How to Reduce Artifactual pH Drifts in Isoelectric Focusing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF pH Drift Background and Objectives
Isoelectric focusing (IEF) represents a cornerstone technique in protein separation and analysis, with applications spanning from basic research to industrial bioprocessing. The technique, developed in the 1960s, leverages the amphoteric nature of proteins to separate them based on their isoelectric points (pI) within a pH gradient. Over the decades, IEF has evolved from conventional gel-based methods to capillary and microchip formats, enabling higher resolution and automation capabilities.
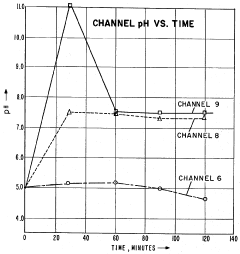
Despite its analytical power, IEF has been persistently challenged by artifactual pH drift phenomena, which compromise reproducibility and resolution. These drifts manifest as temporal instabilities in the established pH gradient, leading to protein band distortion, poor focusing, and ultimately unreliable analytical results. The technical evolution trajectory shows increasing sophistication in addressing these limitations, yet a comprehensive solution remains elusive.
The primary objective of this technical investigation is to systematically analyze the underlying mechanisms of artifactual pH drifts in IEF systems and develop robust strategies for their mitigation. Specifically, we aim to characterize the physicochemical factors contributing to gradient instability, including carrier ampholyte behaviors, electrode reactions, and protein-gradient interactions that collectively destabilize the pH environment.
Recent advances in materials science, microfluidics, and computational modeling have opened new avenues for addressing these challenges. The convergence of these disciplines presents opportunities to reimagine IEF methodologies with inherently stable pH gradients. Our technical goals include developing novel carrier ampholyte formulations with enhanced stability properties, engineering electrode systems that minimize electrochemical perturbations, and designing innovative surface chemistries that reduce protein-wall interactions.
The broader impact of resolving pH drift issues extends beyond analytical improvements. Enhanced IEF stability would enable more reliable protein characterization in pharmaceutical development, more sensitive biomarker detection in clinical diagnostics, and more efficient protein purification in industrial settings. Additionally, stabilized IEF would facilitate integration with other analytical techniques in comprehensive proteomics workflows.
This investigation aligns with the industry-wide trend toward higher precision analytical methods capable of resolving increasingly complex biological samples. As proteomics and biotherapeutics continue their rapid expansion, the demand for reliable, high-resolution protein separation techniques becomes increasingly critical. By addressing the fundamental limitations of pH stability in IEF, we position ourselves at the forefront of analytical innovation with significant implications for both research and commercial applications.
Despite its analytical power, IEF has been persistently challenged by artifactual pH drift phenomena, which compromise reproducibility and resolution. These drifts manifest as temporal instabilities in the established pH gradient, leading to protein band distortion, poor focusing, and ultimately unreliable analytical results. The technical evolution trajectory shows increasing sophistication in addressing these limitations, yet a comprehensive solution remains elusive.
The primary objective of this technical investigation is to systematically analyze the underlying mechanisms of artifactual pH drifts in IEF systems and develop robust strategies for their mitigation. Specifically, we aim to characterize the physicochemical factors contributing to gradient instability, including carrier ampholyte behaviors, electrode reactions, and protein-gradient interactions that collectively destabilize the pH environment.
Recent advances in materials science, microfluidics, and computational modeling have opened new avenues for addressing these challenges. The convergence of these disciplines presents opportunities to reimagine IEF methodologies with inherently stable pH gradients. Our technical goals include developing novel carrier ampholyte formulations with enhanced stability properties, engineering electrode systems that minimize electrochemical perturbations, and designing innovative surface chemistries that reduce protein-wall interactions.
The broader impact of resolving pH drift issues extends beyond analytical improvements. Enhanced IEF stability would enable more reliable protein characterization in pharmaceutical development, more sensitive biomarker detection in clinical diagnostics, and more efficient protein purification in industrial settings. Additionally, stabilized IEF would facilitate integration with other analytical techniques in comprehensive proteomics workflows.
This investigation aligns with the industry-wide trend toward higher precision analytical methods capable of resolving increasingly complex biological samples. As proteomics and biotherapeutics continue their rapid expansion, the demand for reliable, high-resolution protein separation techniques becomes increasingly critical. By addressing the fundamental limitations of pH stability in IEF, we position ourselves at the forefront of analytical innovation with significant implications for both research and commercial applications.
Market Analysis for Improved IEF Technologies
The global market for isoelectric focusing (IEF) technologies is experiencing steady growth, driven by increasing applications in proteomics research, pharmaceutical development, and clinical diagnostics. The current market size for electrophoresis equipment and consumables is estimated at $2.5 billion, with IEF technologies representing approximately 15% of this segment. Annual growth rates for advanced IEF solutions are projected at 6-8% through 2028, outpacing traditional electrophoresis methods.
pH drift reduction technologies represent a particularly valuable niche within this market. Laboratories and research institutions are increasingly willing to invest in premium solutions that minimize artifactual pH drifts, as these technical improvements directly translate to enhanced reproducibility and reliability of results. The average research laboratory spends between $50,000-$100,000 annually on electrophoresis equipment and consumables, with approximately 30% allocated to IEF-specific products.
Pharmaceutical and biotechnology companies constitute the largest market segment (42%), followed by academic and research institutions (35%), clinical laboratories (15%), and other industrial applications (8%). Geographically, North America leads with 38% market share, followed by Europe (30%), Asia-Pacific (25%), and rest of world (7%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory at 10-12% annually.
Key market drivers include the growing emphasis on precision medicine, increased R&D spending in biopharmaceuticals, and the rising importance of protein-based biomarkers in disease diagnosis. The demand for improved pH stability in IEF is particularly strong in applications involving therapeutic protein characterization, where even minor variations in results can have significant regulatory implications.
Market barriers include the high cost of advanced IEF equipment, technical complexity requiring specialized training, and competition from alternative protein separation technologies. However, solutions addressing the pH drift challenge specifically could command premium pricing, with surveys indicating that laboratories would pay 20-30% more for technologies demonstrating significant improvements in pH stability.
Customer pain points consistently highlight reproducibility challenges, with 78% of users reporting pH drift issues as a significant limitation in their current IEF workflows. This represents a clear market opportunity for innovations specifically targeting this technical challenge, with potential for rapid adoption across multiple industry segments.
pH drift reduction technologies represent a particularly valuable niche within this market. Laboratories and research institutions are increasingly willing to invest in premium solutions that minimize artifactual pH drifts, as these technical improvements directly translate to enhanced reproducibility and reliability of results. The average research laboratory spends between $50,000-$100,000 annually on electrophoresis equipment and consumables, with approximately 30% allocated to IEF-specific products.
Pharmaceutical and biotechnology companies constitute the largest market segment (42%), followed by academic and research institutions (35%), clinical laboratories (15%), and other industrial applications (8%). Geographically, North America leads with 38% market share, followed by Europe (30%), Asia-Pacific (25%), and rest of world (7%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory at 10-12% annually.
Key market drivers include the growing emphasis on precision medicine, increased R&D spending in biopharmaceuticals, and the rising importance of protein-based biomarkers in disease diagnosis. The demand for improved pH stability in IEF is particularly strong in applications involving therapeutic protein characterization, where even minor variations in results can have significant regulatory implications.
Market barriers include the high cost of advanced IEF equipment, technical complexity requiring specialized training, and competition from alternative protein separation technologies. However, solutions addressing the pH drift challenge specifically could command premium pricing, with surveys indicating that laboratories would pay 20-30% more for technologies demonstrating significant improvements in pH stability.
Customer pain points consistently highlight reproducibility challenges, with 78% of users reporting pH drift issues as a significant limitation in their current IEF workflows. This represents a clear market opportunity for innovations specifically targeting this technical challenge, with potential for rapid adoption across multiple industry segments.
Current Challenges in pH Stability During IEF
Isoelectric focusing (IEF) represents a powerful analytical technique for protein separation based on their isoelectric points. However, one of the most persistent challenges in IEF is the occurrence of artifactual pH drifts, which significantly compromise the reproducibility and reliability of results. These drifts manifest as temporal changes in the pH gradient during the focusing process, leading to inconsistent protein migration patterns and erroneous pI determinations.
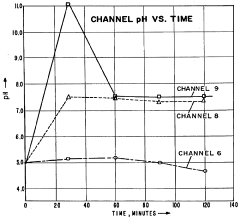
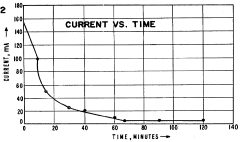
The primary cause of pH instability during IEF is the phenomenon of cathodic drift, where carrier ampholytes migrate toward the cathode over time. This migration results from electroendosmotic flow and the progressive loss of basic carrier ampholytes at the cathodic end. Studies have shown that this drift can cause pH gradient compression of up to 30% within just 2-3 hours of focusing, severely limiting the resolution capability of the technique.
Another significant challenge is the chemical instability of carrier ampholytes under high electric field conditions. Extended exposure to electric fields can lead to ampholyte degradation through oxidation and reduction reactions at the electrodes. This degradation alters the buffering capacity of the ampholytes, resulting in localized pH fluctuations throughout the separation medium.
Temperature gradients represent an additional complication in maintaining pH stability. Joule heating during electrophoresis creates temperature variations across the separation medium, affecting the pKa values of ampholytes and consequently distorting the pH gradient. Even with modern cooling systems, temperature variations of 1-2°C can cause detectable pH shifts, particularly in the alkaline regions of the gradient.
The interaction between sample proteins and carrier ampholytes presents yet another challenge. High-abundance proteins can bind ampholytes, effectively removing them from the system and creating localized disturbances in the pH gradient. This protein-ampholyte interaction is particularly problematic when analyzing complex biological samples with wide dynamic ranges of protein concentrations.
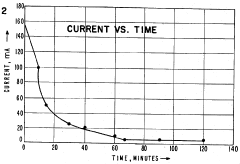
Electrode reactions further contribute to pH instability through the generation of acid at the anode and base at the cathode. These electrolysis products can migrate into the separation medium, particularly in systems without effective ion-exchange membranes, causing progressive acidification or alkalization of the gradient extremes.
Commercial carrier ampholyte formulations exhibit batch-to-batch variations that impact gradient stability. Despite manufacturers' efforts to standardize production, subtle differences in ampholyte composition can lead to unpredictable gradient behaviors, complicating method transfer and validation across laboratories.
The challenges in pH stability during IEF have significant implications for proteomics research, clinical diagnostics, and biopharmaceutical analysis, where reproducible protein characterization is essential. Addressing these challenges requires innovative approaches in ampholyte chemistry, electrode design, and focusing protocols to minimize artifactual pH drifts and enhance the analytical power of IEF.
The primary cause of pH instability during IEF is the phenomenon of cathodic drift, where carrier ampholytes migrate toward the cathode over time. This migration results from electroendosmotic flow and the progressive loss of basic carrier ampholytes at the cathodic end. Studies have shown that this drift can cause pH gradient compression of up to 30% within just 2-3 hours of focusing, severely limiting the resolution capability of the technique.
Another significant challenge is the chemical instability of carrier ampholytes under high electric field conditions. Extended exposure to electric fields can lead to ampholyte degradation through oxidation and reduction reactions at the electrodes. This degradation alters the buffering capacity of the ampholytes, resulting in localized pH fluctuations throughout the separation medium.
Temperature gradients represent an additional complication in maintaining pH stability. Joule heating during electrophoresis creates temperature variations across the separation medium, affecting the pKa values of ampholytes and consequently distorting the pH gradient. Even with modern cooling systems, temperature variations of 1-2°C can cause detectable pH shifts, particularly in the alkaline regions of the gradient.
The interaction between sample proteins and carrier ampholytes presents yet another challenge. High-abundance proteins can bind ampholytes, effectively removing them from the system and creating localized disturbances in the pH gradient. This protein-ampholyte interaction is particularly problematic when analyzing complex biological samples with wide dynamic ranges of protein concentrations.
Electrode reactions further contribute to pH instability through the generation of acid at the anode and base at the cathode. These electrolysis products can migrate into the separation medium, particularly in systems without effective ion-exchange membranes, causing progressive acidification or alkalization of the gradient extremes.
Commercial carrier ampholyte formulations exhibit batch-to-batch variations that impact gradient stability. Despite manufacturers' efforts to standardize production, subtle differences in ampholyte composition can lead to unpredictable gradient behaviors, complicating method transfer and validation across laboratories.
The challenges in pH stability during IEF have significant implications for proteomics research, clinical diagnostics, and biopharmaceutical analysis, where reproducible protein characterization is essential. Addressing these challenges requires innovative approaches in ampholyte chemistry, electrode design, and focusing protocols to minimize artifactual pH drifts and enhance the analytical power of IEF.
Contemporary Approaches to Minimize Artifactual pH Drifts
01 Causes of pH drift in isoelectric focusing
Artifactual pH drifts in isoelectric focusing can be caused by several factors including electroendosmosis, ampholyte migration, and electrode reactions. These phenomena can lead to distortion of the pH gradient over time, affecting the resolution and reproducibility of protein separation. Understanding these causes is crucial for developing methods to minimize or correct pH drift during isoelectric focusing procedures.- Causes of pH drift in isoelectric focusing: Artifactual pH drifts in isoelectric focusing can occur due to various factors including electroendosmosis, carrier ampholyte instability, and electrode reactions. These phenomena can lead to distortion of the pH gradient over time, affecting the accuracy and reproducibility of protein separation. Understanding these causes is essential for developing methods to minimize or correct for pH drift during isoelectric focusing procedures.
- Buffer systems to stabilize pH gradients: Specialized buffer systems can be employed to stabilize pH gradients and reduce artifactual drifts during isoelectric focusing. These systems may include immobilized pH gradients (IPGs), specialized carrier ampholytes, or buffer additives that minimize electroendosmosis and other destabilizing effects. The composition and concentration of these buffer systems play a crucial role in maintaining stable pH gradients throughout the focusing process.
- Instrumentation and monitoring techniques: Advanced instrumentation and monitoring techniques can be used to detect and correct pH drifts during isoelectric focusing. These include real-time pH monitoring systems, automated control mechanisms, and specialized electrodes that minimize electrode reactions. Continuous monitoring allows for adjustments to be made during the focusing process, improving the accuracy and reproducibility of protein separation.
- Mathematical models and algorithms for drift correction: Mathematical models and computational algorithms can be developed to predict, analyze, and correct for artifactual pH drifts in isoelectric focusing. These approaches may involve statistical analysis of drift patterns, simulation of electrophoretic processes, or machine learning algorithms that can identify and compensate for systematic errors. Such computational methods enable more accurate interpretation of results and improved reproducibility.
- Novel materials and gel formulations: Innovative materials and gel formulations can be developed to minimize pH drift during isoelectric focusing. These may include modified acrylamide gels, composite materials with enhanced stability, or novel polymer matrices that reduce electroendosmosis. Additionally, specialized coatings or treatments for electrophoresis chambers can help maintain stable pH gradients by minimizing surface interactions that contribute to drift phenomena.
02 Buffer systems to stabilize pH gradients
Specialized buffer systems can be employed to stabilize pH gradients and reduce artifactual drifts during isoelectric focusing. These systems may include carrier ampholytes with improved properties, immobilized pH gradient (IPG) strips, or buffer additives that minimize electroendosmosis. The composition and concentration of these buffer systems play a critical role in maintaining stable pH gradients throughout the separation process.Expand Specific Solutions03 Instrumentation and equipment modifications
Modifications to isoelectric focusing equipment can help reduce artifactual pH drifts. These include improved electrode designs, temperature control systems, and specialized gel cassettes. Advanced instruments may incorporate real-time monitoring of pH gradients and automated correction mechanisms to maintain stable conditions throughout the separation process, resulting in more reproducible protein focusing.Expand Specific Solutions04 Detection and correction methods for pH drift
Various methods have been developed to detect and correct artifactual pH drifts during or after isoelectric focusing. These include the use of pH markers, mathematical modeling of drift patterns, and post-run calibration techniques. Software algorithms can analyze the drift patterns and apply corrections to the experimental data, improving the accuracy of isoelectric point determinations despite the presence of pH drift.Expand Specific Solutions05 Novel materials and techniques for drift reduction
Innovative materials and techniques have been developed specifically to address the issue of pH drift in isoelectric focusing. These include new gel formulations, membrane-based separation systems, and microfluidic devices. Some approaches incorporate nanomaterials or polymer composites that provide enhanced stability to pH gradients, while others utilize alternative separation principles that are less susceptible to the factors causing drift.Expand Specific Solutions
Leading Companies and Research Groups in IEF
The isoelectric focusing (IEF) pH drift reduction technology landscape is currently in a mature development stage, with established methodologies being refined by key industry players. The market size is estimated to be moderate but growing steadily as proteomics research expands. Leading companies like Bio-Rad Laboratories, Life Technologies (now part of Thermo Fisher), and Becton Dickinson have developed advanced solutions addressing artifactual pH drift issues through improved ampholyte formulations and electrode designs. Academic institutions including MIT, University of Washington, and Xiamen University contribute significant research innovations, while specialized biotechnology firms like ProteoSys AG focus on niche applications. The technology continues to evolve with emphasis on reproducibility and resolution for protein separation applications in both research and clinical diagnostics markets.
Becton, Dickinson & Co.
Technical Solution: Becton Dickinson has developed the BD Proteomics IEF system that addresses artifactual pH drift through a combination of hardware and reagent innovations. Their approach utilizes a patented electrode design with increased surface area that distributes current more evenly across the gel, reducing localized pH disturbances. The company has formulated specialized rehydration buffers containing optimized concentrations of CHAPS, urea, and thiourea that enhance protein solubility while maintaining gradient stability. BD's system incorporates programmable voltage ramping protocols that gradually increase field strength, preventing the sudden pH shifts that occur with rapid voltage changes. Their technology also features specialized strip holders with uniform pressure application that prevents uneven swelling and subsequent gradient distortion that contributes to drift phenomena.
Strengths: Excellent integration with downstream mass spectrometry applications; user-friendly interface requires minimal specialized training; consistent performance across diverse sample types. Weakness: Limited flexibility for customization of protocols; higher consumable costs compared to some competitors; system optimization primarily focused on mammalian samples.
Life Technologies Corp.
Technical Solution: Life Technologies has developed the ZOOM IEF Fractionator system specifically designed to minimize artifactual pH drifts during isoelectric focusing. Their approach utilizes proprietary membrane technology with specialized chemistry that creates discrete pH chambers with minimal ionic migration between compartments. The system incorporates platinum-coated titanium electrodes that reduce electrolysis effects and subsequent pH disturbances. Their solution includes optimized power protocols with stepped voltage increases that prevent overheating and maintain gradient stability. Life Technologies has also formulated specialized carrier ampholytes with enhanced buffering capacity at critical pH regions where drift is most problematic. Their system includes real-time pH monitoring capabilities that allow for dynamic adjustments during the focusing process to counteract developing drift patterns.
Strengths: Innovative chamber-based approach effectively isolates pH regions; integrated monitoring system provides real-time feedback; modular design allows customization for different sample types. Weakness: System complexity requires significant technical expertise; higher initial investment compared to traditional IEF methods; limited compatibility with some downstream applications.
Critical Patents and Literature on pH Stabilization
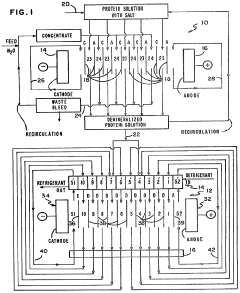
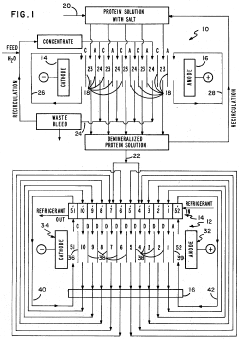
Separation of proteins using electrodialysis - isoelectric focusing combination
PatentInactiveUS4441978A
Innovation
- The integration of electrodialysis before isoelectric focusing allows for controlled salt removal, creating a demineralized protein solution that stabilizes the pH gradient and prevents protein precipitation, thereby enhancing the efficiency of the separation process.
Separation of proteins using electrodialysis-isoelectric focusing combination
PatentInactiveUS4396477A
Innovation
- The integration of electrodialysis before isoelectric focusing allows for controlled salt removal, creating a stable protein mixture that can be efficiently focused, using an electrodialysis cell with ion permselective membranes to separate salts and a subsequent isoelectric focusing cell with a pH gradient and permeable membranes to facilitate protein separation.
Validation Methods for IEF pH Stability
Validation of pH stability in isoelectric focusing (IEF) requires systematic approaches to ensure accurate and reproducible results. Effective validation methods must address the fundamental challenge of artifactual pH drift, which can significantly compromise experimental outcomes. A comprehensive validation protocol typically begins with standard curve generation using calibrated pH markers under identical experimental conditions as the actual samples. These markers, consisting of well-characterized proteins with known isoelectric points, serve as reference points for monitoring pH gradient stability throughout the IEF process.
Real-time pH monitoring represents another critical validation approach, employing specialized microelectrodes or pH-sensitive fluorescent probes that can detect localized pH changes during separation. These tools enable researchers to identify the onset of drift phenomena and implement corrective measures promptly. The integration of imaging techniques with pH-sensitive dyes has further enhanced this capability, allowing for spatial visualization of pH gradients across the entire separation medium.
Statistical validation methods have emerged as essential components of IEF quality control. These include reproducibility assessments through multiple technical replicates and calculation of coefficient of variation (CV) values for pI determinations. Advanced statistical approaches such as ANOVA can help distinguish between normal experimental variation and significant pH drift, particularly when analyzing complex sample sets across multiple experimental runs.
Temperature validation constitutes another crucial aspect of IEF pH stability assessment. Given the temperature-dependent nature of pH in carrier ampholyte systems, continuous temperature monitoring using calibrated thermocouples or infrared sensors helps ensure that thermal fluctuations do not contribute to apparent pH shifts. Many modern IEF systems incorporate built-in temperature control validation features that log temperature data throughout the separation process.
Comparative validation using orthogonal techniques provides additional confidence in IEF results. This approach involves analyzing the same samples using alternative separation methods such as chromatofocusing or capillary electrophoresis, then comparing the observed pI values. Significant discrepancies between techniques may indicate pH instability in the IEF system that requires further investigation and correction.
Documentation and standardization of validation protocols represent the final critical element in ensuring IEF pH stability. Detailed records of validation parameters, including ampholyte batch information, power conditions, and environmental factors, facilitate troubleshooting and method optimization. The implementation of standardized validation workflows across laboratories has significantly improved inter-laboratory reproducibility and reliability of IEF-based analyses in both research and clinical applications.
Real-time pH monitoring represents another critical validation approach, employing specialized microelectrodes or pH-sensitive fluorescent probes that can detect localized pH changes during separation. These tools enable researchers to identify the onset of drift phenomena and implement corrective measures promptly. The integration of imaging techniques with pH-sensitive dyes has further enhanced this capability, allowing for spatial visualization of pH gradients across the entire separation medium.
Statistical validation methods have emerged as essential components of IEF quality control. These include reproducibility assessments through multiple technical replicates and calculation of coefficient of variation (CV) values for pI determinations. Advanced statistical approaches such as ANOVA can help distinguish between normal experimental variation and significant pH drift, particularly when analyzing complex sample sets across multiple experimental runs.
Temperature validation constitutes another crucial aspect of IEF pH stability assessment. Given the temperature-dependent nature of pH in carrier ampholyte systems, continuous temperature monitoring using calibrated thermocouples or infrared sensors helps ensure that thermal fluctuations do not contribute to apparent pH shifts. Many modern IEF systems incorporate built-in temperature control validation features that log temperature data throughout the separation process.
Comparative validation using orthogonal techniques provides additional confidence in IEF results. This approach involves analyzing the same samples using alternative separation methods such as chromatofocusing or capillary electrophoresis, then comparing the observed pI values. Significant discrepancies between techniques may indicate pH instability in the IEF system that requires further investigation and correction.
Documentation and standardization of validation protocols represent the final critical element in ensuring IEF pH stability. Detailed records of validation parameters, including ampholyte batch information, power conditions, and environmental factors, facilitate troubleshooting and method optimization. The implementation of standardized validation workflows across laboratories has significantly improved inter-laboratory reproducibility and reliability of IEF-based analyses in both research and clinical applications.
Regulatory Considerations for IEF Applications
Isoelectric focusing (IEF) applications in pharmaceutical and clinical diagnostics are subject to stringent regulatory oversight due to their critical role in product quality control and patient safety. Regulatory bodies such as the FDA, EMA, and ICH have established comprehensive guidelines governing the validation, implementation, and quality control of IEF methodologies. These frameworks emphasize the importance of pH drift control as a critical quality attribute that directly impacts method reliability and reproducibility.
The FDA's guidance on analytical procedures specifically addresses the need for robust control of pH gradients in IEF applications, requiring manufacturers to demonstrate consistent performance across multiple runs. Method validation protocols must include specific measures to quantify, monitor, and mitigate artifactual pH drifts, with acceptance criteria typically set at ±0.2 pH units from established values.
For IEF applications in biopharmaceutical manufacturing, 21 CFR Part 211 mandates thorough documentation of all factors affecting pH stability, including electrode calibration procedures, buffer composition verification, and environmental controls. Companies must implement change control procedures that evaluate the impact of any modifications to IEF protocols on pH gradient stability.
The ICH Q2(R1) guidelines on validation of analytical procedures provide specific recommendations for establishing pH gradient linearity, range, and stability in IEF methods. These guidelines require manufacturers to demonstrate that artifactual pH drifts remain within predefined limits throughout the shelf-life of the product and under various storage conditions.
Regulatory submissions for novel biotherapeutics must include comprehensive data on pH gradient stability studies, demonstrating that artifactual drifts do not compromise the ability to detect charge variants or impurities. This typically involves comparative analyses using orthogonal methods to verify that observed separations reflect true molecular properties rather than methodological artifacts.
Compliance with ISO 17025 standards for testing laboratories necessitates regular proficiency testing and interlaboratory comparisons specifically focused on pH gradient reproducibility in IEF applications. Laboratories must maintain detailed records of equipment qualification, reagent certification, and personnel training related to pH drift prevention and control.
Recent regulatory trends indicate increasing scrutiny of continuous monitoring approaches for pH gradient stability during IEF runs, with expectations for real-time corrective actions when drifts exceed predetermined thresholds. This reflects the growing regulatory emphasis on process analytical technology (PAT) principles in analytical method execution.
The FDA's guidance on analytical procedures specifically addresses the need for robust control of pH gradients in IEF applications, requiring manufacturers to demonstrate consistent performance across multiple runs. Method validation protocols must include specific measures to quantify, monitor, and mitigate artifactual pH drifts, with acceptance criteria typically set at ±0.2 pH units from established values.
For IEF applications in biopharmaceutical manufacturing, 21 CFR Part 211 mandates thorough documentation of all factors affecting pH stability, including electrode calibration procedures, buffer composition verification, and environmental controls. Companies must implement change control procedures that evaluate the impact of any modifications to IEF protocols on pH gradient stability.
The ICH Q2(R1) guidelines on validation of analytical procedures provide specific recommendations for establishing pH gradient linearity, range, and stability in IEF methods. These guidelines require manufacturers to demonstrate that artifactual pH drifts remain within predefined limits throughout the shelf-life of the product and under various storage conditions.
Regulatory submissions for novel biotherapeutics must include comprehensive data on pH gradient stability studies, demonstrating that artifactual drifts do not compromise the ability to detect charge variants or impurities. This typically involves comparative analyses using orthogonal methods to verify that observed separations reflect true molecular properties rather than methodological artifacts.
Compliance with ISO 17025 standards for testing laboratories necessitates regular proficiency testing and interlaboratory comparisons specifically focused on pH gradient reproducibility in IEF applications. Laboratories must maintain detailed records of equipment qualification, reagent certification, and personnel training related to pH drift prevention and control.
Recent regulatory trends indicate increasing scrutiny of continuous monitoring approaches for pH gradient stability during IEF runs, with expectations for real-time corrective actions when drifts exceed predetermined thresholds. This reflects the growing regulatory emphasis on process analytical technology (PAT) principles in analytical method execution.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!