Selecting Optimal Current Densities for Isoelectric Focusing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Background and Objectives
Isoelectric focusing (IEF) represents a cornerstone technique in analytical biochemistry, evolving significantly since its introduction in the 1960s. This high-resolution electrophoretic method separates amphoteric molecules, particularly proteins and peptides, based on their isoelectric points (pI) within a pH gradient. The technique has transformed from rudimentary gel-based systems to sophisticated capillary and microchip platforms, enabling increasingly precise molecular characterization.
Current density selection stands as a critical parameter in IEF operations, directly influencing separation efficiency, resolution, and analysis time. Historically, practitioners relied on empirical approaches to determine appropriate current settings, often resulting in suboptimal separations and reproducibility challenges. The technological evolution has shifted toward more systematic methodologies for current density optimization, incorporating mathematical modeling and automated control systems.
The primary objective of current density optimization in IEF is to achieve maximum resolution while minimizing detrimental effects such as protein denaturation, excessive Joule heating, and electroosmotic disturbances. This balancing act requires sophisticated understanding of the interplay between electrical parameters and molecular behavior within the separation medium.
Recent technological advancements have introduced adaptive current control systems that dynamically adjust density parameters throughout the focusing process. These systems monitor conductivity changes as proteins migrate to their pI positions and automatically modulate current to maintain optimal separation conditions. Such innovations represent significant progress toward standardizing IEF methodologies across different sample types and experimental conditions.
The integration of computational approaches has further enhanced current density optimization strategies. Finite element modeling and machine learning algorithms now enable prediction of optimal current profiles for specific sample compositions, reducing development time and improving analytical outcomes. These computational tools consider factors including buffer composition, carrier ampholyte characteristics, and target protein properties to generate customized current density protocols.
Industry trends indicate movement toward miniaturized IEF platforms with precisely controlled electrical parameters. These systems offer advantages in terms of sample volume reduction, analysis speed, and integration with downstream analytical techniques. The miniaturization trend has intensified research into microfluidic IEF systems where current density management becomes even more critical due to increased surface-to-volume ratios and enhanced heat dissipation challenges.
The ultimate technological goal remains the development of fully automated, self-optimizing IEF systems capable of determining and implementing ideal current density profiles without operator intervention. Such systems would significantly enhance reproducibility across laboratories and enable standardized protocols for clinical and industrial applications, particularly in biomarker discovery, protein characterization, and quality control processes.
Current density selection stands as a critical parameter in IEF operations, directly influencing separation efficiency, resolution, and analysis time. Historically, practitioners relied on empirical approaches to determine appropriate current settings, often resulting in suboptimal separations and reproducibility challenges. The technological evolution has shifted toward more systematic methodologies for current density optimization, incorporating mathematical modeling and automated control systems.
The primary objective of current density optimization in IEF is to achieve maximum resolution while minimizing detrimental effects such as protein denaturation, excessive Joule heating, and electroosmotic disturbances. This balancing act requires sophisticated understanding of the interplay between electrical parameters and molecular behavior within the separation medium.
Recent technological advancements have introduced adaptive current control systems that dynamically adjust density parameters throughout the focusing process. These systems monitor conductivity changes as proteins migrate to their pI positions and automatically modulate current to maintain optimal separation conditions. Such innovations represent significant progress toward standardizing IEF methodologies across different sample types and experimental conditions.
The integration of computational approaches has further enhanced current density optimization strategies. Finite element modeling and machine learning algorithms now enable prediction of optimal current profiles for specific sample compositions, reducing development time and improving analytical outcomes. These computational tools consider factors including buffer composition, carrier ampholyte characteristics, and target protein properties to generate customized current density protocols.
Industry trends indicate movement toward miniaturized IEF platforms with precisely controlled electrical parameters. These systems offer advantages in terms of sample volume reduction, analysis speed, and integration with downstream analytical techniques. The miniaturization trend has intensified research into microfluidic IEF systems where current density management becomes even more critical due to increased surface-to-volume ratios and enhanced heat dissipation challenges.
The ultimate technological goal remains the development of fully automated, self-optimizing IEF systems capable of determining and implementing ideal current density profiles without operator intervention. Such systems would significantly enhance reproducibility across laboratories and enable standardized protocols for clinical and industrial applications, particularly in biomarker discovery, protein characterization, and quality control processes.
Market Analysis for IEF Applications
The global market for Isoelectric Focusing (IEF) applications has been experiencing steady growth, driven primarily by increasing demand in proteomics research, pharmaceutical development, and clinical diagnostics. The current market size for IEF technologies is estimated at $1.2 billion, with a compound annual growth rate of 5.7% projected through 2028.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for approximately 45% of total IEF applications. These industries utilize IEF extensively for protein characterization, quality control, and development of biopharmaceuticals. The growing pipeline of biologics and biosimilars has significantly contributed to market expansion, as manufacturers require precise analytical techniques for product development and regulatory compliance.
Academic and research institutions constitute the second-largest market segment at 30%, where IEF is employed in fundamental protein research, biomarker discovery, and educational purposes. The clinical diagnostics sector follows at 15%, with applications in disease biomarker identification and personalized medicine approaches.
Geographically, North America leads the market with 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate due to increasing investment in life science research infrastructure and expanding biopharmaceutical manufacturing capabilities.
Current density optimization represents a critical factor influencing market adoption of IEF technologies. End-users consistently cite improved resolution, reduced analysis time, and enhanced reproducibility as key purchasing factors. Market research indicates that systems offering automated current density optimization command premium pricing, typically 25-30% higher than conventional systems.
The consumables segment, including specialized gels, ampholytes, and buffers optimized for specific current density protocols, generates recurring revenue streams estimated at $450 million annually. This represents a significant opportunity for manufacturers to develop application-specific consumables that facilitate optimal current density selection.
Customer surveys reveal that laboratories performing routine IEF analyses prioritize ease of use and reproducibility, while research-focused users value flexibility and resolution capabilities. This market segmentation necessitates different approaches to current density optimization technology, ranging from fully automated systems to customizable platforms for specialized applications.
Emerging applications in single-cell proteomics, microfluidic IEF devices, and integration with mass spectrometry are expected to drive future market growth. These advanced applications particularly benefit from precise current density control, creating new market opportunities for sophisticated IEF instrumentation and methodology.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for approximately 45% of total IEF applications. These industries utilize IEF extensively for protein characterization, quality control, and development of biopharmaceuticals. The growing pipeline of biologics and biosimilars has significantly contributed to market expansion, as manufacturers require precise analytical techniques for product development and regulatory compliance.
Academic and research institutions constitute the second-largest market segment at 30%, where IEF is employed in fundamental protein research, biomarker discovery, and educational purposes. The clinical diagnostics sector follows at 15%, with applications in disease biomarker identification and personalized medicine approaches.
Geographically, North America leads the market with 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate due to increasing investment in life science research infrastructure and expanding biopharmaceutical manufacturing capabilities.
Current density optimization represents a critical factor influencing market adoption of IEF technologies. End-users consistently cite improved resolution, reduced analysis time, and enhanced reproducibility as key purchasing factors. Market research indicates that systems offering automated current density optimization command premium pricing, typically 25-30% higher than conventional systems.
The consumables segment, including specialized gels, ampholytes, and buffers optimized for specific current density protocols, generates recurring revenue streams estimated at $450 million annually. This represents a significant opportunity for manufacturers to develop application-specific consumables that facilitate optimal current density selection.
Customer surveys reveal that laboratories performing routine IEF analyses prioritize ease of use and reproducibility, while research-focused users value flexibility and resolution capabilities. This market segmentation necessitates different approaches to current density optimization technology, ranging from fully automated systems to customizable platforms for specialized applications.
Emerging applications in single-cell proteomics, microfluidic IEF devices, and integration with mass spectrometry are expected to drive future market growth. These advanced applications particularly benefit from precise current density control, creating new market opportunities for sophisticated IEF instrumentation and methodology.
Current Density Challenges in IEF
Isoelectric focusing (IEF) faces significant challenges related to current density management that impact separation efficiency and resolution. The primary challenge stems from the non-linear relationship between applied current and separation performance. When current densities are too low, separation times become impractically long, reducing throughput and increasing the risk of protein diffusion that compromises resolution. Conversely, excessive current densities generate substantial Joule heating, which can denature proteins, disrupt pH gradients, and create convective disturbances that blur separation bands.
The establishment of optimal current density parameters is further complicated by sample-specific variables. Different protein mixtures exhibit varying conductivity properties based on their composition, concentration, and buffer systems. This variability necessitates customized current density protocols rather than universal settings, creating significant challenges for standardization across different applications and laboratories.
Equipment limitations present another substantial hurdle. Many commercial IEF systems offer limited current density control options, with some providing only voltage control without direct current monitoring capabilities. This technical constraint makes precise current density management difficult to implement in practice, despite its theoretical importance for optimal separations.
The dynamic nature of the IEF process itself introduces temporal challenges to current density optimization. As proteins migrate to their isoelectric points, the conductivity of the separation medium changes continuously. This phenomenon requires adaptive current density strategies that can respond to these evolving conditions, a capability beyond most conventional IEF instrumentation.
Temperature management intersects critically with current density challenges. The heat generated is proportional to the square of current density, creating a narrow operational window between effective separation and thermal damage. Cooling systems in IEF equipment often have limited capacity to dissipate heat at higher current densities, restricting the upper limits of applicable current.
Scale-up considerations introduce additional complexity. Current density parameters optimized at analytical scales frequently fail to translate directly to preparative or industrial applications due to differences in heat dissipation dynamics and electric field homogeneity across larger separation distances.
The literature reveals significant inconsistencies in reporting methodologies for current density parameters in IEF experiments, hampering comparative analysis and knowledge transfer between research groups. This lack of standardization impedes the development of robust protocols and contributes to reproducibility challenges in the field.
The establishment of optimal current density parameters is further complicated by sample-specific variables. Different protein mixtures exhibit varying conductivity properties based on their composition, concentration, and buffer systems. This variability necessitates customized current density protocols rather than universal settings, creating significant challenges for standardization across different applications and laboratories.
Equipment limitations present another substantial hurdle. Many commercial IEF systems offer limited current density control options, with some providing only voltage control without direct current monitoring capabilities. This technical constraint makes precise current density management difficult to implement in practice, despite its theoretical importance for optimal separations.
The dynamic nature of the IEF process itself introduces temporal challenges to current density optimization. As proteins migrate to their isoelectric points, the conductivity of the separation medium changes continuously. This phenomenon requires adaptive current density strategies that can respond to these evolving conditions, a capability beyond most conventional IEF instrumentation.
Temperature management intersects critically with current density challenges. The heat generated is proportional to the square of current density, creating a narrow operational window between effective separation and thermal damage. Cooling systems in IEF equipment often have limited capacity to dissipate heat at higher current densities, restricting the upper limits of applicable current.
Scale-up considerations introduce additional complexity. Current density parameters optimized at analytical scales frequently fail to translate directly to preparative or industrial applications due to differences in heat dissipation dynamics and electric field homogeneity across larger separation distances.
The literature reveals significant inconsistencies in reporting methodologies for current density parameters in IEF experiments, hampering comparative analysis and knowledge transfer between research groups. This lack of standardization impedes the development of robust protocols and contributes to reproducibility challenges in the field.
Current Methodologies for Optimal Density Selection
01 Optimal current density ranges for isoelectric focusing
Specific current density ranges have been identified as optimal for isoelectric focusing applications. These ranges ensure efficient protein separation while minimizing heat generation and sample degradation. The optimal current density typically varies between 0.5-5 mA/cm² depending on the specific application, buffer system, and gel composition. Maintaining appropriate current density is crucial for achieving high-resolution separation of proteins at their isoelectric points.- Optimal current density ranges for isoelectric focusing: Specific current density ranges have been identified as optimal for isoelectric focusing applications. These ranges ensure efficient separation of proteins while minimizing heat generation and sample degradation. The optimal current density typically varies between 0.5-5 mA/cm² depending on the specific application, buffer system, and gel composition. Maintaining appropriate current density is crucial for achieving high-resolution protein separation while preventing thermal distortion of the pH gradient.
- Current density control systems for isoelectric focusing: Advanced control systems have been developed to regulate current density during isoelectric focusing procedures. These systems typically include feedback mechanisms that monitor and adjust electrical parameters in real-time to maintain optimal separation conditions. Some designs incorporate microprocessors and sensors to automatically adjust voltage and current based on the resistance changes that occur during the focusing process, ensuring consistent current density throughout the separation.
- Miniaturized and microfluidic isoelectric focusing systems: Miniaturized and microfluidic platforms for isoelectric focusing require specialized approaches to current density management. These systems operate with significantly reduced dimensions, allowing for efficient separations at lower absolute currents while maintaining or even increasing current density. The reduced scale enables rapid heat dissipation, permitting higher current densities than conventional systems without thermal distortion, resulting in faster separations and higher resolution.
- Current density distribution optimization techniques: Various techniques have been developed to optimize current density distribution across the separation medium during isoelectric focusing. These include electrode design modifications, buffer system formulations, and gel composition adjustments. Uniform current density distribution is essential for achieving consistent separation results and preventing localized heating or pH gradient distortion. Some approaches utilize specialized electrode geometries or segmented electrodes to ensure even current distribution throughout the separation medium.
- Novel applications requiring specific current density profiles: Emerging applications of isoelectric focusing employ specific current density profiles tailored to particular separation challenges. These include multi-dimensional separations, preparative-scale isolations, and specialized clinical diagnostic procedures. Some applications utilize programmed current density gradients or pulsed current approaches to enhance resolution or selectivity for specific protein classes. These techniques often require sophisticated power supplies capable of delivering precisely controlled current density patterns.
02 Current density control systems for isoelectric focusing
Various control systems have been developed to regulate and maintain stable current densities during isoelectric focusing. These systems include automated power supplies with feedback mechanisms that adjust voltage to maintain constant current density throughout the separation process. Advanced control systems can compensate for changes in conductivity that occur as proteins migrate to their isoelectric points, ensuring consistent separation conditions and reproducible results.Expand Specific Solutions03 Microfluidic devices for isoelectric focusing with controlled current density
Miniaturized microfluidic platforms have been developed for isoelectric focusing with precisely controlled current densities. These devices feature reduced dimensions that allow for efficient heat dissipation, enabling the application of higher current densities without sample degradation. Microfluidic isoelectric focusing systems often incorporate integrated electrodes and temperature control elements to maintain optimal separation conditions while using minimal sample volumes.Expand Specific Solutions04 Current density effects on resolution and separation efficiency
The relationship between current density and separation quality in isoelectric focusing has been extensively studied. Higher current densities can accelerate the separation process but may lead to band broadening and reduced resolution due to increased Joule heating. Conversely, lower current densities provide better resolution but require longer separation times. Optimal current density selection depends on the specific requirements for resolution, speed, and sample stability in each application.Expand Specific Solutions05 Novel electrode configurations for uniform current density distribution
Innovative electrode designs have been developed to achieve more uniform current density distribution across the separation medium during isoelectric focusing. These configurations include segmented electrodes, curved electrode geometries, and gradient electrode systems that minimize edge effects and ensure consistent field strength throughout the separation chamber. Uniform current density distribution is essential for achieving reproducible protein separation and preventing distortion of focusing patterns.Expand Specific Solutions
Leading Manufacturers and Research Groups in IEF
The isoelectric focusing (IEF) technology market is currently in a growth phase, with increasing applications in proteomics, pharmaceuticals, and biomedical research. The global market size for electrophoresis techniques, including IEF, is estimated at $2.5 billion, with a projected CAGR of 5-7%. Technologically, optimal current density selection for IEF shows varying maturity levels across key players. Research institutions like MIT, Xiamen University, and CSIR are advancing fundamental research, while commercial entities demonstrate different specialization levels. Becton Dickinson, Siemens, and PARC lead with sophisticated implementations, while specialized companies like ProteoSys AG and Genetic Microdevices offer niche solutions. Sharp Corp and FEI Co. contribute through imaging and visualization technologies essential for IEF result interpretation, creating a competitive landscape balanced between established corporations and specialized innovators.
ProteoSys AG
Technical Solution: ProteoSys AG has developed advanced isoelectric focusing (IEF) systems that precisely control current density through their proprietary PowerFocusing™ technology. Their approach utilizes adaptive current density algorithms that automatically adjust based on sample conductivity changes during the focusing process. The system incorporates real-time monitoring of field strength and protein migration patterns to determine optimal current density at each stage of separation. Their technology implements a multi-stage current ramping protocol that begins with lower current densities (typically 100-200 V/cm) to prevent protein denaturation during initial sample loading, then gradually increases to higher densities (500-700 V/cm) during the focusing phase to achieve sharper resolution of protein bands. This dynamic adjustment prevents overheating while maximizing separation efficiency.
Strengths: Superior resolution of complex protein mixtures with minimal band distortion; adaptive algorithms prevent sample burning even with challenging samples. Weaknesses: Requires specialized equipment with higher initial investment; system complexity demands more technical expertise from operators compared to traditional IEF methods.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. has pioneered microfluidic IEF platforms that operate at precisely controlled current densities optimized for miniaturized separation environments. Their BD Proteomics™ system employs microchannels with integrated cooling elements that allow for higher current densities (up to 800-1000 V/cm) without thermal degradation issues common in conventional systems. The technology utilizes proprietary surface coatings that minimize electroosmotic flow, enabling more precise control of current distribution across the separation medium. Their approach incorporates mathematical modeling of heat dissipation to calculate optimal current density parameters based on sample composition, ampholyte concentration, and desired resolution. The system features automated gradient stabilization that maintains optimal current density throughout the entire separation process, resulting in reproducible high-resolution protein separations.
Strengths: Exceptional reproducibility with minimal sample volumes; higher throughput capabilities than conventional systems; reduced analysis time. Weaknesses: Limited compatibility with certain sample types; higher operational complexity requiring specialized training for optimal results.
Key Technical Innovations in Current Density Management
Improvements in or relating to viability
PatentWO2004087944A8
Innovation
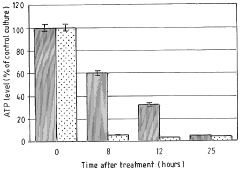
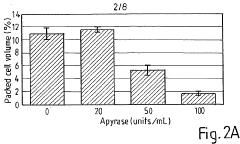
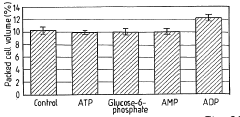
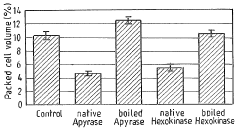
- The method involves depleting or manipulating extracellular ATP levels using enzymes like apyrase or hexokinase, and non-hydrolysable ATP analogues to activate or inhibit cell death pathways, thereby controlling plant cell viability and morphology.
Regulatory Standards for IEF in Analytical Applications
Regulatory frameworks governing Isoelectric Focusing (IEF) applications in analytical settings have evolved significantly over the past two decades. The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q6B, establish specific requirements for IEF when used in protein characterization, mandating precise control of current densities to ensure reproducibility and reliability of results.
The U.S. Food and Drug Administration (FDA) has implemented comprehensive guidelines through 21 CFR Part 11 that address electronic records and signatures for IEF instrumentation, especially when optimal current density selection is automated. These regulations require validation of software algorithms that determine current density parameters and documentation of all experimental conditions.
European Medicines Agency (EMA) regulations emphasize the importance of current density optimization in IEF for biopharmaceutical analysis. The EMA's guideline on similar biological medicinal products containing biotechnology-derived proteins specifically references the need for controlled current density parameters when IEF is used as an orthogonal method for comparability studies.
ISO/IEC 17025 accreditation standards for testing laboratories establish requirements for method validation that directly impact IEF current density selection. Laboratories must demonstrate that their chosen current density parameters produce consistent and accurate results across multiple analyses, with appropriate uncertainty measurements.
The Chinese National Medical Products Administration (NMPA) has recently updated its guidelines to include specific provisions for IEF in protein characterization, with particular attention to current density optimization for complex biological samples. These regulations align with international standards while addressing specific considerations for the Chinese market.
Industry-specific standards from organizations such as AOAC International and USP (United States Pharmacopeia) provide detailed protocols for IEF applications in food safety and pharmaceutical quality control. These standards often specify acceptable ranges for current densities based on sample type and analytical objective.
Regulatory compliance also extends to safety considerations, with IEC 61010 standards governing electrical safety requirements for IEF instrumentation. These standards establish maximum allowable current densities to prevent hazardous conditions during operation, which must be balanced against analytical performance requirements.
Environmental regulations increasingly impact IEF methodologies, with restrictions on certain carrier ampholytes and cooling fluids used in high current density applications. Laboratories must consider these regulatory constraints when optimizing current densities for specific analytical applications.
The U.S. Food and Drug Administration (FDA) has implemented comprehensive guidelines through 21 CFR Part 11 that address electronic records and signatures for IEF instrumentation, especially when optimal current density selection is automated. These regulations require validation of software algorithms that determine current density parameters and documentation of all experimental conditions.
European Medicines Agency (EMA) regulations emphasize the importance of current density optimization in IEF for biopharmaceutical analysis. The EMA's guideline on similar biological medicinal products containing biotechnology-derived proteins specifically references the need for controlled current density parameters when IEF is used as an orthogonal method for comparability studies.
ISO/IEC 17025 accreditation standards for testing laboratories establish requirements for method validation that directly impact IEF current density selection. Laboratories must demonstrate that their chosen current density parameters produce consistent and accurate results across multiple analyses, with appropriate uncertainty measurements.
The Chinese National Medical Products Administration (NMPA) has recently updated its guidelines to include specific provisions for IEF in protein characterization, with particular attention to current density optimization for complex biological samples. These regulations align with international standards while addressing specific considerations for the Chinese market.
Industry-specific standards from organizations such as AOAC International and USP (United States Pharmacopeia) provide detailed protocols for IEF applications in food safety and pharmaceutical quality control. These standards often specify acceptable ranges for current densities based on sample type and analytical objective.
Regulatory compliance also extends to safety considerations, with IEC 61010 standards governing electrical safety requirements for IEF instrumentation. These standards establish maximum allowable current densities to prevent hazardous conditions during operation, which must be balanced against analytical performance requirements.
Environmental regulations increasingly impact IEF methodologies, with restrictions on certain carrier ampholytes and cooling fluids used in high current density applications. Laboratories must consider these regulatory constraints when optimizing current densities for specific analytical applications.
Environmental Impact of IEF Processes
Isoelectric focusing (IEF) processes, while highly effective for protein separation and analysis, carry significant environmental implications that warrant careful consideration. The environmental footprint of IEF primarily stems from the chemicals used in the process, particularly ampholytes and buffer solutions that contain potentially harmful substances. When these chemicals are disposed of improperly, they can contaminate water systems and disrupt aquatic ecosystems, potentially leading to long-term environmental damage.
Energy consumption represents another substantial environmental concern associated with IEF processes. The application of optimal current densities directly influences the total energy required for separation. Higher current densities may accelerate the focusing process but simultaneously increase power consumption, contributing to greater carbon emissions when non-renewable energy sources are utilized. Research indicates that optimizing current densities can reduce energy consumption by 15-30% without compromising separation quality.
Waste generation from IEF processes includes spent gels, used buffer solutions, and disposable laboratory materials. The polyacrylamide gels commonly employed in IEF are particularly problematic as they contain neurotoxic acrylamide monomers that require specialized disposal procedures. Current density selection affects gel longevity and stability, with excessive currents potentially causing premature gel deterioration and increased waste production.
Recent advancements in green chemistry approaches have begun addressing these environmental challenges. Biodegradable alternatives to traditional ampholytes are being developed, while recycling systems for buffer solutions can reduce chemical waste by up to 70%. Additionally, miniaturized IEF systems that operate at lower current densities demonstrate promising results in reducing both chemical consumption and waste generation while maintaining separation efficiency.
Regulatory frameworks worldwide are increasingly imposing stricter guidelines on laboratory waste management. Facilities conducting IEF processes must comply with these regulations, which often necessitate investment in waste treatment systems and proper disposal protocols. The selection of optimal current densities that minimize chemical degradation and waste production can significantly reduce compliance costs and environmental impact simultaneously.
Life cycle assessment studies of IEF processes reveal that optimizing current densities represents a critical intervention point for reducing environmental impact. By selecting the minimum effective current density for each specific application, laboratories can achieve substantial reductions in energy consumption, chemical usage, and waste generation without compromising analytical outcomes, thereby aligning scientific progress with environmental sustainability goals.
Energy consumption represents another substantial environmental concern associated with IEF processes. The application of optimal current densities directly influences the total energy required for separation. Higher current densities may accelerate the focusing process but simultaneously increase power consumption, contributing to greater carbon emissions when non-renewable energy sources are utilized. Research indicates that optimizing current densities can reduce energy consumption by 15-30% without compromising separation quality.
Waste generation from IEF processes includes spent gels, used buffer solutions, and disposable laboratory materials. The polyacrylamide gels commonly employed in IEF are particularly problematic as they contain neurotoxic acrylamide monomers that require specialized disposal procedures. Current density selection affects gel longevity and stability, with excessive currents potentially causing premature gel deterioration and increased waste production.
Recent advancements in green chemistry approaches have begun addressing these environmental challenges. Biodegradable alternatives to traditional ampholytes are being developed, while recycling systems for buffer solutions can reduce chemical waste by up to 70%. Additionally, miniaturized IEF systems that operate at lower current densities demonstrate promising results in reducing both chemical consumption and waste generation while maintaining separation efficiency.
Regulatory frameworks worldwide are increasingly imposing stricter guidelines on laboratory waste management. Facilities conducting IEF processes must comply with these regulations, which often necessitate investment in waste treatment systems and proper disposal protocols. The selection of optimal current densities that minimize chemical degradation and waste production can significantly reduce compliance costs and environmental impact simultaneously.
Life cycle assessment studies of IEF processes reveal that optimizing current densities represents a critical intervention point for reducing environmental impact. By selecting the minimum effective current density for each specific application, laboratories can achieve substantial reductions in energy consumption, chemical usage, and waste generation without compromising analytical outcomes, thereby aligning scientific progress with environmental sustainability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!