols that enhance reproducibility across laboratories and enable reliable quantitative analysis.
For complex protein samples specifically, the technical goals include achieving higher loading capacity without compromising resolution, developing specialized pH gradients for challenging sample types, and creating integrated workflows that maintain protein solubility throughout the analytical process. The ultimate aim is to establish IEF as a robust, high-resolution technique capable of comprehensive proteome coverage, particularly for samples containing proteins with diverse physicochemical properties and wide dynamic range of concentrations.
Market Demand for Protein Separation Technologies
The protein separation technology market has witnessed substantial growth in recent years, driven primarily by advancements in proteomics research, biopharmaceutical development, and diagnostic applications. The global market for protein separation technologies was valued at approximately $11.5 billion in 2022 and is projected to reach $19.8 billion by 2028, representing a compound annual growth rate (CAGR) of 9.5%.
Isoelectric focusing (IEF), as a high-resolution technique for separating proteins based on their isoelectric points, occupies a significant segment within this market. The demand for IEF and related technologies has been particularly strong in the biopharmaceutical sector, where the need for efficient characterization of complex protein mixtures continues to grow with the expansion of biologics development.
Research institutions and academic laboratories constitute another major market segment, with increasing focus on proteome analysis driving demand for sophisticated separation technologies. The ability to analyze complex protein samples effectively has become crucial in various research fields, including cancer research, neurodegenerative disease studies, and systems biology.
Clinical diagnostics represents an emerging application area with substantial growth potential. The identification of protein biomarkers for disease diagnosis, prognosis, and treatment monitoring has created new opportunities for protein separation technologies. IEF, with its high resolution capabilities, is increasingly being incorporated into diagnostic workflows for detecting subtle protein variations associated with pathological conditions.
Geographically, North America dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments, expanding biotechnology sectors in China and India, and growing awareness about proteomics applications.
End-user preferences indicate a shift toward integrated systems that combine multiple separation techniques, including IEF, to achieve comprehensive protein characterization. This trend reflects the growing complexity of protein samples being analyzed and the need for multidimensional separation approaches.
Market challenges include the high cost of advanced separation systems, technical complexity requiring specialized expertise, and competition from alternative technologies such as mass spectrometry-based approaches. Nevertheless, continuous innovations in IEF technology, including the development of improved ampholytes, specialized gels, and automated systems, are addressing these challenges and expanding market opportunities.
Industry surveys indicate that approximately 78% of proteomics researchers consider protein separation capabilities for complex samples as "very important" or "critical" to their work, highlighting the significant market demand for effective solutions in this area.
Isoelectric focusing (IEF), as a high-resolution technique for separating proteins based on their isoelectric points, occupies a significant segment within this market. The demand for IEF and related technologies has been particularly strong in the biopharmaceutical sector, where the need for efficient characterization of complex protein mixtures continues to grow with the expansion of biologics development.
Research institutions and academic laboratories constitute another major market segment, with increasing focus on proteome analysis driving demand for sophisticated separation technologies. The ability to analyze complex protein samples effectively has become crucial in various research fields, including cancer research, neurodegenerative disease studies, and systems biology.
Clinical diagnostics represents an emerging application area with substantial growth potential. The identification of protein biomarkers for disease diagnosis, prognosis, and treatment monitoring has created new opportunities for protein separation technologies. IEF, with its high resolution capabilities, is increasingly being incorporated into diagnostic workflows for detecting subtle protein variations associated with pathological conditions.
Geographically, North America dominates the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing R&D investments, expanding biotechnology sectors in China and India, and growing awareness about proteomics applications.
End-user preferences indicate a shift toward integrated systems that combine multiple separation techniques, including IEF, to achieve comprehensive protein characterization. This trend reflects the growing complexity of protein samples being analyzed and the need for multidimensional separation approaches.
Market challenges include the high cost of advanced separation systems, technical complexity requiring specialized expertise, and competition from alternative technologies such as mass spectrometry-based approaches. Nevertheless, continuous innovations in IEF technology, including the development of improved ampholytes, specialized gels, and automated systems, are addressing these challenges and expanding market opportunities.
Industry surveys indicate that approximately 78% of proteomics researchers consider protein separation capabilities for complex samples as "very important" or "critical" to their work, highlighting the significant market demand for effective solutions in this area.
Current Challenges in Complex Protein Sample Analysis
Despite significant advancements in proteomics technologies, complex protein sample analysis continues to present formidable challenges for researchers. The heterogeneity of protein mixtures derived from biological samples often contains thousands of different proteins with varying abundances spanning several orders of magnitude. This dynamic range issue severely hampers the detection and quantification of low-abundance proteins, which are frequently the most biologically significant in disease biomarker discovery and systems biology studies.
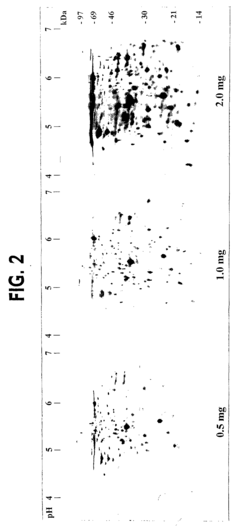
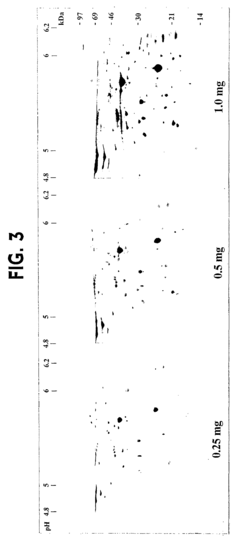
Isoelectric focusing (IEF), while powerful for separating proteins based on their isoelectric points, faces substantial limitations when applied to complex samples. The resolving power of traditional IEF becomes overwhelmed when handling samples containing more than a few hundred distinct proteins. This results in co-migration of multiple proteins to the same position on the pH gradient, creating overlapping bands that compromise downstream analysis and identification.
Sample preparation represents another critical challenge, as complex biological matrices contain interfering compounds such as lipids, nucleic acids, and metabolites that can disrupt the establishment of stable pH gradients during IEF. These contaminants often cause horizontal streaking, poor resolution, and irreproducible results. Furthermore, highly hydrophobic membrane proteins, which constitute approximately 30% of the proteome and include many therapeutic targets, are notoriously difficult to solubilize in IEF-compatible buffers without precipitation at their isoelectric points.
The limited loading capacity of IEF gels presents an additional obstacle when analyzing complex samples. This constraint particularly affects the detection of low-abundance proteins, which may be present at concentrations several orders of magnitude lower than high-abundance proteins. Despite various depletion strategies for removing abundant proteins, these approaches inevitably introduce biases and potential loss of important protein-protein interactions.
Post-translational modifications (PTMs) further complicate IEF analysis by altering proteins' isoelectric points, creating multiple spots for the same protein. While this property can be advantageous for studying PTMs, it significantly increases the complexity of the protein pattern and complicates quantitative analysis in already complex samples.
Integration with downstream analytical techniques poses additional challenges. The compatibility of IEF with subsequent mass spectrometry analysis requires careful consideration of ampholytes and other additives that may interfere with ionization efficiency. Moreover, the recovery of focused proteins from IEF gels for further analysis often results in significant sample loss, particularly problematic when working with limited biological material.
Recent technological innovations have attempted to address these limitations through the development of multimodal separation approaches, improved ampholytes, and specialized immobilized pH gradient strips. However, significant challenges remain in achieving comprehensive coverage of complex proteomes using IEF-based techniques.
Isoelectric focusing (IEF), while powerful for separating proteins based on their isoelectric points, faces substantial limitations when applied to complex samples. The resolving power of traditional IEF becomes overwhelmed when handling samples containing more than a few hundred distinct proteins. This results in co-migration of multiple proteins to the same position on the pH gradient, creating overlapping bands that compromise downstream analysis and identification.
Sample preparation represents another critical challenge, as complex biological matrices contain interfering compounds such as lipids, nucleic acids, and metabolites that can disrupt the establishment of stable pH gradients during IEF. These contaminants often cause horizontal streaking, poor resolution, and irreproducible results. Furthermore, highly hydrophobic membrane proteins, which constitute approximately 30% of the proteome and include many therapeutic targets, are notoriously difficult to solubilize in IEF-compatible buffers without precipitation at their isoelectric points.
The limited loading capacity of IEF gels presents an additional obstacle when analyzing complex samples. This constraint particularly affects the detection of low-abundance proteins, which may be present at concentrations several orders of magnitude lower than high-abundance proteins. Despite various depletion strategies for removing abundant proteins, these approaches inevitably introduce biases and potential loss of important protein-protein interactions.
Post-translational modifications (PTMs) further complicate IEF analysis by altering proteins' isoelectric points, creating multiple spots for the same protein. While this property can be advantageous for studying PTMs, it significantly increases the complexity of the protein pattern and complicates quantitative analysis in already complex samples.
Integration with downstream analytical techniques poses additional challenges. The compatibility of IEF with subsequent mass spectrometry analysis requires careful consideration of ampholytes and other additives that may interfere with ionization efficiency. Moreover, the recovery of focused proteins from IEF gels for further analysis often results in significant sample loss, particularly problematic when working with limited biological material.
Recent technological innovations have attempted to address these limitations through the development of multimodal separation approaches, improved ampholytes, and specialized immobilized pH gradient strips. However, significant challenges remain in achieving comprehensive coverage of complex proteomes using IEF-based techniques.
Current IEF Methodologies for Complex Samples
01 Improved gel compositions for isoelectric focusing
Advanced gel compositions have been developed to enhance the effectiveness of isoelectric focusing. These compositions include specialized polymers, buffer systems, and additives that improve resolution and separation of proteins. The improved gels allow for better stability during the focusing process, reduced protein aggregation, and enhanced visualization of separated components. These developments have significantly increased the sensitivity and reproducibility of isoelectric focusing techniques.- Improved gel compositions for isoelectric focusing: Advanced gel compositions have been developed to enhance the effectiveness of isoelectric focusing. These compositions include specialized polymers, buffer systems, and additives that improve resolution and separation of proteins. The improved gels allow for better stability during the focusing process, reduced protein aggregation, and enhanced visualization of separated components. These advancements lead to more precise protein separation based on their isoelectric points.
- Microfluidic and miniaturized IEF systems: Miniaturized isoelectric focusing systems utilize microfluidic technology to perform separations with higher efficiency and reduced sample volumes. These systems incorporate microchannels, specialized electrodes, and integrated detection methods to achieve rapid analysis with minimal reagent consumption. The miniaturized format allows for automation, parallelization, and integration with other analytical techniques, making isoelectric focusing more accessible for point-of-care diagnostics and high-throughput applications.
- Novel detection methods for IEF analysis: Innovative detection methods have been developed to enhance the visualization and quantification of proteins separated by isoelectric focusing. These include fluorescent labeling techniques, digital imaging systems, and spectroscopic methods that improve sensitivity and dynamic range. Some approaches incorporate real-time monitoring capabilities to observe the focusing process as it occurs. These detection advancements allow for the identification of low-abundance proteins and provide more accurate quantitative analysis.
- Multi-dimensional separation techniques incorporating IEF: Combining isoelectric focusing with other separation techniques creates powerful multi-dimensional analysis systems. These approaches typically use IEF as a first dimension separation followed by methods such as gel electrophoresis, chromatography, or mass spectrometry. The orthogonal separation mechanisms provide significantly improved resolution of complex protein mixtures. These integrated systems enable comprehensive proteome analysis with enhanced identification of protein variants and post-translational modifications.
- Specialized IEF applications for specific biomolecules: Tailored isoelectric focusing methods have been developed for specific types of biomolecules including antibodies, enzymes, and nucleic acids. These specialized applications incorporate optimized pH gradients, carrier ampholytes, and separation conditions designed for particular molecular characteristics. Some approaches focus on native protein analysis to maintain biological activity, while others are optimized for denatured proteins to achieve maximum resolution. These specialized techniques enhance the effectiveness of isoelectric focusing for targeted analytical purposes.
02 Microfluidic and miniaturized IEF systems
Miniaturized and microfluidic systems have revolutionized isoelectric focusing by allowing for analysis with minimal sample volumes and faster separation times. These systems incorporate microchannels, specialized electrodes, and integrated detection methods to perform high-resolution separations in compact formats. The miniaturization enables higher electric field strengths while minimizing Joule heating effects, resulting in improved focusing efficiency and resolution of closely related protein variants.Expand Specific Solutions03 Integration with other analytical techniques
The effectiveness of isoelectric focusing has been enhanced through integration with complementary analytical techniques. Combining IEF with mass spectrometry, chromatography, or electrophoresis in multidimensional separation approaches provides comprehensive protein characterization. These integrated systems allow for automated sample processing, improved detection sensitivity, and enhanced data analysis capabilities, making it possible to analyze complex biological samples with greater depth and accuracy.Expand Specific Solutions04 Advanced detection and imaging methods
Novel detection and imaging technologies have significantly improved the effectiveness of isoelectric focusing. These include fluorescent labeling strategies, digital imaging systems, and real-time monitoring capabilities that enhance sensitivity and enable quantitative analysis. Advanced detection methods allow for visualization of low-abundance proteins, improved dynamic range, and better discrimination between closely migrating protein bands, thereby increasing the overall analytical power of isoelectric focusing techniques.Expand Specific Solutions05 Optimization of focusing conditions and parameters
Research has focused on optimizing various parameters that affect isoelectric focusing effectiveness, including pH gradient formation, electric field application, temperature control, and sample preparation methods. Systematic approaches to parameter optimization have led to improved reproducibility, reduced focusing times, and enhanced resolution. These optimizations include the development of specialized ampholytes, carrier molecules, and protocols that minimize protein modifications during the focusing process, resulting in more accurate protein characterization.Expand Specific Solutions
Major Players in Protein Separation Industry
Isoelectric focusing (IEF) for complex protein samples is currently in a mature development stage, with significant market growth driven by proteomics research and biopharmaceutical applications. The global market is estimated at $1.5-2 billion, expanding at 6-8% annually. Leading companies like Bio-Rad Laboratories, ProteinSimple, and Life Technologies have developed advanced IEF platforms offering high resolution for complex samples. Philips, Regeneron, and MedImmune are investing in IEF applications for clinical diagnostics, while academic institutions including MIT, University of Washington, and Shanghai Jiao Tong University are advancing the technology through innovative approaches for handling sample complexity. Recent improvements in 2D electrophoresis integration and automation have significantly enhanced IEF's effectiveness for complex protein mixture analysis.
ProteinSimple
Technical Solution: ProteinSimple has revolutionized isoelectric focusing for complex protein samples with their automated capillary IEF (cIEF) systems, particularly the Maurice and Jess platforms. These systems utilize capillary electrophoresis principles combined with whole-column detection to achieve high-resolution separation of proteins based on their isoelectric points. Their proprietary technology addresses key challenges in complex sample analysis by incorporating automated sample handling that minimizes manual intervention and reduces variability. The cIEF approach requires minimal sample volumes (typically 1-4 μL) while delivering exceptional resolution of charge variants, making it particularly valuable for analyzing complex biological samples with limited availability[2]. ProteinSimple's systems employ specialized ampholyte mixtures optimized for different pH ranges, allowing researchers to focus on specific subsets of proteins within complex mixtures. Their technology also features real-time monitoring of the focusing process, enabling dynamic adjustment of electric field parameters to optimize separation of challenging samples.
Strengths: Exceptional automation reducing hands-on time and variability; minimal sample consumption; superior quantitative capabilities for charge variant analysis. Weaknesses: Limited throughput compared to gel-based methods; higher initial investment cost; requires specialized reagents and consumables from the manufacturer.
Amersham Biosciences AB
Technical Solution: Amersham Biosciences (now part of Cytiva/GE Healthcare) has developed sophisticated IEF technologies specifically engineered for complex protein sample analysis. Their Multiphor II and Ettan IPGphor systems represent significant advancements in handling challenging protein mixtures through immobilized pH gradient (IPG) technology. Amersham's approach incorporates covalently immobilized ampholytes within polyacrylamide gels, creating stable pH gradients that resist cathodic drift—a critical advantage when analyzing complex samples over extended separation times. Their technology includes specialized sample application methods that ensure uniform protein loading across the pH gradient, enhancing reproducibility with heterogeneous samples[3]. Amersham has also pioneered narrow-range IPG strips that provide enhanced resolution within specific pH intervals, allowing researchers to zoom in on crowded regions of complex proteomes. Their systems feature precise temperature control mechanisms that prevent protein precipitation during focusing, a common challenge with complex biological samples containing diverse protein classes.
Strengths: Exceptional pH gradient stability for extended run times; high loading capacity accommodating complex sample diversity; compatible with downstream mass spectrometry analysis. Weaknesses: Requires significant technical expertise; time-consuming protocol compared to newer automated systems; potential for protein loss during strip rehydration with very complex samples.
Key Innovations in IEF Technology
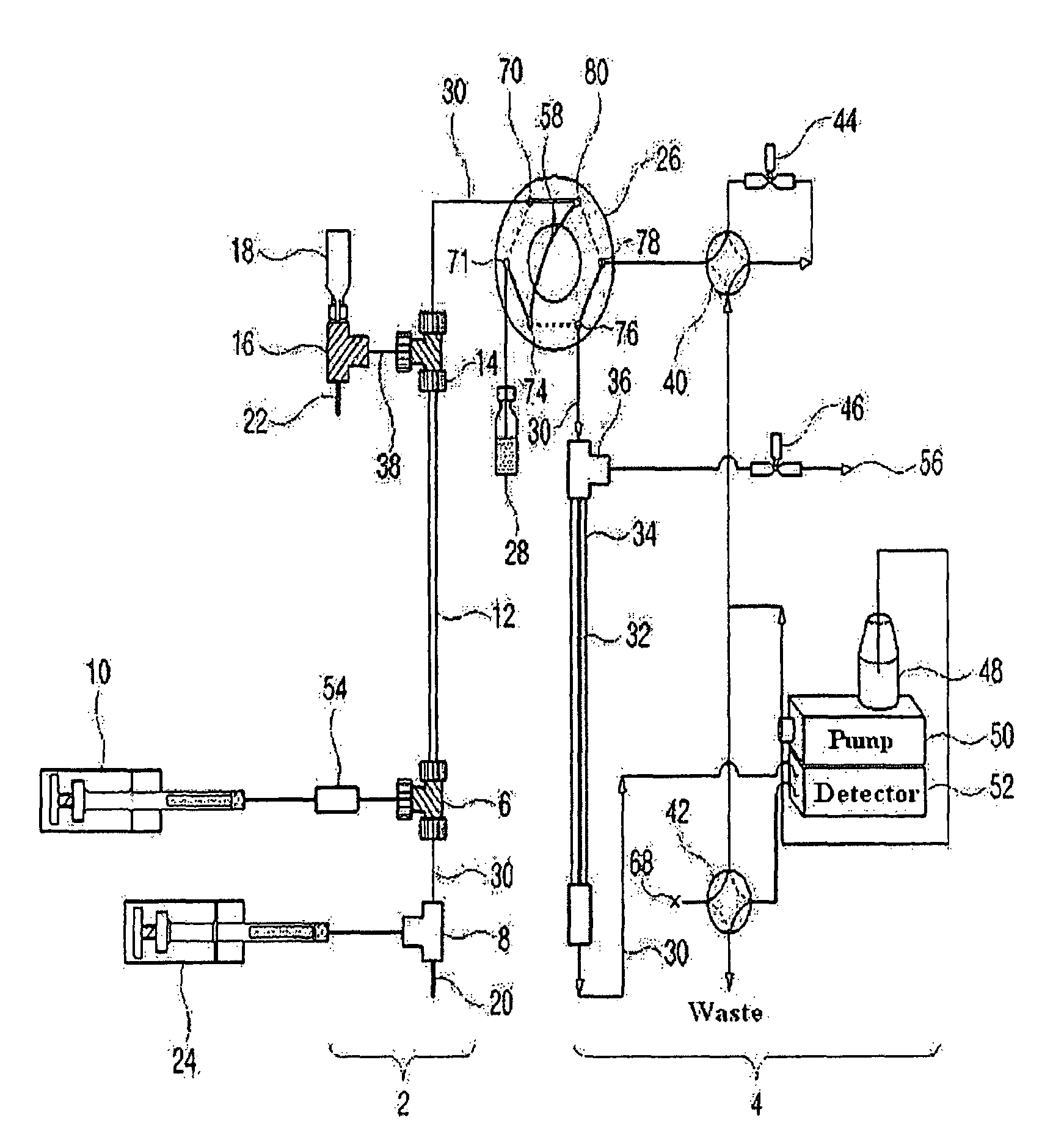
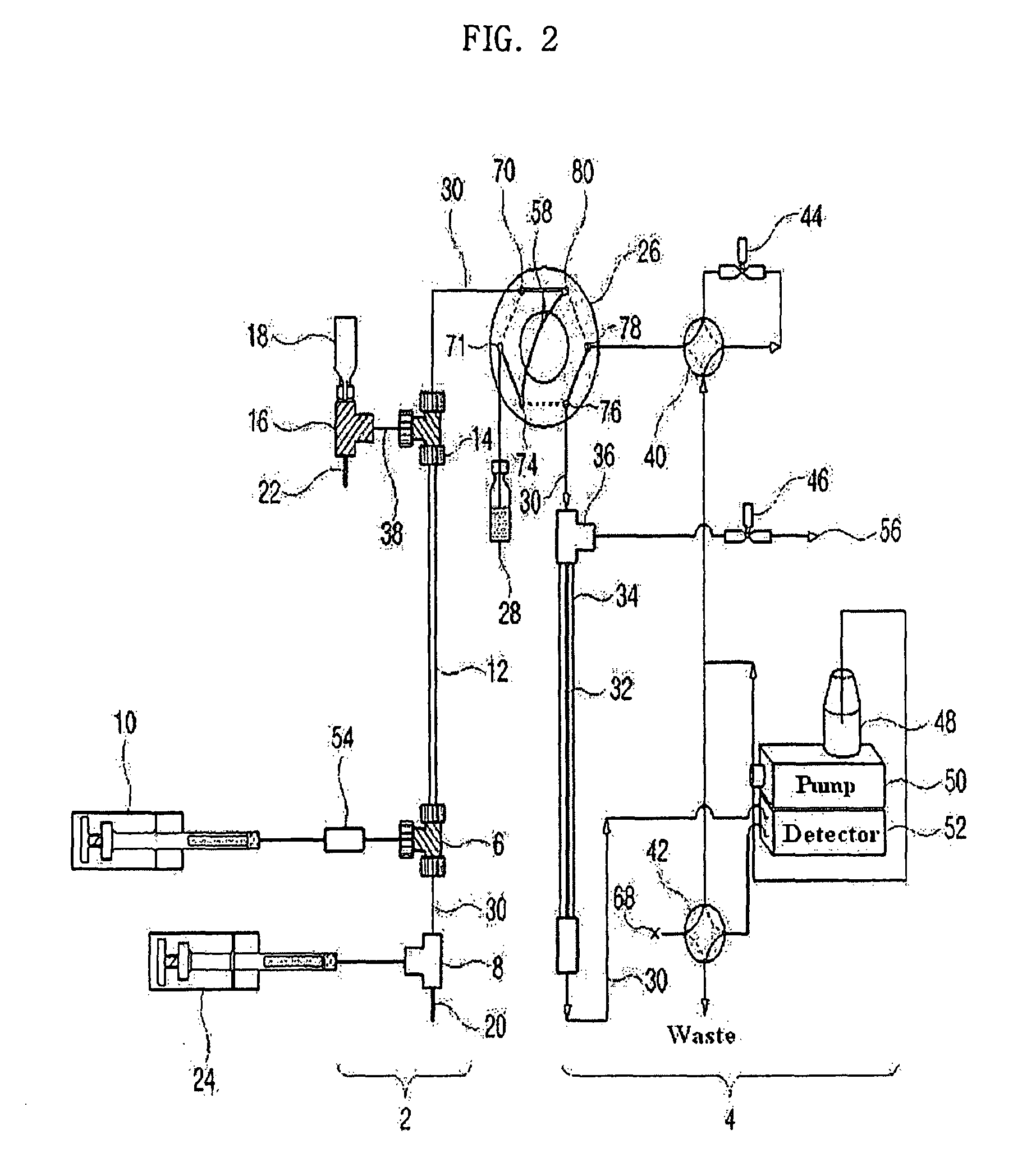
A method and device for analysis of charged molecules by solution isoelectric focusing
PatentInactiveEP1269178B1
Innovation
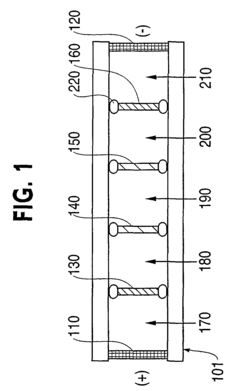
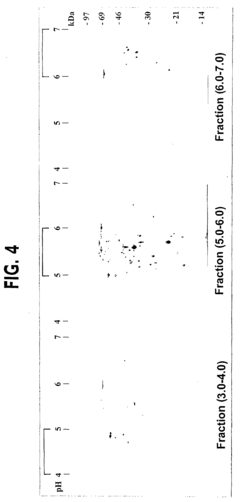
- A novel small-scale solution isofocusing device with multiple compartments separated by pH gradient gel membranes, allowing for reproducible fractionation of charged molecules into well-defined pools, suitable for complex eukaryotic proteome samples, enabling the resolution and quantitation of over 10,000 protein components.
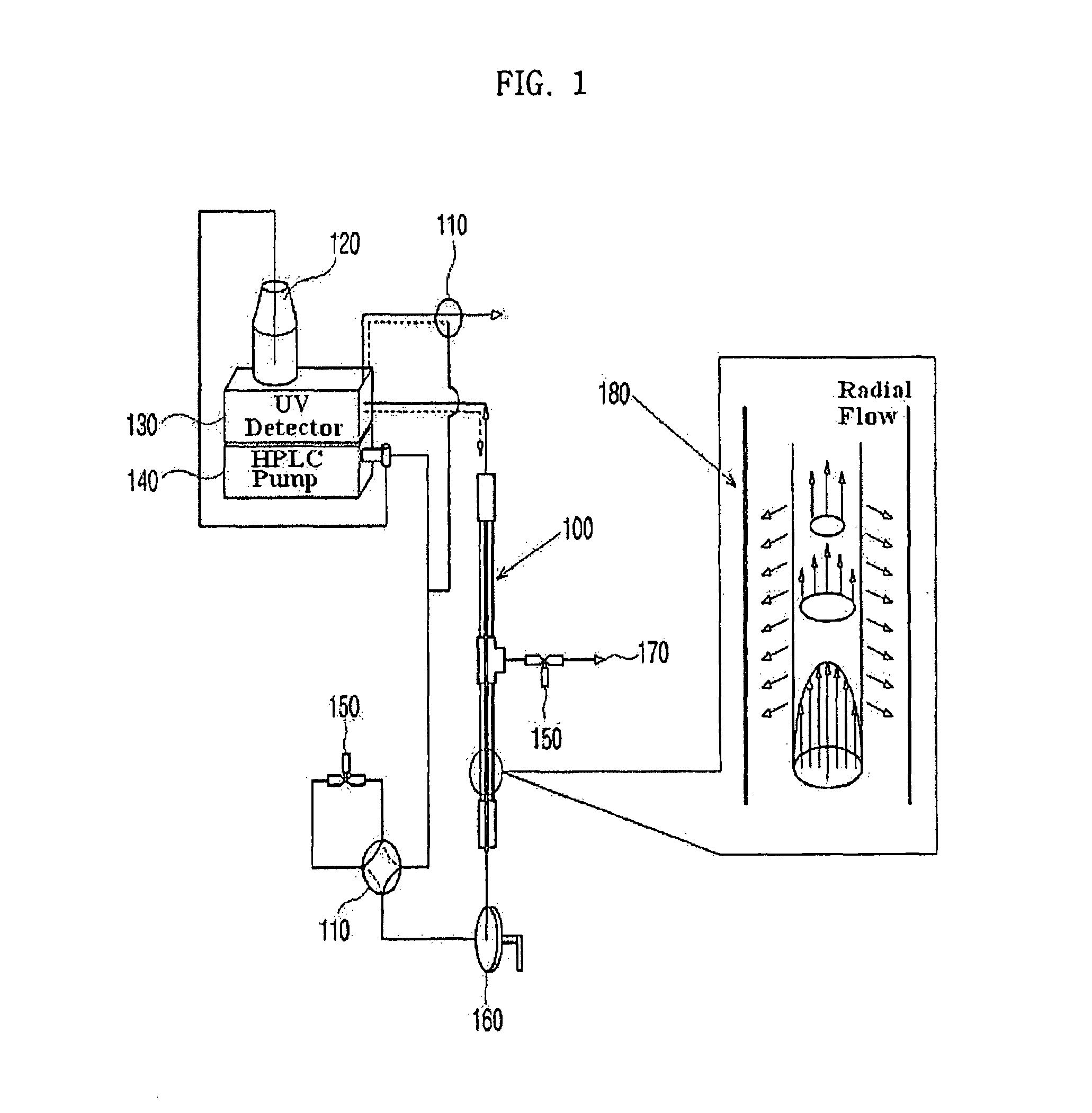
Apparatus for protein separation using capillary isoelectric focusing-hollow fiber flow field flow fractionation and method thereof
PatentInactiveUS8585884B2
Innovation
- A capillary isoelectric focusing-hollow fiber flow field flow fractionation apparatus that separates proteins based on isoelectric point (pI) and molecular weight in a two-dimensional, non-gel, and liquid phase manner, using a capillary isoelectric focusing unit connected to a hollow fiber flow field flow fractionation unit, allowing for automatic removal of ampholytes and avoiding protein denaturation.
Comparative Analysis of Alternative Separation Methods
When evaluating isoelectric focusing (IEF) for complex protein samples, it is essential to consider alternative separation methods that may offer complementary or superior performance characteristics. Two-dimensional gel electrophoresis (2-DE), which combines IEF with SDS-PAGE, has been the gold standard for protein separation for decades, but newer technologies have emerged to address its limitations.
Liquid chromatography (LC) techniques, particularly high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC), offer excellent resolution for complex protein mixtures. When coupled with mass spectrometry (LC-MS/MS), these methods provide superior sensitivity and quantification capabilities compared to IEF alone. LC-based approaches also overcome sample handling challenges associated with IEF gels and allow for automation, increasing throughput and reproducibility.
Capillary electrophoresis (CE) represents another powerful alternative, combining high resolution with minimal sample requirements. CE systems can separate proteins based on size, charge, and hydrophobicity, making them versatile for complex sample analysis. The integration of CE with MS detection (CE-MS) further enhances identification capabilities while maintaining separation efficiency.
Size exclusion chromatography (SEC) offers advantages for native protein complex analysis, preserving protein-protein interactions that might be disrupted during IEF. While SEC provides lower resolution than IEF for similarly sized proteins, it excels at separating protein complexes and aggregates from monomeric forms, which is particularly valuable for biopharmaceutical applications.
Affinity-based separation methods, including immunoaffinity chromatography and aptamer-based techniques, provide highly selective isolation of target proteins from complex mixtures. These approaches can be used as pre-fractionation steps before IEF to reduce sample complexity and enhance detection of low-abundance proteins.
Multi-dimensional separation strategies that combine orthogonal techniques have demonstrated superior performance for complex proteomes. For instance, protein fractionation by solution isoelectric focusing followed by reversed-phase chromatography significantly increases proteome coverage compared to single-dimension approaches. These hybrid methods leverage the strengths of individual techniques while mitigating their limitations.
Emerging microfluidic platforms offer miniaturized alternatives to conventional separation methods, requiring minimal sample volumes while maintaining separation efficiency. These systems show promise for point-of-care applications where rapid analysis of complex protein samples is needed with limited resources.
Liquid chromatography (LC) techniques, particularly high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC), offer excellent resolution for complex protein mixtures. When coupled with mass spectrometry (LC-MS/MS), these methods provide superior sensitivity and quantification capabilities compared to IEF alone. LC-based approaches also overcome sample handling challenges associated with IEF gels and allow for automation, increasing throughput and reproducibility.
Capillary electrophoresis (CE) represents another powerful alternative, combining high resolution with minimal sample requirements. CE systems can separate proteins based on size, charge, and hydrophobicity, making them versatile for complex sample analysis. The integration of CE with MS detection (CE-MS) further enhances identification capabilities while maintaining separation efficiency.
Size exclusion chromatography (SEC) offers advantages for native protein complex analysis, preserving protein-protein interactions that might be disrupted during IEF. While SEC provides lower resolution than IEF for similarly sized proteins, it excels at separating protein complexes and aggregates from monomeric forms, which is particularly valuable for biopharmaceutical applications.
Affinity-based separation methods, including immunoaffinity chromatography and aptamer-based techniques, provide highly selective isolation of target proteins from complex mixtures. These approaches can be used as pre-fractionation steps before IEF to reduce sample complexity and enhance detection of low-abundance proteins.
Multi-dimensional separation strategies that combine orthogonal techniques have demonstrated superior performance for complex proteomes. For instance, protein fractionation by solution isoelectric focusing followed by reversed-phase chromatography significantly increases proteome coverage compared to single-dimension approaches. These hybrid methods leverage the strengths of individual techniques while mitigating their limitations.
Emerging microfluidic platforms offer miniaturized alternatives to conventional separation methods, requiring minimal sample volumes while maintaining separation efficiency. These systems show promise for point-of-care applications where rapid analysis of complex protein samples is needed with limited resources.
Reproducibility and Validation Considerations
Reproducibility and validation represent critical challenges in the application of isoelectric focusing (IEF) for complex protein samples. The inherent variability in IEF experiments demands rigorous validation protocols to ensure consistent and reliable results. When analyzing complex protein mixtures, researchers must implement standardized procedures that address both technical and biological variability.
Technical reproducibility in IEF requires careful control of experimental parameters including pH gradient stability, temperature fluctuations, and sample loading consistency. Studies have shown that even minor variations in these factors can significantly alter protein migration patterns, particularly in complex samples where protein-protein interactions may influence isoelectric point determination. Implementing internal standards and reference markers throughout the focusing process enables quantitative assessment of run-to-run variability.
Validation strategies for IEF methods applied to complex samples should incorporate orthogonal techniques such as mass spectrometry or western blotting to confirm protein identifications. This multi-platform approach strengthens confidence in results by providing complementary data on protein characteristics beyond isoelectric points. Researchers have demonstrated that combining IEF with LC-MS/MS can substantially improve validation rates for proteins identified in complex biological samples.
Statistical validation frameworks specifically designed for IEF data analysis have emerged as essential tools for complex sample analysis. These frameworks typically include measures of technical variance, limits of detection, and confidence intervals for protein identification. Recent advances in computational approaches have enhanced the ability to distinguish true biological differences from technical artifacts in IEF separations of complex proteomes.
Inter-laboratory validation studies have highlighted challenges in standardizing IEF protocols across different research environments. These collaborative efforts reveal that while basic separation principles remain consistent, subtle differences in equipment calibration and reagent quality can impact reproducibility when analyzing complex samples. Establishing community-wide standards and quality control metrics has become increasingly important for advancing IEF applications in proteomics research.
Sample preparation reproducibility presents a particular challenge for complex protein mixtures. Variations in protein extraction efficiency, solubilization conditions, and removal of interfering compounds can dramatically affect IEF performance. Validated protocols that include detailed sample handling procedures and quality control checkpoints are essential for meaningful comparisons across experiments and laboratories.
Automation technologies have significantly improved reproducibility in IEF applications by reducing human error and standardizing critical steps. Modern integrated systems that combine sample preparation, focusing, and detection have demonstrated superior reproducibility metrics compared to manual methods, particularly when handling complex protein samples with wide dynamic ranges of abundance.
Technical reproducibility in IEF requires careful control of experimental parameters including pH gradient stability, temperature fluctuations, and sample loading consistency. Studies have shown that even minor variations in these factors can significantly alter protein migration patterns, particularly in complex samples where protein-protein interactions may influence isoelectric point determination. Implementing internal standards and reference markers throughout the focusing process enables quantitative assessment of run-to-run variability.
Validation strategies for IEF methods applied to complex samples should incorporate orthogonal techniques such as mass spectrometry or western blotting to confirm protein identifications. This multi-platform approach strengthens confidence in results by providing complementary data on protein characteristics beyond isoelectric points. Researchers have demonstrated that combining IEF with LC-MS/MS can substantially improve validation rates for proteins identified in complex biological samples.
Statistical validation frameworks specifically designed for IEF data analysis have emerged as essential tools for complex sample analysis. These frameworks typically include measures of technical variance, limits of detection, and confidence intervals for protein identification. Recent advances in computational approaches have enhanced the ability to distinguish true biological differences from technical artifacts in IEF separations of complex proteomes.
Inter-laboratory validation studies have highlighted challenges in standardizing IEF protocols across different research environments. These collaborative efforts reveal that while basic separation principles remain consistent, subtle differences in equipment calibration and reagent quality can impact reproducibility when analyzing complex samples. Establishing community-wide standards and quality control metrics has become increasingly important for advancing IEF applications in proteomics research.
Sample preparation reproducibility presents a particular challenge for complex protein mixtures. Variations in protein extraction efficiency, solubilization conditions, and removal of interfering compounds can dramatically affect IEF performance. Validated protocols that include detailed sample handling procedures and quality control checkpoints are essential for meaningful comparisons across experiments and laboratories.
Automation technologies have significantly improved reproducibility in IEF applications by reducing human error and standardizing critical steps. Modern integrated systems that combine sample preparation, focusing, and detection have demonstrated superior reproducibility metrics compared to manual methods, particularly when handling complex protein samples with wide dynamic ranges of abundance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!