How to Improve Ampholyte Selection for Isoelectric Focusing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ampholyte Technology Background and Objectives
Isoelectric focusing (IEF) emerged in the 1960s as a groundbreaking electrophoretic technique for protein separation based on their isoelectric points. The evolution of this technology has been closely tied to advancements in ampholyte development, which serve as the critical pH gradient-forming components. Initially, carrier ampholytes were derived from simple mixtures of polyamino-polycarboxylic acids, but significant improvements in synthesis methods and purification techniques have dramatically enhanced their performance over the past decades.
The ampholyte landscape has transformed from rudimentary mixtures to sophisticated, precisely engineered compounds with specific pH ranges and improved conductivity characteristics. This evolution has paralleled the growing demands for higher resolution protein separation in proteomics, diagnostics, and biopharmaceutical applications. The technical trajectory shows a clear trend toward ampholytes with narrower pH ranges, greater stability, and reduced batch-to-batch variability.
Current technical objectives in ampholyte development focus on addressing several persistent challenges. Researchers aim to create ampholytes with enhanced resolution capabilities, particularly for separating proteins with minimal pI differences (ΔpI < 0.01). Another critical goal is developing ampholytes that maintain stable pH gradients over extended separation times, reducing the "cathodic drift" phenomenon that compromises reproducibility in IEF experiments.
The field is also pursuing ampholytes with reduced protein-ampholyte interactions, as these non-specific bindings can alter protein migration patterns and complicate downstream analysis. Additionally, there is significant interest in developing ampholytes compatible with mass spectrometry, addressing the long-standing challenge of ampholyte interference in MS-based protein identification workflows.
Recent technological advances have opened new possibilities for synthetic approaches to ampholyte design. Computational modeling now enables the prediction of ampholyte behavior in electric fields, facilitating rational design rather than empirical formulation. Polymer chemistry innovations have introduced novel carrier ampholyte backbones with improved properties, while advances in analytical techniques allow for more precise characterization of ampholyte mixtures.
The ultimate technical objective remains the development of "ideal" ampholytes that combine high buffering capacity, low conductivity, good solubility, zero mobility at their pI, and minimal interaction with separated biomolecules. Progress toward this goal continues to drive innovation in the field, with significant implications for proteomics research, clinical diagnostics, and biopharmaceutical quality control processes.
The ampholyte landscape has transformed from rudimentary mixtures to sophisticated, precisely engineered compounds with specific pH ranges and improved conductivity characteristics. This evolution has paralleled the growing demands for higher resolution protein separation in proteomics, diagnostics, and biopharmaceutical applications. The technical trajectory shows a clear trend toward ampholytes with narrower pH ranges, greater stability, and reduced batch-to-batch variability.
Current technical objectives in ampholyte development focus on addressing several persistent challenges. Researchers aim to create ampholytes with enhanced resolution capabilities, particularly for separating proteins with minimal pI differences (ΔpI < 0.01). Another critical goal is developing ampholytes that maintain stable pH gradients over extended separation times, reducing the "cathodic drift" phenomenon that compromises reproducibility in IEF experiments.
The field is also pursuing ampholytes with reduced protein-ampholyte interactions, as these non-specific bindings can alter protein migration patterns and complicate downstream analysis. Additionally, there is significant interest in developing ampholytes compatible with mass spectrometry, addressing the long-standing challenge of ampholyte interference in MS-based protein identification workflows.
Recent technological advances have opened new possibilities for synthetic approaches to ampholyte design. Computational modeling now enables the prediction of ampholyte behavior in electric fields, facilitating rational design rather than empirical formulation. Polymer chemistry innovations have introduced novel carrier ampholyte backbones with improved properties, while advances in analytical techniques allow for more precise characterization of ampholyte mixtures.
The ultimate technical objective remains the development of "ideal" ampholytes that combine high buffering capacity, low conductivity, good solubility, zero mobility at their pI, and minimal interaction with separated biomolecules. Progress toward this goal continues to drive innovation in the field, with significant implications for proteomics research, clinical diagnostics, and biopharmaceutical quality control processes.
Market Analysis for Advanced Isoelectric Focusing Applications
The global market for isoelectric focusing (IEF) applications continues to expand, driven by increasing demand in proteomics research, pharmaceutical development, and clinical diagnostics. The market size for IEF technologies was valued at approximately $1.2 billion in 2022 and is projected to grow at a compound annual growth rate of 5.8% through 2028, reflecting the rising importance of protein separation techniques in various scientific fields.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for nearly 45% of the total IEF market. This dominance stems from the critical role of protein analysis in drug development, particularly for biologics and biosimilars. The increasing complexity of therapeutic proteins has heightened the need for more precise and efficient separation techniques, creating substantial market opportunities for advanced ampholyte solutions.
Academic and research institutions constitute the second-largest market segment, contributing approximately 30% of market revenue. These institutions primarily utilize IEF for fundamental protein research, biomarker discovery, and educational purposes. The remaining market share is distributed among clinical diagnostic laboratories, food safety testing facilities, and environmental monitoring agencies.
Geographically, North America leads the market with a 38% share, followed by Europe (32%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, demonstrates the highest growth potential due to expanding biotechnology sectors, increasing research funding, and growing adoption of advanced protein analysis techniques.
Customer needs analysis reveals several key market drivers for improved ampholyte selection. First, there is significant demand for ampholytes that enable higher resolution separation, particularly for closely related protein isoforms and post-translational modifications. Second, customers seek ampholytes with enhanced stability and reproducibility to ensure consistent results across multiple experiments and laboratories.
The market also shows strong interest in specialized ampholytes designed for specific pH ranges and sample types. This trend reflects the growing customization requirements in proteomics research and clinical applications. Additionally, there is increasing demand for ampholytes compatible with downstream analytical techniques such as mass spectrometry, enabling integrated workflow solutions.
Cost considerations remain important, with many laboratories seeking more economical alternatives to premium ampholyte products without compromising performance. This price sensitivity is particularly evident in academic settings and emerging markets, creating opportunities for cost-effective innovations in ampholyte formulation and production.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for nearly 45% of the total IEF market. This dominance stems from the critical role of protein analysis in drug development, particularly for biologics and biosimilars. The increasing complexity of therapeutic proteins has heightened the need for more precise and efficient separation techniques, creating substantial market opportunities for advanced ampholyte solutions.
Academic and research institutions constitute the second-largest market segment, contributing approximately 30% of market revenue. These institutions primarily utilize IEF for fundamental protein research, biomarker discovery, and educational purposes. The remaining market share is distributed among clinical diagnostic laboratories, food safety testing facilities, and environmental monitoring agencies.
Geographically, North America leads the market with a 38% share, followed by Europe (32%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, demonstrates the highest growth potential due to expanding biotechnology sectors, increasing research funding, and growing adoption of advanced protein analysis techniques.
Customer needs analysis reveals several key market drivers for improved ampholyte selection. First, there is significant demand for ampholytes that enable higher resolution separation, particularly for closely related protein isoforms and post-translational modifications. Second, customers seek ampholytes with enhanced stability and reproducibility to ensure consistent results across multiple experiments and laboratories.
The market also shows strong interest in specialized ampholytes designed for specific pH ranges and sample types. This trend reflects the growing customization requirements in proteomics research and clinical applications. Additionally, there is increasing demand for ampholytes compatible with downstream analytical techniques such as mass spectrometry, enabling integrated workflow solutions.
Cost considerations remain important, with many laboratories seeking more economical alternatives to premium ampholyte products without compromising performance. This price sensitivity is particularly evident in academic settings and emerging markets, creating opportunities for cost-effective innovations in ampholyte formulation and production.
Current Challenges in Ampholyte Selection and Development
Despite significant advancements in isoelectric focusing (IEF) technology, ampholyte selection remains a critical bottleneck in achieving optimal protein separation. Current commercial carrier ampholytes suffer from batch-to-batch variability, which compromises experimental reproducibility and reliability. This inconsistency stems from manufacturing processes that cannot guarantee identical molecular distributions across production runs, resulting in shifted pH gradients and altered protein migration patterns.
The limited pH range coverage of available ampholytes presents another significant challenge. While broad-range ampholytes (pH 3-10) are widely available, narrow-range ampholytes for specific applications often lack sufficient resolution in critical pH regions. This deficiency is particularly problematic when analyzing proteins with closely similar isoelectric points or when working with samples containing predominantly basic or acidic proteins.
Ampholyte conductivity issues continue to plague IEF applications, especially during extended focusing times. Current ampholytes can contribute to excessive joule heating and gradient drift, leading to protein precipitation and loss of resolution. This phenomenon is exacerbated in high-throughput applications where rapid, reliable separations are essential for downstream analysis.
Chemical interactions between ampholytes and target proteins represent another unresolved challenge. Some ampholytes contain reactive groups that can form adducts with proteins, altering their migration patterns and complicating subsequent mass spectrometry analysis. These interactions are often unpredictable and can vary depending on sample composition and experimental conditions.
The cost-effectiveness of high-quality ampholytes remains problematic for many laboratories. Premium ampholyte formulations that offer improved resolution and stability come at significantly higher prices, limiting their routine use in research settings with budget constraints. This economic barrier has slowed the adoption of advanced IEF techniques in many fields.
Environmental and safety concerns associated with traditional ampholyte production and disposal have gained increasing attention. Many conventional ampholytes contain toxic components that require special handling and disposal procedures. The development of more environmentally friendly alternatives has been slow, partly due to the technical challenges in maintaining performance while reducing toxicity.
The integration of ampholytes with emerging microfluidic and lab-on-a-chip technologies presents additional challenges. Miniaturized IEF systems require ampholytes with specific physical properties, including lower viscosity and improved stability under high electric field strengths. Current ampholyte formulations are not optimized for these cutting-edge applications, limiting the potential of these promising technologies.
The limited pH range coverage of available ampholytes presents another significant challenge. While broad-range ampholytes (pH 3-10) are widely available, narrow-range ampholytes for specific applications often lack sufficient resolution in critical pH regions. This deficiency is particularly problematic when analyzing proteins with closely similar isoelectric points or when working with samples containing predominantly basic or acidic proteins.
Ampholyte conductivity issues continue to plague IEF applications, especially during extended focusing times. Current ampholytes can contribute to excessive joule heating and gradient drift, leading to protein precipitation and loss of resolution. This phenomenon is exacerbated in high-throughput applications where rapid, reliable separations are essential for downstream analysis.
Chemical interactions between ampholytes and target proteins represent another unresolved challenge. Some ampholytes contain reactive groups that can form adducts with proteins, altering their migration patterns and complicating subsequent mass spectrometry analysis. These interactions are often unpredictable and can vary depending on sample composition and experimental conditions.
The cost-effectiveness of high-quality ampholytes remains problematic for many laboratories. Premium ampholyte formulations that offer improved resolution and stability come at significantly higher prices, limiting their routine use in research settings with budget constraints. This economic barrier has slowed the adoption of advanced IEF techniques in many fields.
Environmental and safety concerns associated with traditional ampholyte production and disposal have gained increasing attention. Many conventional ampholytes contain toxic components that require special handling and disposal procedures. The development of more environmentally friendly alternatives has been slow, partly due to the technical challenges in maintaining performance while reducing toxicity.
The integration of ampholytes with emerging microfluidic and lab-on-a-chip technologies presents additional challenges. Miniaturized IEF systems require ampholytes with specific physical properties, including lower viscosity and improved stability under high electric field strengths. Current ampholyte formulations are not optimized for these cutting-edge applications, limiting the potential of these promising technologies.
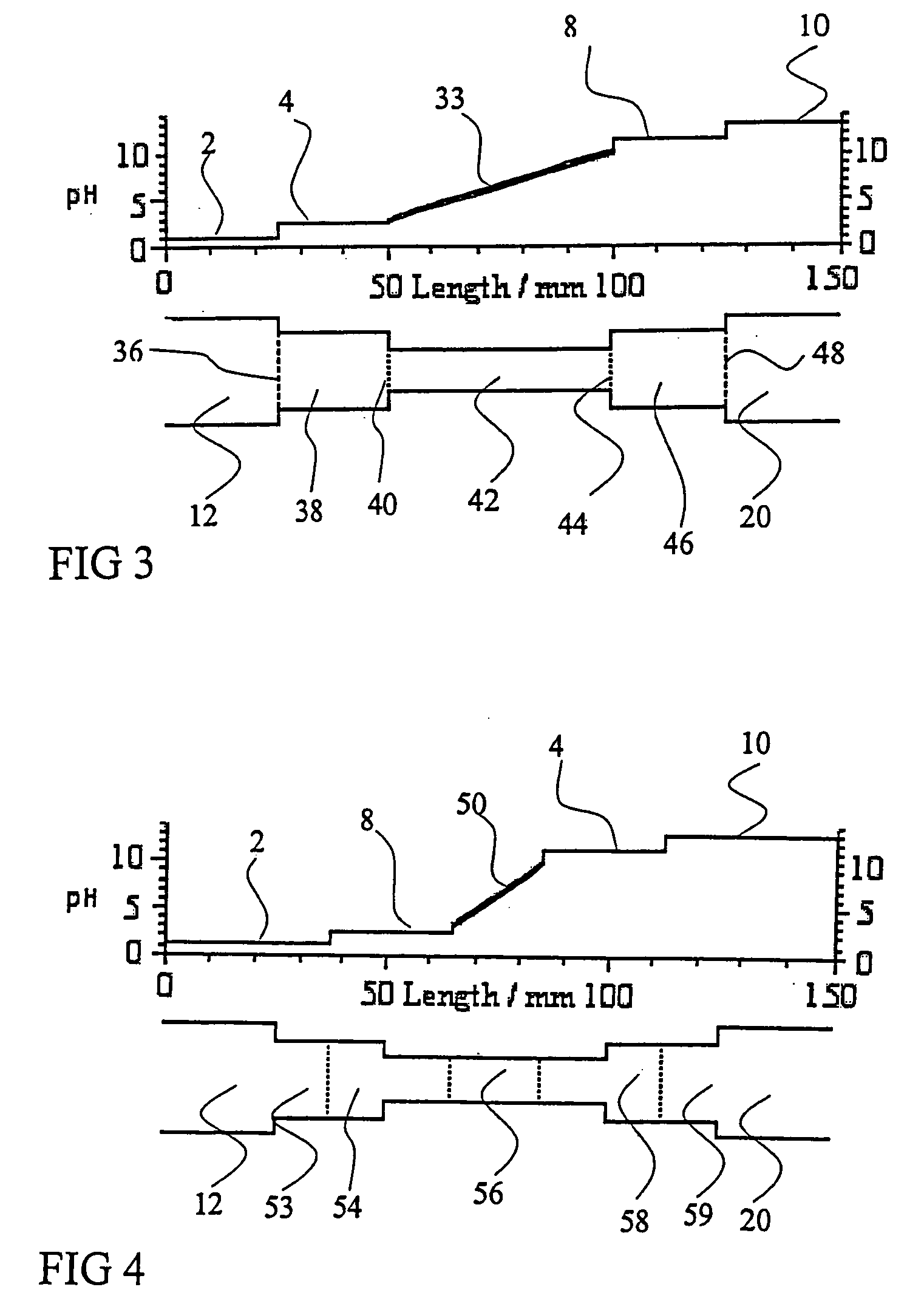
Contemporary Ampholyte Selection Methodologies
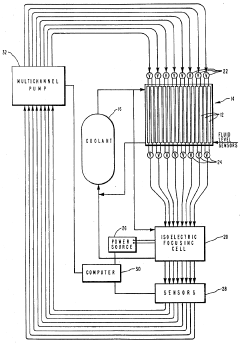
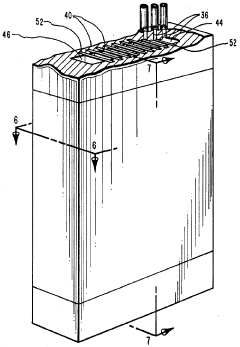
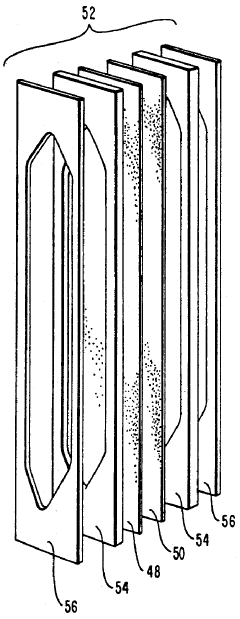
01 Ampholytes for isoelectric focusing
Ampholytes are used in isoelectric focusing techniques for protein separation and analysis. These carrier ampholytes create a stable pH gradient in electrophoresis, allowing proteins to migrate to their isoelectric points. Selection of appropriate ampholytes with specific pH ranges is crucial for achieving high-resolution separation of biomolecules in analytical and preparative applications.- Ampholytes for isoelectric focusing: Ampholytes are used in isoelectric focusing techniques for protein separation and analysis. These carrier ampholytes create a stable pH gradient in electrophoresis, allowing proteins to migrate to their isoelectric points. Selection of appropriate ampholytes with specific pH ranges is crucial for achieving high-resolution separation of biomolecules in analytical and preparative applications.
- Ampholytes in electrophoretic systems: Ampholytes play a critical role in various electrophoretic systems beyond isoelectric focusing. The selection of ampholytes affects the performance of capillary electrophoresis, gel electrophoresis, and other separation techniques. Specific ampholytes are chosen based on their buffering capacity, conductivity, and compatibility with detection methods to optimize separation efficiency and reproducibility.
- Computational methods for ampholyte selection: Advanced computational methods are employed for the selection and optimization of ampholytes in analytical applications. These methods include algorithm-based approaches for predicting ampholyte behavior, simulation of pH gradients, and data analysis tools for optimizing separation conditions. Computational techniques help in designing custom ampholyte mixtures tailored to specific separation challenges.
- Novel ampholyte formulations: Development of novel ampholyte formulations with improved properties for specific applications. These include synthetic ampholytes with enhanced stability, reduced conductivity, or specialized pH ranges. Innovative formulations may incorporate modified chemical structures, hybrid materials, or carrier molecules to achieve superior separation performance or compatibility with challenging sample types.
- Ampholytes in microfluidic and miniaturized systems: Selection and adaptation of ampholytes for use in microfluidic and miniaturized separation systems. These applications require specialized ampholytes that function effectively at microscale dimensions, with considerations for surface interactions, evaporation effects, and integration with detection systems. Optimized ampholyte selection enables high-performance separations in lab-on-a-chip devices and portable analytical instruments.
02 Ampholytic polymers in separation technologies
Ampholytic polymers containing both acidic and basic functional groups are selected for various separation technologies. These polymers can be tailored with specific charge distributions and molecular weights to enhance separation efficiency. Applications include chromatography, membrane filtration, and electrophoretic techniques where the ampholytic nature provides unique selectivity based on charge interactions with target molecules.Expand Specific Solutions03 Computational methods for ampholyte selection
Advanced computational algorithms and data analysis methods are employed for optimal ampholyte selection. These approaches include machine learning techniques, molecular modeling, and simulation tools that predict ampholyte behavior in various systems. The computational methods help in screening large libraries of potential ampholytes and identifying candidates with desired properties for specific applications.Expand Specific Solutions04 Ampholytes in pharmaceutical and biomedical applications
Selection of ampholytes for pharmaceutical and biomedical applications focuses on biocompatibility, stability, and specific interactions with biological systems. These ampholytes are used in drug delivery systems, diagnostic assays, and therapeutic formulations. The selection criteria include pH responsiveness, solubility profiles, and ability to interact with biological membranes or target molecules.Expand Specific Solutions05 Industrial applications of ampholytes
Ampholytes are selected for various industrial applications based on their performance in specific environments. These applications include water treatment, surface modification, emulsion stabilization, and as additives in manufacturing processes. Selection criteria focus on stability under processing conditions, cost-effectiveness, environmental impact, and compatibility with other components in industrial formulations.Expand Specific Solutions
Leading Manufacturers and Research Institutions in IEF Field
The isoelectric focusing (IEF) ampholyte selection landscape is currently in a mature growth phase, with a global market estimated at $350-400 million annually. The technology has evolved from early experimental stages to standardized applications in proteomics and biopharmaceutical analysis. Leading companies like Becton Dickinson, Regeneron Pharmaceuticals, and Life Technologies have established dominant positions through proprietary ampholyte formulations offering superior resolution and reproducibility. Academic institutions including Texas A&M University and University of Washington contribute significant research innovations, while specialized manufacturers like Sharp Corp. and Hamamatsu Photonics provide complementary instrumentation. The competitive environment is characterized by increasing focus on application-specific ampholyte mixtures and integration with downstream analytical techniques, driving continued innovation despite the technology's maturity.
Becton, Dickinson & Co.
Technical Solution: Becton Dickinson has developed an advanced ampholyte selection platform for isoelectric focusing that integrates microfluidic technology with sophisticated ampholyte chemistry. Their approach utilizes a proprietary algorithm to create optimized ampholyte mixtures tailored to specific protein families or sample types. The BD system employs a combination of synthetic and natural ampholytes with carefully controlled molecular weights and pKa distributions to achieve superior resolution across challenging pH ranges. Their technology incorporates real-time pH monitoring during the focusing process, allowing dynamic adjustment of electric field parameters to optimize separation. BD's ampholyte formulations include specialized additives that minimize protein-wall interactions in capillary electrophoresis formats, reducing band broadening and improving detection sensitivity for low-abundance proteins.
Strengths: Highly customizable for specific applications; excellent performance in microfluidic formats; reduced sample requirements; superior detection sensitivity. Weaknesses: Proprietary nature limits flexibility for researchers; higher cost than traditional methods; requires specialized instrumentation; optimization process can be time-consuming for novel applications.
Life Technologies Corp.
Technical Solution: Life Technologies has pioneered a systematic approach to ampholyte selection through their ZOOM® IEF Fractionator system. This technology employs a series of discrete pH membranes with specific ampholyte compositions to create well-defined pH compartments for protein separation. Their innovation lies in the development of membrane-specific ampholyte formulations that maintain stable pH values at membrane interfaces while minimizing protein precipitation. Life Technologies' approach includes a proprietary ampholyte screening platform that evaluates hundreds of potential carrier ampholyte combinations for specific applications, optimizing for parameters such as buffering capacity, conductivity, and protein solubility. Their system incorporates machine learning algorithms to predict optimal ampholyte compositions based on protein sequence data, allowing for application-specific customization. The technology also features ampholyte regeneration capabilities, reducing costs for high-throughput applications.
Strengths: Excellent reproducibility; modular design allows flexible pH range selection; reduced sample complexity improves downstream analysis; compatible with various proteomics workflows. Weaknesses: Discrete pH steps rather than continuous gradients may miss some proteins; higher initial investment; requires specialized equipment; optimization needed for each new sample type.
Critical Patents and Innovations in Carrier Ampholyte Technology
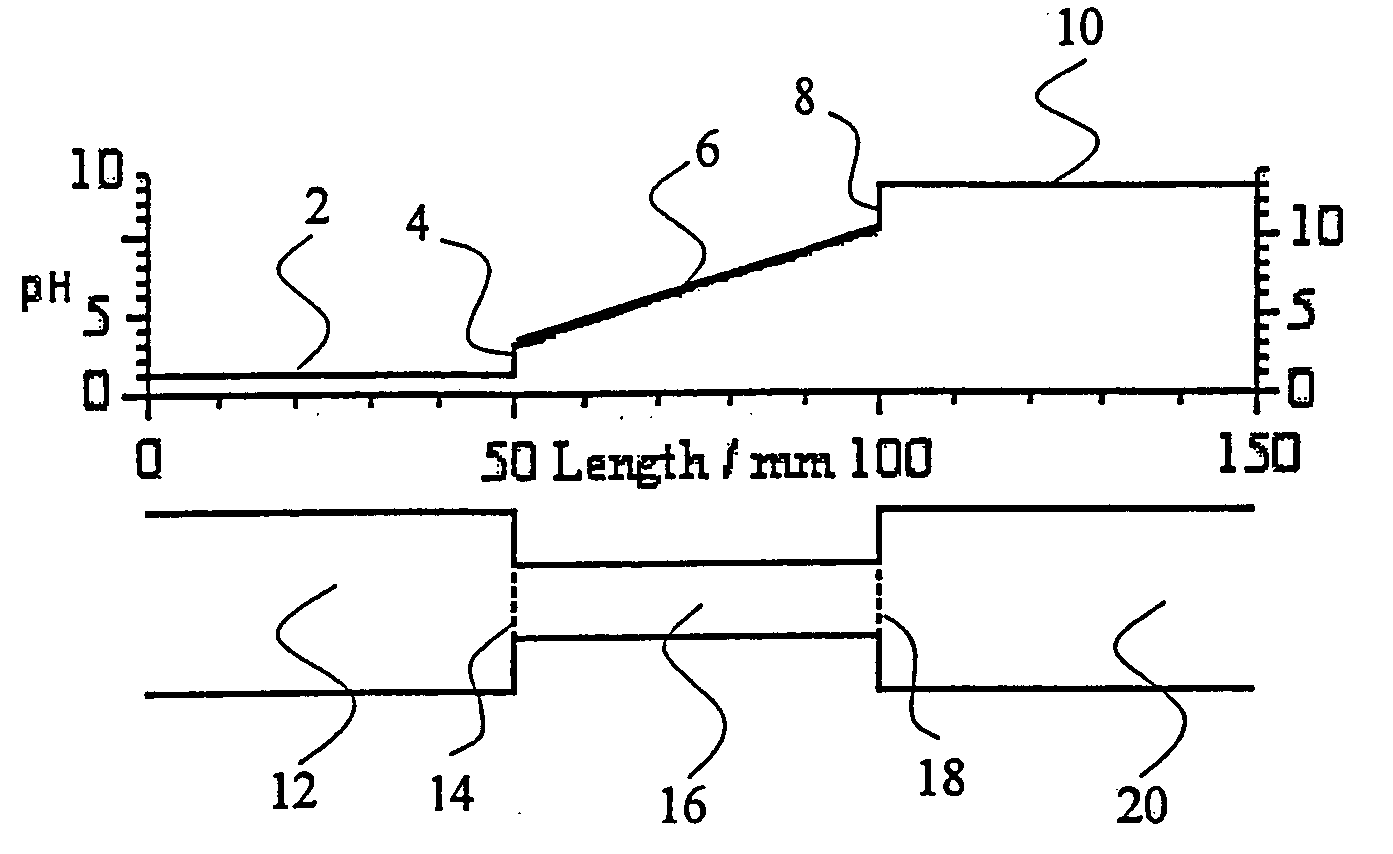
Apparatus and methods for isoelectric focusing of amphoteric substances incorporating ion selective membranes in electrode chambers
PatentInactiveUS5160594A
Innovation
- The use of bipolar membranes or a combination of anion and cation selective membranes to separate electrode compartments from the focusing cell compartments, preventing the passage of ions and maintaining a stable, narrow pH gradient.
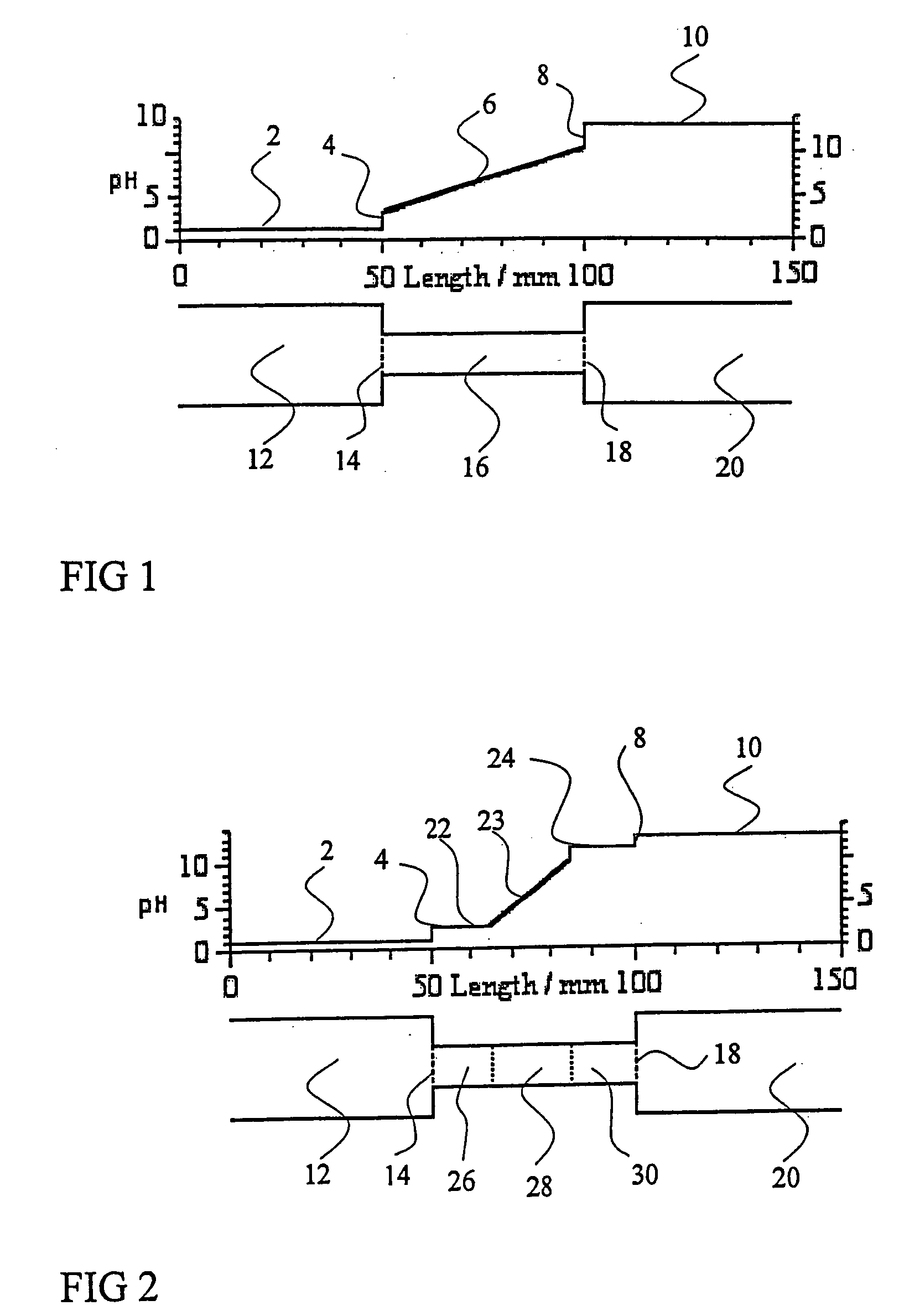
Method and apparatus to improve the concentration detection sensitivity in isoelectric focusing systems
PatentInactiveUS20050161332A1
Innovation
- The addition of an auxiliary compartment to the separation capillary, combined with suitable auxiliary agents such as strong or weak electrolytes or ampholytic substances, increases the sample holding volume and forces ampholytic components into the detection area, improving concentration detection limits and correcting salt-induced pH gradient shifts.
Quality Control Standards for Ampholyte Production
Quality control standards for ampholyte production represent a critical framework for ensuring consistent and reliable performance in isoelectric focusing (IEF) applications. The establishment of rigorous quality parameters directly impacts separation efficiency, resolution, and reproducibility of results. Current industry standards typically encompass several key dimensions of ampholyte quality that manufacturers must adhere to.
Purity specifications constitute the foundation of ampholyte quality control, with most premium commercial preparations requiring >99% chemical purity. Contaminants, particularly those with charged groups, can significantly distort pH gradients and introduce artifacts in separation patterns. Modern quality control protocols employ high-performance liquid chromatography (HPLC), mass spectrometry, and capillary electrophoresis to verify purity levels and identify potential impurities.
Conductivity measurements serve as another essential quality parameter, as variations in ionic strength can dramatically affect the establishment and stability of pH gradients. Standard specifications typically limit conductivity to <50 μS/cm for carrier ampholytes intended for high-resolution applications. Regular batch testing using calibrated conductivity meters ensures compliance with these specifications.
pH range accuracy represents perhaps the most critical quality attribute for ampholytes. Industry standards now require manufacturers to certify the actual pH range covered by each ampholyte preparation, with acceptable deviation typically limited to ±0.2 pH units from the stated range. This certification involves rigorous testing using standardized IEF protocols and reference markers.
Batch-to-batch consistency has emerged as a paramount concern for research and industrial applications. Current standards mandate comprehensive documentation of manufacturing processes and quality control testing to ensure reproducibility between production lots. Leading manufacturers now provide certificates of analysis detailing lot-specific performance characteristics.
Shelf-life stability constitutes another dimension of quality control, with standards requiring ampholyte preparations to maintain their specified pH range and separation characteristics for a minimum of 12-18 months under recommended storage conditions. Accelerated aging tests and real-time stability studies support these shelf-life claims.
The implementation of these quality control standards has significantly advanced the reliability of IEF techniques across various applications. However, opportunities remain for further standardization, particularly in defining application-specific quality parameters for specialized fields such as proteomics and clinical diagnostics, where performance requirements may differ substantially from general research applications.
Purity specifications constitute the foundation of ampholyte quality control, with most premium commercial preparations requiring >99% chemical purity. Contaminants, particularly those with charged groups, can significantly distort pH gradients and introduce artifacts in separation patterns. Modern quality control protocols employ high-performance liquid chromatography (HPLC), mass spectrometry, and capillary electrophoresis to verify purity levels and identify potential impurities.
Conductivity measurements serve as another essential quality parameter, as variations in ionic strength can dramatically affect the establishment and stability of pH gradients. Standard specifications typically limit conductivity to <50 μS/cm for carrier ampholytes intended for high-resolution applications. Regular batch testing using calibrated conductivity meters ensures compliance with these specifications.
pH range accuracy represents perhaps the most critical quality attribute for ampholytes. Industry standards now require manufacturers to certify the actual pH range covered by each ampholyte preparation, with acceptable deviation typically limited to ±0.2 pH units from the stated range. This certification involves rigorous testing using standardized IEF protocols and reference markers.
Batch-to-batch consistency has emerged as a paramount concern for research and industrial applications. Current standards mandate comprehensive documentation of manufacturing processes and quality control testing to ensure reproducibility between production lots. Leading manufacturers now provide certificates of analysis detailing lot-specific performance characteristics.
Shelf-life stability constitutes another dimension of quality control, with standards requiring ampholyte preparations to maintain their specified pH range and separation characteristics for a minimum of 12-18 months under recommended storage conditions. Accelerated aging tests and real-time stability studies support these shelf-life claims.
The implementation of these quality control standards has significantly advanced the reliability of IEF techniques across various applications. However, opportunities remain for further standardization, particularly in defining application-specific quality parameters for specialized fields such as proteomics and clinical diagnostics, where performance requirements may differ substantially from general research applications.
Environmental Impact of Ampholyte Manufacturing Processes
The manufacturing processes of ampholytes for isoelectric focusing (IEF) present significant environmental considerations that warrant careful examination. Traditional ampholyte production typically involves complex chemical synthesis routes utilizing various solvents, catalysts, and reagents that may pose environmental hazards. These processes often generate substantial waste streams containing unreacted precursors, byproducts, and spent catalysts that require proper treatment and disposal.
Carrier ampholyte production frequently employs organic solvents such as dimethylformamide (DMF), acetonitrile, and tetrahydrofuran, which are associated with air quality concerns and potential groundwater contamination if improperly managed. The synthesis pathways also commonly utilize formaldehyde and other aldehydes, compounds known for their environmental persistence and toxicity to aquatic organisms.
Energy consumption represents another critical environmental aspect of ampholyte manufacturing. The multiple reaction steps, purification processes, and quality control procedures demand considerable energy inputs, contributing to the carbon footprint of these specialized chemicals. Particularly energy-intensive are the high-temperature reactions and vacuum distillation techniques often required to achieve the precise molecular characteristics necessary for effective IEF performance.
Water usage in ampholyte production presents additional environmental challenges. Purification steps typically consume significant volumes of water, while generating contaminated wastewater streams that require specialized treatment. The high purity requirements for IEF-grade ampholytes necessitate extensive washing and purification cycles, further increasing water consumption and treatment needs.
Recent industry trends show movement toward greener manufacturing approaches for ampholytes. Several leading manufacturers have begun implementing solvent recovery systems, catalytic process improvements, and waste minimization strategies. For instance, the adoption of continuous flow chemistry techniques has demonstrated potential for reducing solvent usage by up to 60% while simultaneously decreasing energy requirements through more efficient heat transfer.
Regulatory frameworks increasingly influence ampholyte manufacturing practices. The European REACH regulations and similar initiatives worldwide have prompted manufacturers to seek alternatives to substances of very high concern (SVHCs) in their production processes. This regulatory pressure has accelerated research into bio-based precursors and environmentally benign synthesis routes that maintain the required ampholyte performance characteristics while reducing environmental impact.
Life cycle assessment (LCA) studies of ampholyte production reveal that environmental impacts extend beyond manufacturing to include raw material extraction, transportation, and end-of-life considerations. These analyses suggest that improvements in ampholyte selection for IEF should incorporate environmental criteria alongside performance parameters to achieve truly sustainable laboratory practices.
Carrier ampholyte production frequently employs organic solvents such as dimethylformamide (DMF), acetonitrile, and tetrahydrofuran, which are associated with air quality concerns and potential groundwater contamination if improperly managed. The synthesis pathways also commonly utilize formaldehyde and other aldehydes, compounds known for their environmental persistence and toxicity to aquatic organisms.
Energy consumption represents another critical environmental aspect of ampholyte manufacturing. The multiple reaction steps, purification processes, and quality control procedures demand considerable energy inputs, contributing to the carbon footprint of these specialized chemicals. Particularly energy-intensive are the high-temperature reactions and vacuum distillation techniques often required to achieve the precise molecular characteristics necessary for effective IEF performance.
Water usage in ampholyte production presents additional environmental challenges. Purification steps typically consume significant volumes of water, while generating contaminated wastewater streams that require specialized treatment. The high purity requirements for IEF-grade ampholytes necessitate extensive washing and purification cycles, further increasing water consumption and treatment needs.
Recent industry trends show movement toward greener manufacturing approaches for ampholytes. Several leading manufacturers have begun implementing solvent recovery systems, catalytic process improvements, and waste minimization strategies. For instance, the adoption of continuous flow chemistry techniques has demonstrated potential for reducing solvent usage by up to 60% while simultaneously decreasing energy requirements through more efficient heat transfer.
Regulatory frameworks increasingly influence ampholyte manufacturing practices. The European REACH regulations and similar initiatives worldwide have prompted manufacturers to seek alternatives to substances of very high concern (SVHCs) in their production processes. This regulatory pressure has accelerated research into bio-based precursors and environmentally benign synthesis routes that maintain the required ampholyte performance characteristics while reducing environmental impact.
Life cycle assessment (LCA) studies of ampholyte production reveal that environmental impacts extend beyond manufacturing to include raw material extraction, transportation, and end-of-life considerations. These analyses suggest that improvements in ampholyte selection for IEF should incorporate environmental criteria alongside performance parameters to achieve truly sustainable laboratory practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!