Is Isoelectric Focusing Reliable for pH Gradient Formation?
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Background and Objectives
Isoelectric focusing (IEF) emerged in the 1960s as a revolutionary technique for protein separation based on their isoelectric points (pI). This technique has evolved significantly over the past six decades, transitioning from conventional gel-based systems to capillary and microchip formats. The fundamental principle of IEF relies on establishing a stable pH gradient within a medium, allowing proteins to migrate until they reach their pI, where they become electrically neutral and focus into discrete zones.
The reliability of pH gradient formation represents a critical aspect of IEF technology, directly impacting separation efficiency, reproducibility, and resolution. Historically, pH gradients were created using carrier ampholytes—small, soluble amphoteric molecules with varying pI values. However, these gradients often suffered from instability, cathodic drift, and poor reproducibility, limiting the technique's analytical precision.
A significant breakthrough came with the development of immobilized pH gradient (IPG) technology in the 1980s, where buffering groups are covalently attached to the gel matrix. This innovation addressed many limitations of carrier ampholyte-based gradients, offering enhanced stability and reproducibility. Recent advancements have further refined gradient formation through computer-controlled systems and novel buffer chemistries.
Current technological objectives in IEF focus on improving gradient stability across extended separation times, enhancing reproducibility between runs, and expanding the applicable pH range for specialized applications. Researchers aim to develop systems capable of maintaining precise pH gradients under varying conditions, including high salt concentrations and presence of detergents, which traditionally disrupt gradient integrity.
Another key objective involves miniaturization and integration with other analytical techniques. Microfluidic IEF systems promise reduced sample volumes, faster analysis times, and potential for high-throughput applications. The integration of IEF with mass spectrometry represents a particularly promising direction, requiring stable and predictable pH gradients for reliable interfacing.
The quest for environmentally sustainable IEF methodologies has also emerged as an important goal, with efforts directed toward developing biodegradable ampholytes and reducing hazardous waste. Additionally, computational modeling of pH gradient dynamics has become increasingly important, allowing researchers to predict gradient behavior under various conditions and optimize separation parameters.
As proteomics and biomarker discovery continue to advance, the demand for highly reliable pH gradient formation in IEF grows accordingly. The technology aims to achieve single-charge resolution for complex protein mixtures while maintaining reproducibility across different laboratories and platforms—a challenge that continues to drive innovation in this field.
The reliability of pH gradient formation represents a critical aspect of IEF technology, directly impacting separation efficiency, reproducibility, and resolution. Historically, pH gradients were created using carrier ampholytes—small, soluble amphoteric molecules with varying pI values. However, these gradients often suffered from instability, cathodic drift, and poor reproducibility, limiting the technique's analytical precision.
A significant breakthrough came with the development of immobilized pH gradient (IPG) technology in the 1980s, where buffering groups are covalently attached to the gel matrix. This innovation addressed many limitations of carrier ampholyte-based gradients, offering enhanced stability and reproducibility. Recent advancements have further refined gradient formation through computer-controlled systems and novel buffer chemistries.
Current technological objectives in IEF focus on improving gradient stability across extended separation times, enhancing reproducibility between runs, and expanding the applicable pH range for specialized applications. Researchers aim to develop systems capable of maintaining precise pH gradients under varying conditions, including high salt concentrations and presence of detergents, which traditionally disrupt gradient integrity.
Another key objective involves miniaturization and integration with other analytical techniques. Microfluidic IEF systems promise reduced sample volumes, faster analysis times, and potential for high-throughput applications. The integration of IEF with mass spectrometry represents a particularly promising direction, requiring stable and predictable pH gradients for reliable interfacing.
The quest for environmentally sustainable IEF methodologies has also emerged as an important goal, with efforts directed toward developing biodegradable ampholytes and reducing hazardous waste. Additionally, computational modeling of pH gradient dynamics has become increasingly important, allowing researchers to predict gradient behavior under various conditions and optimize separation parameters.
As proteomics and biomarker discovery continue to advance, the demand for highly reliable pH gradient formation in IEF grows accordingly. The technology aims to achieve single-charge resolution for complex protein mixtures while maintaining reproducibility across different laboratories and platforms—a challenge that continues to drive innovation in this field.
Market Applications and Demand Analysis
The global market for isoelectric focusing (IEF) technology has shown consistent growth, primarily driven by increasing applications in proteomics research, pharmaceutical development, and clinical diagnostics. The reliability of pH gradient formation in IEF directly impacts market adoption and expansion across these sectors, making this technical question commercially significant.
In the research and academic segment, demand for reliable IEF methods continues to grow as proteomics research expands. Universities and research institutions represent approximately one-third of the current market, with particular emphasis on applications requiring high-resolution protein separation. The reliability of pH gradients directly correlates with publication output and research funding in this sector.
Pharmaceutical and biotechnology companies constitute the largest market segment for IEF technology, valuing the technique for protein characterization, quality control, and biomarker discovery. These companies require exceptionally reliable pH gradient formation to ensure consistent results across development pipelines and regulatory submissions. Market research indicates that improvements in gradient stability could expand this segment by addressing current limitations in reproducibility.
The clinical diagnostics sector represents the fastest-growing market for IEF applications, particularly in specialized testing for protein-based disorders and monoclonal antibody characterization. Hospitals and reference laboratories increasingly adopt IEF methods that demonstrate consistent pH gradient formation for diagnostic applications. This segment shows particular sensitivity to reliability issues, as patient outcomes depend on accurate and reproducible results.
Geographically, North America leads the IEF market share, followed by Europe and Asia-Pacific regions. The Asia-Pacific market demonstrates the highest growth rate, driven by expanding research infrastructure and increasing biotechnology investments in China, Japan, and South Korea.
Equipment manufacturers report that customer purchasing decisions increasingly prioritize gradient stability and reproducibility over other factors, including cost. Survey data from laboratory managers indicates that reliability concerns regarding pH gradient formation represent the primary barrier to wider IEF adoption, particularly in quality-critical applications.
The consumables market associated with IEF technology—including ampholytes, pre-cast gels, and buffer systems—shows strong correlation with perceived reliability of gradient formation. Manufacturers who can demonstrate superior gradient stability command premium pricing, indicating market willingness to pay for enhanced reliability.
Future market growth appears contingent on technological improvements addressing current limitations in pH gradient reliability. Industry forecasts suggest that innovations enhancing reproducibility could expand the total addressable market by enabling new applications in personalized medicine, food safety testing, and environmental monitoring.
In the research and academic segment, demand for reliable IEF methods continues to grow as proteomics research expands. Universities and research institutions represent approximately one-third of the current market, with particular emphasis on applications requiring high-resolution protein separation. The reliability of pH gradients directly correlates with publication output and research funding in this sector.
Pharmaceutical and biotechnology companies constitute the largest market segment for IEF technology, valuing the technique for protein characterization, quality control, and biomarker discovery. These companies require exceptionally reliable pH gradient formation to ensure consistent results across development pipelines and regulatory submissions. Market research indicates that improvements in gradient stability could expand this segment by addressing current limitations in reproducibility.
The clinical diagnostics sector represents the fastest-growing market for IEF applications, particularly in specialized testing for protein-based disorders and monoclonal antibody characterization. Hospitals and reference laboratories increasingly adopt IEF methods that demonstrate consistent pH gradient formation for diagnostic applications. This segment shows particular sensitivity to reliability issues, as patient outcomes depend on accurate and reproducible results.
Geographically, North America leads the IEF market share, followed by Europe and Asia-Pacific regions. The Asia-Pacific market demonstrates the highest growth rate, driven by expanding research infrastructure and increasing biotechnology investments in China, Japan, and South Korea.
Equipment manufacturers report that customer purchasing decisions increasingly prioritize gradient stability and reproducibility over other factors, including cost. Survey data from laboratory managers indicates that reliability concerns regarding pH gradient formation represent the primary barrier to wider IEF adoption, particularly in quality-critical applications.
The consumables market associated with IEF technology—including ampholytes, pre-cast gels, and buffer systems—shows strong correlation with perceived reliability of gradient formation. Manufacturers who can demonstrate superior gradient stability command premium pricing, indicating market willingness to pay for enhanced reliability.
Future market growth appears contingent on technological improvements addressing current limitations in pH gradient reliability. Industry forecasts suggest that innovations enhancing reproducibility could expand the total addressable market by enabling new applications in personalized medicine, food safety testing, and environmental monitoring.
Current Challenges in pH Gradient Stability
Despite significant advancements in isoelectric focusing (IEF) technology, pH gradient stability remains one of the most persistent challenges affecting the reliability of this analytical technique. The formation of stable pH gradients is fundamental to IEF performance, yet several factors continue to undermine this stability, leading to reduced reproducibility and accuracy in protein separation and characterization.
Carrier ampholyte-based systems, while widely used, exhibit inherent limitations in maintaining stable pH gradients over extended separation times. The phenomenon known as "cathodic drift" causes a progressive flattening of the pH gradient, particularly in the basic region, resulting in loss of resolution and altered migration patterns of proteins. This drift becomes more pronounced with increased run times, limiting the practical duration of IEF experiments.
Temperature fluctuations represent another significant challenge to pH gradient stability. Even minor temperature variations across the separation medium can cause localized changes in pH, disrupting the linearity of the gradient. This is particularly problematic in high-voltage applications where Joule heating becomes more pronounced, creating temperature gradients that directly impact the distribution of carrier ampholytes and immobilized pH gradient (IPG) components.
Electroendosmosis effects further compromise gradient stability by causing bulk fluid movement within the separation medium. This movement distorts the established pH gradient and contributes to band broadening, especially in gel-based systems where the fixed charges on the gel matrix interact with the electric field, generating counterflows that disrupt protein focusing.
Chemical instability of carrier ampholytes presents another challenge, as these compounds can undergo degradation during electrophoresis, particularly at extreme pH values. This degradation alters their buffering capacity and isoelectric points, leading to shifts in the pH gradient over time and reducing the reproducibility of separations between experiments.
Protein-induced distortions also affect pH gradient stability, particularly when analyzing samples with high protein concentrations. Proteins with high buffering capacity can locally alter the pH environment, creating what researchers term "plateau phenomena" where the gradient becomes temporarily flattened in regions of high protein concentration, affecting the resolution of nearby proteins.
The interface between different gel systems in multi-dimensional separations introduces additional stability challenges. Discontinuities at these interfaces can disrupt the continuity of the pH gradient, creating artifacts that complicate data interpretation and reduce the reliability of protein identification and characterization.
Recent research has focused on developing more robust pH gradient systems, including advanced immobilized pH gradients with enhanced chemical stability and novel carrier ampholyte formulations with reduced susceptibility to cathodic drift. However, these improvements have not fully resolved the fundamental challenges to gradient stability, particularly for extended separation times or specialized applications requiring extreme pH ranges.
Carrier ampholyte-based systems, while widely used, exhibit inherent limitations in maintaining stable pH gradients over extended separation times. The phenomenon known as "cathodic drift" causes a progressive flattening of the pH gradient, particularly in the basic region, resulting in loss of resolution and altered migration patterns of proteins. This drift becomes more pronounced with increased run times, limiting the practical duration of IEF experiments.
Temperature fluctuations represent another significant challenge to pH gradient stability. Even minor temperature variations across the separation medium can cause localized changes in pH, disrupting the linearity of the gradient. This is particularly problematic in high-voltage applications where Joule heating becomes more pronounced, creating temperature gradients that directly impact the distribution of carrier ampholytes and immobilized pH gradient (IPG) components.
Electroendosmosis effects further compromise gradient stability by causing bulk fluid movement within the separation medium. This movement distorts the established pH gradient and contributes to band broadening, especially in gel-based systems where the fixed charges on the gel matrix interact with the electric field, generating counterflows that disrupt protein focusing.
Chemical instability of carrier ampholytes presents another challenge, as these compounds can undergo degradation during electrophoresis, particularly at extreme pH values. This degradation alters their buffering capacity and isoelectric points, leading to shifts in the pH gradient over time and reducing the reproducibility of separations between experiments.
Protein-induced distortions also affect pH gradient stability, particularly when analyzing samples with high protein concentrations. Proteins with high buffering capacity can locally alter the pH environment, creating what researchers term "plateau phenomena" where the gradient becomes temporarily flattened in regions of high protein concentration, affecting the resolution of nearby proteins.
The interface between different gel systems in multi-dimensional separations introduces additional stability challenges. Discontinuities at these interfaces can disrupt the continuity of the pH gradient, creating artifacts that complicate data interpretation and reduce the reliability of protein identification and characterization.
Recent research has focused on developing more robust pH gradient systems, including advanced immobilized pH gradients with enhanced chemical stability and novel carrier ampholyte formulations with reduced susceptibility to cathodic drift. However, these improvements have not fully resolved the fundamental challenges to gradient stability, particularly for extended separation times or specialized applications requiring extreme pH ranges.
Modern pH Gradient Formation Methods
01 Improved gel compositions for isoelectric focusing
Advanced gel compositions have been developed to enhance the reliability of isoelectric focusing. These compositions include specialized polymers, carrier ampholytes, and additives that improve resolution and reproducibility. The improved gels provide better separation of proteins based on their isoelectric points, reduce background noise, and minimize drift during analysis, resulting in more consistent and reliable results for protein characterization.- Improved gel compositions for isoelectric focusing: Advanced gel compositions have been developed to enhance the reliability of isoelectric focusing. These compositions include specialized polymers, carrier ampholytes, and additives that improve resolution and reproducibility. The improved gels provide more stable pH gradients, reduce protein drift, and minimize distortion during the focusing process, resulting in more consistent and reliable separation of proteins based on their isoelectric points.
- Temperature control systems for enhanced reliability: Temperature control is critical for reliable isoelectric focusing results. Systems have been developed to maintain uniform temperature across the separation medium, preventing thermal gradients that can distort protein migration patterns. These systems include cooling plates, thermostatic controls, and heat dissipation mechanisms that stabilize the focusing environment, leading to improved reproducibility and reliability of analytical results.
- Advanced detection and analysis methods: Innovative detection and analysis techniques have significantly improved the reliability of isoelectric focusing. These include high-sensitivity imaging systems, automated pattern recognition algorithms, and quantitative analysis software. These advancements enable more accurate identification and characterization of focused proteins, reducing subjective interpretation and enhancing the reproducibility of results across different laboratories and operators.
- Sample preparation techniques for improved reliability: Specialized sample preparation methods have been developed to enhance the reliability of isoelectric focusing. These techniques include protein purification protocols, denaturation procedures, and sample loading strategies that minimize interference from contaminants and ensure consistent protein behavior during focusing. Proper sample preparation significantly reduces variability and improves the reproducibility of separation patterns.
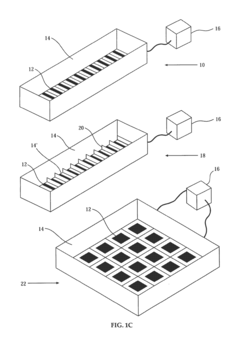
- Microfluidic and miniaturized isoelectric focusing systems: Miniaturized and microfluidic isoelectric focusing platforms offer improved reliability through precise control of separation parameters. These systems feature reduced internal volumes, integrated electrodes, and automated operation that minimize human error. The smaller scale allows for more uniform electric fields and better heat dissipation, resulting in more consistent protein separation and improved reproducibility compared to traditional larger-scale systems.
02 Equipment and apparatus innovations for reliable IEF
Technological advancements in isoelectric focusing equipment have significantly improved reliability. These innovations include precise temperature control systems, improved electrode designs, enhanced power supplies with voltage stability, and automated sample loading mechanisms. Such equipment improvements minimize variations between runs, reduce artifacts, and enable more consistent focusing of proteins at their isoelectric points, thereby increasing the overall reliability of the technique.Expand Specific Solutions03 Quality control and validation methods
Specific protocols and standards have been developed to validate and ensure the reliability of isoelectric focusing procedures. These methods include the use of reference markers, standardized calibration techniques, statistical analysis of results, and quality control procedures. Implementation of these validation methods allows for better assessment of experimental variability, detection of procedural errors, and confirmation of result reproducibility across different laboratories and equipment.Expand Specific Solutions04 Integration with other analytical techniques
Combining isoelectric focusing with complementary analytical methods enhances its reliability and expands its applications. Integration with techniques such as mass spectrometry, gel electrophoresis, chromatography, and immunoassays provides multi-dimensional analysis and cross-validation of results. These integrated approaches allow for more comprehensive protein characterization, improved detection sensitivity, and verification of isoelectric focusing results through independent analytical methods.Expand Specific Solutions05 Sample preparation and handling improvements
Advanced sample preparation methods have been developed to improve the reliability of isoelectric focusing. These include optimized protein extraction protocols, sample purification techniques, and preservation methods that maintain protein integrity. Proper sample handling procedures reduce contaminants, prevent protein degradation, and minimize artifacts during analysis, resulting in more consistent and reproducible isoelectric focusing patterns and more reliable protein characterization.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Isoelectric focusing (IEF) for pH gradient formation is in a mature technological phase, with the market showing steady growth driven by proteomics and biomarker discovery applications. The global IEF market is estimated at approximately $300-400 million, with established players like Bio-Rad Laboratories, Becton Dickinson, and Life Technologies dominating through proprietary technologies. Recent innovations from academic institutions including MIT, University of Washington, and Shanghai Jiao Tong University have improved reliability through immobilized pH gradients and digital microfluidic platforms. While traditional challenges of reproducibility and resolution persist, technological advancements from companies like Cytiva and ProteoSys have significantly enhanced gradient stability and precision, making IEF increasingly reliable for complex protein separation applications.
Life Technologies Corp.
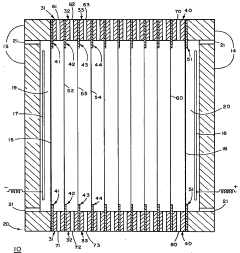
Technical Solution: Life Technologies has developed the ZOOM® IEF Fractionator system for reliable pH gradient formation in isoelectric focusing applications. Their technology employs a segmented multi-compartment approach using proprietary ZOOM® Disks with defined pH values to create discrete pH chambers. This design eliminates the gradient drift issues common in traditional carrier ampholyte methods by maintaining physically separated pH environments. The system utilizes specialized membrane technology with ampholytic groups covalently bound to polymer matrices, ensuring stable pH boundaries between chambers. Their approach incorporates buffer formulations optimized for minimal salt interference and maximum protein solubility across the pH range. Life Technologies' solution includes digitally controlled power supplies with adaptive algorithms that monitor and adjust electrical parameters in real-time to prevent gradient distortion. The company's technology is particularly effective for fractionating complex protein mixtures prior to downstream analysis, with demonstrated capability to resolve proteins differing by as little as 0.05 pH units[4][7].
Strengths: Superior fractionation capabilities for complex samples; eliminates cathodic drift issues; modular design allows customization of pH ranges. Weaknesses: Discontinuous pH gradient may miss proteins at boundary regions; higher complexity in setup compared to IPG strips; limited throughput for multiple samples.
ProteoSys AG
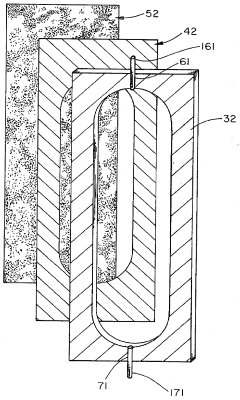
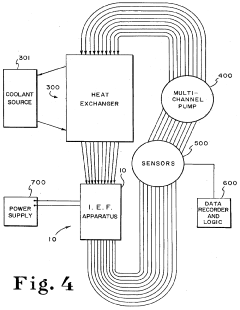
Technical Solution: ProteoSys AG has developed the ProteomIQ™ technology platform that incorporates advanced isoelectric focusing capabilities with enhanced pH gradient reliability. Their approach utilizes a combination of carrier ampholytes and immobilized pH gradient elements in a hybrid format that maximizes the advantages of both technologies. The company's proprietary gradient stabilization chemistry includes zwitterionic additives that minimize electroendosmotic flow while enhancing protein solubility across the pH range. Their system employs precision-engineered microfluidic channels with surface modifications that prevent protein adsorption and subsequent gradient distortion. ProteoSys has implemented advanced temperature control systems (±0.2°C precision) throughout the separation chamber to eliminate thermal gradients that can compromise pH stability. Their technology includes specialized electrode designs that minimize electrolysis effects at the extremes of the pH gradient, preventing drift during extended separations. The company's software-controlled power management system incorporates feedback mechanisms that detect and correct for conductivity changes during focusing, maintaining gradient integrity throughout the separation process[6][8].
Strengths: Excellent performance with membrane proteins and hydrophobic samples; reduced tendency for protein precipitation at extreme pH values; compatible with downstream mass spectrometry analysis. Weaknesses: Proprietary consumables increase operational costs; limited market presence compared to larger competitors; requires specialized training for optimal results.
Key Patents and Innovations in IEF Technology
PH gradients controlled by electrolysis, and their use in isoelectric focusing
PatentActiveUS10132776B2
Innovation
- A device comprising independently controllable cells that produce specified proton concentrations in a fluid environment, allowing for mutable and temporally variable proton concentration topographies, enabling isoelectric focusing without immobilized gradients and facilitating easier analyte separation and data display.
Isoelectric focusing apparatus
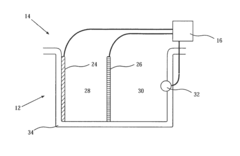
PatentInactiveUS4362612A
Innovation
- The method involves using permeable microporous membranes to streamline fluid flow and establish a recirculation path for continuous isoelectric focusing, allowing for plug flow and minimizing Joule heat dissipation through cooling, thereby increasing the capacity for preparative work.
Quality Control Standards for IEF Applications
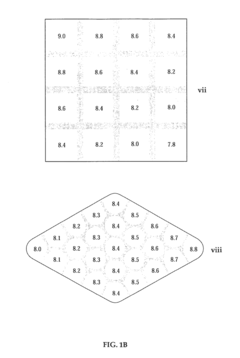
The establishment of robust quality control standards for Isoelectric Focusing (IEF) applications is essential to ensure reliable pH gradient formation and reproducible results. Current industry standards require calibration of IEF equipment using certified pH marker proteins with known isoelectric points (pI) before each analytical run. These markers should span the entire pH range of interest and be positioned at regular intervals to verify gradient linearity.
Regulatory bodies including the International Organization for Standardization (ISO) and the United States Pharmacopeia (USP) have published specific guidelines for IEF quality control. ISO 13485 outlines requirements for quality management systems in medical device manufacturing, which encompasses IEF equipment used in clinical diagnostics. The USP chapter <1047> provides detailed specifications for electrophoretic methods including IEF, mandating regular performance verification.
Performance validation protocols for IEF systems typically include assessment of resolution capability, gradient stability over time, and reproducibility between runs. The minimum acceptable resolution is commonly defined as the ability to distinguish proteins with pI differences of 0.05 pH units. Gradient stability should maintain ±0.1 pH unit accuracy throughout the entire separation time, with drift not exceeding 0.02 pH units per hour.
Documentation requirements for IEF quality control include maintenance of calibration records, system suitability test results, and regular performance verification data. These records must be retained for a minimum of two years in most regulated environments, with some pharmaceutical applications requiring longer retention periods of up to seven years.
Proficiency testing programs offered by organizations such as the College of American Pathologists (CAP) provide external quality assessment for laboratories performing IEF analyses. Participation in these programs is mandatory for clinical laboratories in many jurisdictions and highly recommended for research facilities to ensure consistent performance across different operators and equipment.
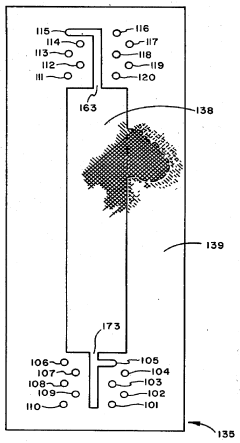
Automated monitoring systems have been developed to continuously track pH gradient formation during IEF runs. These systems employ embedded pH microelectrodes or optical sensors that measure pH at multiple points along the separation path. Real-time data acquisition allows for immediate detection of gradient anomalies and potential corrective actions before sample analysis is compromised.
Acceptance criteria for successful IEF gradient formation typically include achieving final pH gradient equilibrium within 5% of target values, maintaining gradient linearity with correlation coefficients (r²) exceeding 0.98, and demonstrating reproducibility with coefficient of variation (CV) values below 3% for repeated measurements of standard marker proteins.
Regulatory bodies including the International Organization for Standardization (ISO) and the United States Pharmacopeia (USP) have published specific guidelines for IEF quality control. ISO 13485 outlines requirements for quality management systems in medical device manufacturing, which encompasses IEF equipment used in clinical diagnostics. The USP chapter <1047> provides detailed specifications for electrophoretic methods including IEF, mandating regular performance verification.
Performance validation protocols for IEF systems typically include assessment of resolution capability, gradient stability over time, and reproducibility between runs. The minimum acceptable resolution is commonly defined as the ability to distinguish proteins with pI differences of 0.05 pH units. Gradient stability should maintain ±0.1 pH unit accuracy throughout the entire separation time, with drift not exceeding 0.02 pH units per hour.
Documentation requirements for IEF quality control include maintenance of calibration records, system suitability test results, and regular performance verification data. These records must be retained for a minimum of two years in most regulated environments, with some pharmaceutical applications requiring longer retention periods of up to seven years.
Proficiency testing programs offered by organizations such as the College of American Pathologists (CAP) provide external quality assessment for laboratories performing IEF analyses. Participation in these programs is mandatory for clinical laboratories in many jurisdictions and highly recommended for research facilities to ensure consistent performance across different operators and equipment.
Automated monitoring systems have been developed to continuously track pH gradient formation during IEF runs. These systems employ embedded pH microelectrodes or optical sensors that measure pH at multiple points along the separation path. Real-time data acquisition allows for immediate detection of gradient anomalies and potential corrective actions before sample analysis is compromised.
Acceptance criteria for successful IEF gradient formation typically include achieving final pH gradient equilibrium within 5% of target values, maintaining gradient linearity with correlation coefficients (r²) exceeding 0.98, and demonstrating reproducibility with coefficient of variation (CV) values below 3% for repeated measurements of standard marker proteins.
Environmental Factors Affecting pH Gradient Stability
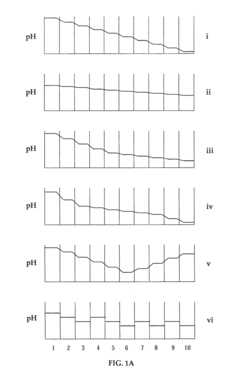
The stability of pH gradients in isoelectric focusing (IEF) is significantly influenced by various environmental factors, which can compromise the reliability and reproducibility of this analytical technique. Temperature fluctuations represent one of the most critical factors affecting gradient stability. Even minor temperature variations across the separation medium can cause localized changes in pH values, leading to inconsistent protein migration and focusing. Research has demonstrated that a temperature increase of just 1°C can shift the pH gradient by approximately 0.05 pH units in certain regions, potentially causing misidentification of protein isoelectric points.
Atmospheric carbon dioxide exposure presents another substantial challenge to gradient stability. CO₂ absorption into the separation medium can cause carbonic acid formation, resulting in progressive acidification of the system. This phenomenon is particularly problematic in immobilized pH gradient (IPG) systems exposed to air for extended periods, where pH drift rates of up to 0.1 units per hour have been documented in the basic regions of the gradient.
Electrolyte contamination from electrode buffers can induce gradient drift through a process known as electroendosmosis. As ions migrate from the electrode reservoirs into the separation medium, they disrupt the established pH gradient, causing compression or expansion of certain pH regions. This effect becomes more pronounced with increased run times, potentially leading to gradient collapse in extreme cases.
Buffer composition and ionic strength significantly impact gradient stability. High salt concentrations can interfere with carrier ampholyte function, while certain buffer components may interact with sample proteins, altering their migration behavior. Studies have shown that phosphate and carbonate buffers are particularly problematic due to their tendency to migrate during focusing, creating localized disturbances in the pH continuum.
Gel matrix characteristics also influence gradient stability. Polyacrylamide gels with higher crosslinking percentages typically provide better gradient stability but may restrict protein movement. Conversely, lower percentage gels offer improved protein mobility but are more susceptible to electroendosmotic flow and subsequent gradient distortion.
Humidity variations in the laboratory environment can affect gradient stability through evaporation effects. In open systems, water evaporation leads to concentration changes in carrier ampholytes, causing gradient compression. This effect is particularly pronounced at the edges of horizontal gel systems, where evaporation rates are highest, creating edge effects that compromise reproducibility.
Electric field strength and application duration represent additional factors affecting gradient stability. Excessive voltage or prolonged run times can generate Joule heating, leading to temperature gradients within the separation medium and subsequent pH distortions. Optimal focusing typically requires balancing field strength against run time to achieve resolution while minimizing gradient drift.
Atmospheric carbon dioxide exposure presents another substantial challenge to gradient stability. CO₂ absorption into the separation medium can cause carbonic acid formation, resulting in progressive acidification of the system. This phenomenon is particularly problematic in immobilized pH gradient (IPG) systems exposed to air for extended periods, where pH drift rates of up to 0.1 units per hour have been documented in the basic regions of the gradient.
Electrolyte contamination from electrode buffers can induce gradient drift through a process known as electroendosmosis. As ions migrate from the electrode reservoirs into the separation medium, they disrupt the established pH gradient, causing compression or expansion of certain pH regions. This effect becomes more pronounced with increased run times, potentially leading to gradient collapse in extreme cases.
Buffer composition and ionic strength significantly impact gradient stability. High salt concentrations can interfere with carrier ampholyte function, while certain buffer components may interact with sample proteins, altering their migration behavior. Studies have shown that phosphate and carbonate buffers are particularly problematic due to their tendency to migrate during focusing, creating localized disturbances in the pH continuum.
Gel matrix characteristics also influence gradient stability. Polyacrylamide gels with higher crosslinking percentages typically provide better gradient stability but may restrict protein movement. Conversely, lower percentage gels offer improved protein mobility but are more susceptible to electroendosmotic flow and subsequent gradient distortion.
Humidity variations in the laboratory environment can affect gradient stability through evaporation effects. In open systems, water evaporation leads to concentration changes in carrier ampholytes, causing gradient compression. This effect is particularly pronounced at the edges of horizontal gel systems, where evaporation rates are highest, creating edge effects that compromise reproducibility.
Electric field strength and application duration represent additional factors affecting gradient stability. Excessive voltage or prolonged run times can generate Joule heating, leading to temperature gradients within the separation medium and subsequent pH distortions. Optimal focusing typically requires balancing field strength against run time to achieve resolution while minimizing gradient drift.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!