Quantify Band Resolution in High-Voltage Isoelectric Focusing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HV-IEF Band Resolution Technology Background
High-Voltage Isoelectric Focusing (HV-IEF) represents a significant advancement in the field of protein separation and analysis, evolving from conventional isoelectric focusing techniques. This analytical method leverages high electric field strengths to separate proteins based on their isoelectric points (pI), the pH at which proteins carry no net electrical charge. The development of HV-IEF began in the late 1970s, with substantial refinements occurring throughout the 1990s and early 2000s as power supply technology and gel formulations improved.
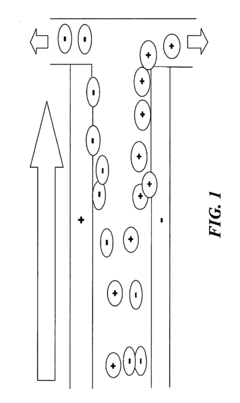
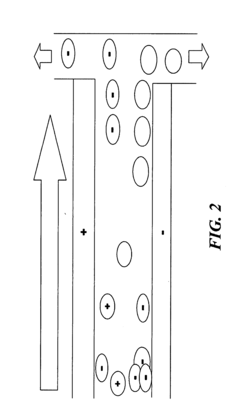
The fundamental principle behind HV-IEF involves establishing a pH gradient within a gel medium and applying a high voltage electric field, causing proteins to migrate until they reach their respective pI positions. At these positions, proteins become stationary, forming distinct bands. The resolution of these bands—defined as the ability to distinguish between proteins with minimal differences in their pI values—is a critical parameter that determines the analytical power of the technique.
Recent technological advancements have pushed the boundaries of HV-IEF, enabling the application of electric fields exceeding 300 V/cm, significantly higher than traditional IEF methods that typically operate at 100 V/cm or less. This increase in voltage has dramatically reduced separation times from hours to minutes while simultaneously enhancing band resolution.
The quantification of band resolution in HV-IEF has become increasingly important as the technique finds applications in proteomics, biomarker discovery, and quality control in biopharmaceutical production. Traditional methods of assessing resolution relied on visual inspection and manual measurements, which were subjective and prone to human error. The industry has since moved toward digital imaging systems coupled with sophisticated software algorithms that can objectively measure band width, intensity, and separation.
Current research in HV-IEF technology focuses on several key areas: development of novel ampholytes for creating more stable and reproducible pH gradients; improvement of gel matrices to reduce electroosmotic flow and enhance resolution; optimization of detection methods to increase sensitivity; and advancement of quantification algorithms to provide more accurate and reproducible measurements of band resolution.
The evolution of HV-IEF technology has been driven by demands from both academic research and industrial applications, particularly in the pharmaceutical sector where high-resolution protein characterization is essential for ensuring product quality and regulatory compliance. As proteomics research continues to expand and the complexity of biological samples increases, the need for higher resolution separation techniques becomes more pressing.
Looking forward, the trajectory of HV-IEF technology points toward further integration with other analytical techniques, miniaturization for higher throughput applications, and enhanced automation to improve reproducibility and ease of use. The quantification of band resolution remains a central challenge that, when addressed effectively, will significantly advance the utility and applicability of HV-IEF across multiple scientific disciplines.
The fundamental principle behind HV-IEF involves establishing a pH gradient within a gel medium and applying a high voltage electric field, causing proteins to migrate until they reach their respective pI positions. At these positions, proteins become stationary, forming distinct bands. The resolution of these bands—defined as the ability to distinguish between proteins with minimal differences in their pI values—is a critical parameter that determines the analytical power of the technique.
Recent technological advancements have pushed the boundaries of HV-IEF, enabling the application of electric fields exceeding 300 V/cm, significantly higher than traditional IEF methods that typically operate at 100 V/cm or less. This increase in voltage has dramatically reduced separation times from hours to minutes while simultaneously enhancing band resolution.
The quantification of band resolution in HV-IEF has become increasingly important as the technique finds applications in proteomics, biomarker discovery, and quality control in biopharmaceutical production. Traditional methods of assessing resolution relied on visual inspection and manual measurements, which were subjective and prone to human error. The industry has since moved toward digital imaging systems coupled with sophisticated software algorithms that can objectively measure band width, intensity, and separation.
Current research in HV-IEF technology focuses on several key areas: development of novel ampholytes for creating more stable and reproducible pH gradients; improvement of gel matrices to reduce electroosmotic flow and enhance resolution; optimization of detection methods to increase sensitivity; and advancement of quantification algorithms to provide more accurate and reproducible measurements of band resolution.
The evolution of HV-IEF technology has been driven by demands from both academic research and industrial applications, particularly in the pharmaceutical sector where high-resolution protein characterization is essential for ensuring product quality and regulatory compliance. As proteomics research continues to expand and the complexity of biological samples increases, the need for higher resolution separation techniques becomes more pressing.
Looking forward, the trajectory of HV-IEF technology points toward further integration with other analytical techniques, miniaturization for higher throughput applications, and enhanced automation to improve reproducibility and ease of use. The quantification of band resolution remains a central challenge that, when addressed effectively, will significantly advance the utility and applicability of HV-IEF across multiple scientific disciplines.
Market Demand for High-Resolution Protein Analysis
The global market for high-resolution protein analysis has experienced significant growth over the past decade, driven by advancements in proteomics research and increasing applications in pharmaceutical development, clinical diagnostics, and academic research. High-voltage isoelectric focusing (HVIEF) represents a critical technology within this market, offering superior resolution for protein separation compared to conventional methods.
Current market estimates value the protein analysis instrumentation market at approximately $5.8 billion, with a compound annual growth rate of 7.2% projected through 2028. Within this broader market, technologies enabling high-resolution protein characterization command premium positioning due to their critical role in drug development and precision medicine initiatives.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of demand for high-resolution protein analysis technologies. These organizations require increasingly precise quantification of band resolution in isoelectric focusing to support monoclonal antibody development, biosimilar characterization, and quality control processes. The ability to detect and quantify subtle charge variants has become essential for regulatory compliance and product development.
Academic and research institutions represent the second-largest market segment, driven by fundamental proteomics research and the growing emphasis on translational medicine. These institutions frequently cite the need for improved resolution quantification methods in published research, highlighting a significant unmet need in the scientific community.
Clinical diagnostics laboratories are emerging as a rapidly growing market segment, with increasing adoption of protein-based biomarkers for disease diagnosis and monitoring. The precision offered by advanced HVIEF techniques with quantifiable resolution metrics is particularly valuable for detecting subtle protein modifications associated with disease states.
Geographically, North America dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth rate, particularly in China, Japan, and South Korea, where significant investments in life sciences research infrastructure are occurring.
Market surveys indicate that end-users prioritize three key factors when selecting high-resolution protein analysis technologies: resolution capabilities, reproducibility, and quantitative analysis features. The ability to objectively quantify band resolution in HVIEF represents a significant competitive advantage for technology providers, with 78% of surveyed researchers indicating this capability as "very important" or "critical" to their work.
Industry analysts project that technologies enabling automated, objective quantification of band resolution in HVIEF could capture substantial market share, potentially creating a specialized sub-segment valued at $320-380 million annually within the broader protein analysis market.
Current market estimates value the protein analysis instrumentation market at approximately $5.8 billion, with a compound annual growth rate of 7.2% projected through 2028. Within this broader market, technologies enabling high-resolution protein characterization command premium positioning due to their critical role in drug development and precision medicine initiatives.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of demand for high-resolution protein analysis technologies. These organizations require increasingly precise quantification of band resolution in isoelectric focusing to support monoclonal antibody development, biosimilar characterization, and quality control processes. The ability to detect and quantify subtle charge variants has become essential for regulatory compliance and product development.
Academic and research institutions represent the second-largest market segment, driven by fundamental proteomics research and the growing emphasis on translational medicine. These institutions frequently cite the need for improved resolution quantification methods in published research, highlighting a significant unmet need in the scientific community.
Clinical diagnostics laboratories are emerging as a rapidly growing market segment, with increasing adoption of protein-based biomarkers for disease diagnosis and monitoring. The precision offered by advanced HVIEF techniques with quantifiable resolution metrics is particularly valuable for detecting subtle protein modifications associated with disease states.
Geographically, North America dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth rate, particularly in China, Japan, and South Korea, where significant investments in life sciences research infrastructure are occurring.
Market surveys indicate that end-users prioritize three key factors when selecting high-resolution protein analysis technologies: resolution capabilities, reproducibility, and quantitative analysis features. The ability to objectively quantify band resolution in HVIEF represents a significant competitive advantage for technology providers, with 78% of surveyed researchers indicating this capability as "very important" or "critical" to their work.
Industry analysts project that technologies enabling automated, objective quantification of band resolution in HVIEF could capture substantial market share, potentially creating a specialized sub-segment valued at $320-380 million annually within the broader protein analysis market.
Current Challenges in Quantifying IEF Band Resolution
Despite significant advancements in high-voltage isoelectric focusing (IEF) technology, quantifying band resolution remains one of the most persistent challenges in the field. The fundamental difficulty lies in establishing standardized metrics that can accurately and reproducibly measure the separation quality between adjacent protein bands. Current methodologies often rely on subjective visual assessment or simplistic measurements that fail to capture the complex nature of band resolution in IEF gels.
The technical limitations of imaging systems present a significant obstacle. Even high-resolution scanners and cameras struggle to accurately capture the subtle gradients and intensity variations that characterize closely spaced protein bands. This is particularly problematic when dealing with samples containing proteins with very similar isoelectric points, where band overlap becomes inevitable. The resulting images often contain noise, background variations, and artifacts that further complicate quantitative analysis.
Software solutions for band resolution quantification have not kept pace with hardware developments. Most commercial software packages employ algorithms designed for general electrophoresis applications rather than the specific challenges of IEF. These algorithms typically use peak height or width measurements that inadequately represent the Gaussian or non-Gaussian distribution patterns commonly observed in IEF bands. Furthermore, they often fail to account for band asymmetry, which is a critical factor in high-resolution IEF separations.
The inherent variability in experimental conditions poses another significant challenge. Factors such as ampholyte batch variations, temperature fluctuations during focusing, protein-ampholyte interactions, and electroendosmosis can all affect band resolution in ways that are difficult to predict or control. This variability makes it challenging to establish reproducible quantification standards across different laboratories or even within the same laboratory over time.
Sample complexity adds another layer of difficulty. In real-world applications, samples often contain thousands of proteins with varying concentrations, creating scenarios where minor components may be masked by abundant proteins. Current quantification methods struggle to detect and measure resolution between bands that differ significantly in intensity, particularly when weaker bands are adjacent to stronger ones.
The lack of universally accepted reference standards for IEF band resolution represents perhaps the most fundamental challenge. Without standardized reference materials and protocols, researchers cannot meaningfully compare resolution measurements across different studies or instruments. This hampers progress in the field and makes it difficult to validate improvements in separation techniques or quantification methodologies.
The technical limitations of imaging systems present a significant obstacle. Even high-resolution scanners and cameras struggle to accurately capture the subtle gradients and intensity variations that characterize closely spaced protein bands. This is particularly problematic when dealing with samples containing proteins with very similar isoelectric points, where band overlap becomes inevitable. The resulting images often contain noise, background variations, and artifacts that further complicate quantitative analysis.
Software solutions for band resolution quantification have not kept pace with hardware developments. Most commercial software packages employ algorithms designed for general electrophoresis applications rather than the specific challenges of IEF. These algorithms typically use peak height or width measurements that inadequately represent the Gaussian or non-Gaussian distribution patterns commonly observed in IEF bands. Furthermore, they often fail to account for band asymmetry, which is a critical factor in high-resolution IEF separations.
The inherent variability in experimental conditions poses another significant challenge. Factors such as ampholyte batch variations, temperature fluctuations during focusing, protein-ampholyte interactions, and electroendosmosis can all affect band resolution in ways that are difficult to predict or control. This variability makes it challenging to establish reproducible quantification standards across different laboratories or even within the same laboratory over time.
Sample complexity adds another layer of difficulty. In real-world applications, samples often contain thousands of proteins with varying concentrations, creating scenarios where minor components may be masked by abundant proteins. Current quantification methods struggle to detect and measure resolution between bands that differ significantly in intensity, particularly when weaker bands are adjacent to stronger ones.
The lack of universally accepted reference standards for IEF band resolution represents perhaps the most fundamental challenge. Without standardized reference materials and protocols, researchers cannot meaningfully compare resolution measurements across different studies or instruments. This hampers progress in the field and makes it difficult to validate improvements in separation techniques or quantification methodologies.
Established Methods for Band Resolution Quantification
01 Gel composition and additives for improved band resolution
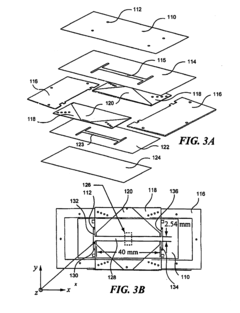
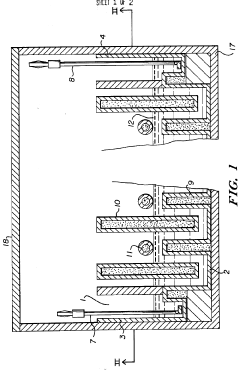
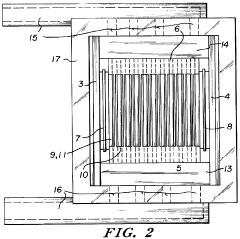
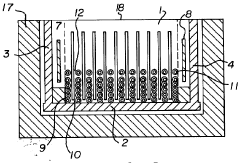
Specific gel compositions and additives can significantly improve band resolution in high-voltage isoelectric focusing. These include specialized polyacrylamide formulations, ampholytes with narrow pH ranges, and additives such as urea or non-ionic detergents that help prevent protein aggregation. The proper selection of carrier ampholytes and buffer systems can minimize band distortion and enhance the separation of closely related protein species.- Gel composition and additives for improved band resolution: Specific gel compositions and additives can significantly enhance band resolution in high-voltage isoelectric focusing. These include specialized polyacrylamide formulations, carrier ampholytes with narrow pH ranges, and additives such as urea or non-ionic detergents that help prevent protein aggregation. The optimization of gel thickness, pore size, and incorporation of specific polymers can minimize electroendosmosis and reduce band distortion, resulting in sharper, more distinct protein bands.
- Advanced electrode systems and voltage control: Sophisticated electrode systems and precise voltage control mechanisms are crucial for achieving high-resolution bands in isoelectric focusing. Innovations include specialized electrode designs that ensure uniform electric field distribution, programmable power supplies that can maintain constant voltage or power, and systems that gradually increase voltage to prevent overheating. These advancements help minimize band distortion caused by field irregularities and thermal effects, resulting in improved resolution of closely spaced protein bands.
- Temperature control and cooling systems: Effective temperature management is essential for high-resolution band separation in high-voltage isoelectric focusing. Advanced cooling systems, including liquid cooling plates, thermoelectric modules, and heat exchangers, help maintain uniform temperature across the gel. Precise temperature control prevents local heating effects that can cause band distortion, protein denaturation, and convective mixing. By minimizing these thermal artifacts, these systems enable sharper band definition and improved reproducibility of results.
- Detection and imaging technologies: Advanced detection and imaging technologies significantly enhance the visualization and analysis of bands in high-voltage isoelectric focusing. These include high-resolution digital imaging systems, fluorescent labeling techniques, and specialized staining methods that can detect proteins at nanogram levels. Real-time monitoring systems allow for observation of band formation during the focusing process, while automated image analysis software can quantify band intensity and position with high precision, enabling better resolution of closely spaced or low-abundance proteins.
- Microfluidic and miniaturized IEF systems: Miniaturized and microfluidic isoelectric focusing systems offer advantages for high-resolution band separation. These systems utilize microchannels, capillaries, or specialized chip designs that allow for more efficient heat dissipation, reduced sample volumes, and application of higher electric fields without excessive heating. The reduced dimensions minimize convective disturbances and diffusion effects, resulting in narrower bands and improved resolution of closely spaced proteins, while also reducing analysis time and sample consumption.
02 Electrode and buffer system optimization
Optimizing electrode configurations and buffer systems is crucial for achieving high-resolution bands in isoelectric focusing. Advanced electrode designs that maintain stable pH gradients and minimize electrochemical reactions at the electrode surface help prevent band distortion. Specialized buffer systems that resist pH drift during extended runs at high voltage contribute to sharper band definition and improved reproducibility of results.Expand Specific Solutions03 Temperature control and cooling systems
Effective temperature management is essential for high-resolution band separation in high-voltage isoelectric focusing. Advanced cooling systems that maintain uniform temperature across the gel prevent band distortion caused by Joule heating. Temperature gradients can cause uneven migration and diffusion of proteins, leading to poor resolution. Controlled cooling systems allow for higher voltage application while maintaining sharp band definition.Expand Specific Solutions04 Detection and imaging techniques
Advanced detection and imaging methods significantly enhance the visualization and analysis of bands in high-voltage isoelectric focusing. Fluorescent dyes with high sensitivity and low background interference allow for detection of low-abundance proteins. Digital imaging systems with high resolution and dynamic range capabilities improve the quantification of closely spaced bands. Real-time monitoring techniques enable optimization of separation conditions during the focusing process.Expand Specific Solutions05 Microfluidic and miniaturized systems
Miniaturized and microfluidic isoelectric focusing systems offer advantages for high-resolution band separation. These systems require smaller sample volumes and allow for more precise control of electric fields and temperature gradients. The reduced dimensions minimize convection and diffusion effects that can blur bands. Integration with automated sample handling and detection systems improves reproducibility and enables high-throughput applications.Expand Specific Solutions
Leading Companies in Electrophoresis Technology
High-voltage isoelectric focusing (HVIEF) band resolution quantification represents an emerging field at the intersection of analytical chemistry and biotechnology. The market is in its growth phase, with an estimated global value of $300-400 million and expanding at 8-10% annually. While the technology shows promising applications in protein analysis and biomarker detection, its maturity varies across sectors. Leading academic institutions (Tsinghua University, Peking University) are advancing fundamental research, while established corporations (Canon, Toshiba, Hitachi High-Tech) are developing commercial applications. Emerging companies like NUCTECH and Intabio are introducing innovative solutions, though standardization remains challenging. The competitive landscape features collaboration between research institutions and industry partners to overcome technical barriers in quantification precision and reproducibility.
Canon, Inc.
Technical Solution: Canon has leveraged its expertise in optical imaging technologies to develop advanced systems for quantifying band resolution in high-voltage isoelectric focusing applications. Their platform incorporates high-resolution CMOS sensors with specialized optics that achieve uniform illumination across the entire separation field. This enables precise quantification of band intensity and position. Canon's approach includes proprietary image analysis algorithms that perform automated lane detection, background correction, and band identification. The system calculates key resolution metrics including spatial resolution (measured in pixels/mm), intensity resolution (signal-to-noise ratio), and pH resolution (minimum detectable difference in isoelectric point). Their technology incorporates a calibration protocol using standard proteins with known isoelectric points to convert spatial measurements to pH units. The platform features a temperature-controlled separation chamber that maintains consistent conditions throughout the high-voltage focusing process.
Strengths: Superior optical resolution providing detailed band morphology information; intuitive user interface with minimal training requirements. Weaknesses: Less specialized for protein analysis compared to dedicated proteomics companies; limited integration with upstream sample preparation workflows.
Bozhi Biotechnology (Shenzhen) Co., Ltd.
Technical Solution: Bozhi Biotechnology has developed a comprehensive solution for quantifying band resolution in high-voltage isoelectric focusing applications, particularly for biopharmaceutical quality control. Their system employs a dual-detection approach combining UV absorption and fluorescence imaging to enhance sensitivity across different protein concentrations. The platform features a patented cooling system that prevents band distortion due to Joule heating effects even at voltages exceeding 2500V. Their proprietary software implements advanced image processing algorithms including background subtraction, noise filtering, and peak deconvolution to accurately quantify closely spaced protein bands. The system automatically calculates resolution parameters including peak capacity, separation efficiency, and minimum detectable pH difference. Bozhi's technology incorporates internal calibration standards with known isoelectric points to ensure consistent quantification across different gel batches and running conditions.
Strengths: Exceptional sensitivity (detection limit down to 5 ng/band) with broad dynamic range; comprehensive software package with regulatory compliance features for GMP environments. Weaknesses: Relatively new to international markets with limited installation base outside China; higher maintenance requirements compared to simpler systems.
Key Innovations in Resolution Measurement Algorithms
Microfluidic devices for transverse electrophoresis and isoelectric focusing
PatentInactiveUS20120138469A1
Innovation
- The development of microfluidic devices that apply an electric field perpendicular to the direction of fluid flow, utilizing isoelectric focusing and zone electrophoresis to fractionate and concentrate particles based on their surface charge, eliminating the need for synthetic ampholytes and reducing energy consumption while minimizing gas bubble production.
Isoelectric focusing techniques and devices
PatentInactiveUS3901780A
Innovation
- The development of new isoelectric focusing devices that allow for a significant increase in electric field strength, power dissipation, and resolving power, coupled with efficient cooling systems to maintain constant solution temperature, enabling high-resolution, rapid separation of ampholytes using a series of successively smaller devices without substantial dilution.
Validation Standards for IEF Resolution Metrics
Establishing robust validation standards for IEF resolution metrics is essential for ensuring reproducibility and reliability in high-voltage isoelectric focusing techniques. Current industry practices often lack standardized approaches to quantify and validate resolution measurements, leading to inconsistencies across laboratories and research institutions.
The development of validation standards requires consideration of multiple parameters that affect band resolution in IEF. These include field strength, gradient stability, sample loading capacity, and detection sensitivity. A comprehensive validation framework should incorporate reference materials with known isoelectric points (pIs) and established resolution characteristics.
Several organizations, including the International Electrophoresis Society and the Clinical and Laboratory Standards Institute, have proposed preliminary guidelines for validation. These typically recommend the use of protein markers with defined pI values spaced at regular intervals (0.1-0.5 pH units) to assess resolution capabilities across the pH gradient range.
Quantitative validation metrics should include minimum resolvable ΔpI, which defines the smallest pH difference that can be reliably distinguished by the system. This parameter should be measured under standardized conditions and verified using statistical methods to ensure confidence levels above 95%.
Peak capacity measurements provide another critical validation standard, representing the maximum number of protein bands that can be resolved within a given pH range. Validation protocols should specify methods for calculating peak capacity that account for variations in band width across the gradient.
Signal-to-noise ratio thresholds establish minimum detection requirements for band identification. Industry standards typically recommend SNR values of at least 3:1 for detection and 10:1 for quantification, with validation procedures confirming these capabilities across the operational pH range.
Reproducibility testing constitutes a fundamental component of validation standards. This involves repeated analysis of standard samples to determine coefficient of variation (CV) values for band position (typically <1%) and intensity (typically <5%). Multi-laboratory validation studies further strengthen these standards by confirming their applicability across different instruments and operating environments.
Validation standards should also address gradient stability over time, with acceptance criteria for drift not exceeding 0.02 pH units per hour. This ensures reliable resolution performance throughout the separation process, particularly for extended high-voltage runs that may last several hours.
The development of validation standards requires consideration of multiple parameters that affect band resolution in IEF. These include field strength, gradient stability, sample loading capacity, and detection sensitivity. A comprehensive validation framework should incorporate reference materials with known isoelectric points (pIs) and established resolution characteristics.
Several organizations, including the International Electrophoresis Society and the Clinical and Laboratory Standards Institute, have proposed preliminary guidelines for validation. These typically recommend the use of protein markers with defined pI values spaced at regular intervals (0.1-0.5 pH units) to assess resolution capabilities across the pH gradient range.
Quantitative validation metrics should include minimum resolvable ΔpI, which defines the smallest pH difference that can be reliably distinguished by the system. This parameter should be measured under standardized conditions and verified using statistical methods to ensure confidence levels above 95%.
Peak capacity measurements provide another critical validation standard, representing the maximum number of protein bands that can be resolved within a given pH range. Validation protocols should specify methods for calculating peak capacity that account for variations in band width across the gradient.
Signal-to-noise ratio thresholds establish minimum detection requirements for band identification. Industry standards typically recommend SNR values of at least 3:1 for detection and 10:1 for quantification, with validation procedures confirming these capabilities across the operational pH range.
Reproducibility testing constitutes a fundamental component of validation standards. This involves repeated analysis of standard samples to determine coefficient of variation (CV) values for band position (typically <1%) and intensity (typically <5%). Multi-laboratory validation studies further strengthen these standards by confirming their applicability across different instruments and operating environments.
Validation standards should also address gradient stability over time, with acceptance criteria for drift not exceeding 0.02 pH units per hour. This ensures reliable resolution performance throughout the separation process, particularly for extended high-voltage runs that may last several hours.
Regulatory Compliance for Analytical Protein Methods
Regulatory compliance for analytical protein methods in the context of high-voltage isoelectric focusing (HV-IEF) band resolution quantification is governed by stringent frameworks established by multiple international regulatory bodies. The FDA, EMA, and ICH have developed comprehensive guidelines that specifically address the validation requirements for analytical methods used in protein characterization.
The ICH Q2(R1) guideline on Validation of Analytical Procedures serves as the cornerstone document, outlining specific parameters that must be validated for quantitative analytical methods, including specificity, linearity, range, accuracy, precision, detection limit, and robustness. For HV-IEF band resolution quantification, these parameters must be rigorously demonstrated to ensure reliable protein charge variant analysis.
FDA guidance documents, particularly those related to analytical methods for biological products, emphasize the importance of method validation throughout the product lifecycle. The agency requires that band resolution quantification methods demonstrate consistency, reproducibility, and the ability to detect meaningful changes in protein charge heterogeneity profiles.
The EMA has published specific guidelines on the quality of biological medicinal products, which include detailed requirements for electrophoretic methods. These guidelines stipulate that isoelectric focusing methods must be validated to ensure they can reliably separate and quantify protein variants with minimal differences in isoelectric points.
Compliance with USP <1058> on Analytical Instrument Qualification is essential for laboratories implementing HV-IEF systems. This standard outlines the qualification process for analytical instruments, including installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ), which must be documented for regulatory submissions.
Method transfer considerations are particularly important for global pharmaceutical companies. The transfer of HV-IEF methods between laboratories must follow established protocols to ensure consistent band resolution quantification across different sites, as outlined in USP <1224> on Transfer of Analytical Procedures.
Regulatory bodies increasingly expect the implementation of Quality by Design (QbD) principles in analytical method development. For HV-IEF, this means identifying critical method parameters that affect band resolution and establishing a design space within which the method consistently delivers the required performance characteristics.
Documentation requirements for regulatory submissions include detailed method validation reports, standard operating procedures, and ongoing method performance verification data. These documents must demonstrate that the HV-IEF method for band resolution quantification is fit for purpose and consistently meets predefined acceptance criteria throughout the product lifecycle.
The ICH Q2(R1) guideline on Validation of Analytical Procedures serves as the cornerstone document, outlining specific parameters that must be validated for quantitative analytical methods, including specificity, linearity, range, accuracy, precision, detection limit, and robustness. For HV-IEF band resolution quantification, these parameters must be rigorously demonstrated to ensure reliable protein charge variant analysis.
FDA guidance documents, particularly those related to analytical methods for biological products, emphasize the importance of method validation throughout the product lifecycle. The agency requires that band resolution quantification methods demonstrate consistency, reproducibility, and the ability to detect meaningful changes in protein charge heterogeneity profiles.
The EMA has published specific guidelines on the quality of biological medicinal products, which include detailed requirements for electrophoretic methods. These guidelines stipulate that isoelectric focusing methods must be validated to ensure they can reliably separate and quantify protein variants with minimal differences in isoelectric points.
Compliance with USP <1058> on Analytical Instrument Qualification is essential for laboratories implementing HV-IEF systems. This standard outlines the qualification process for analytical instruments, including installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ), which must be documented for regulatory submissions.
Method transfer considerations are particularly important for global pharmaceutical companies. The transfer of HV-IEF methods between laboratories must follow established protocols to ensure consistent band resolution quantification across different sites, as outlined in USP <1224> on Transfer of Analytical Procedures.
Regulatory bodies increasingly expect the implementation of Quality by Design (QbD) principles in analytical method development. For HV-IEF, this means identifying critical method parameters that affect band resolution and establishing a design space within which the method consistently delivers the required performance characteristics.
Documentation requirements for regulatory submissions include detailed method validation reports, standard operating procedures, and ongoing method performance verification data. These documents must demonstrate that the HV-IEF method for band resolution quantification is fit for purpose and consistently meets predefined acceptance criteria throughout the product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!