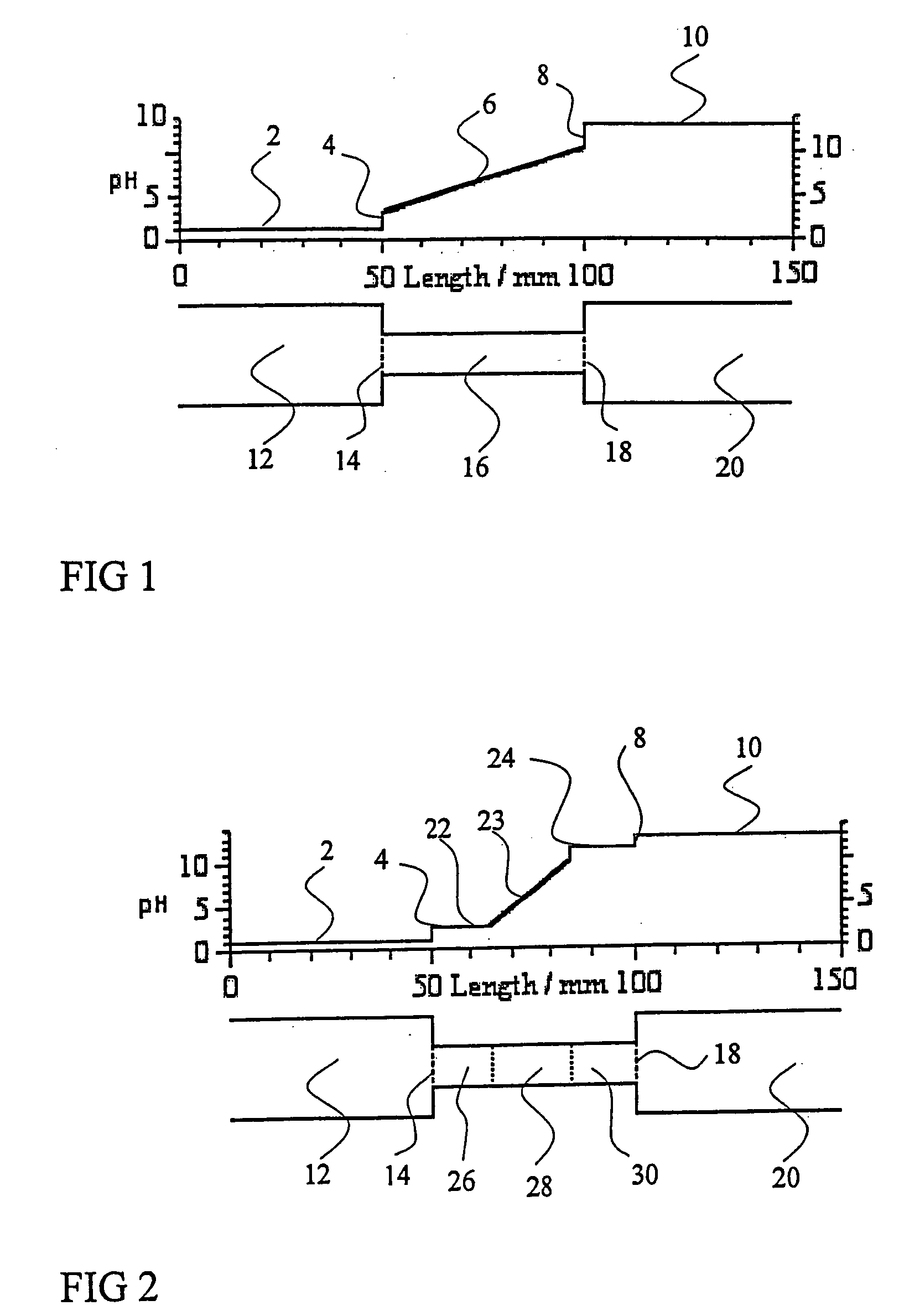
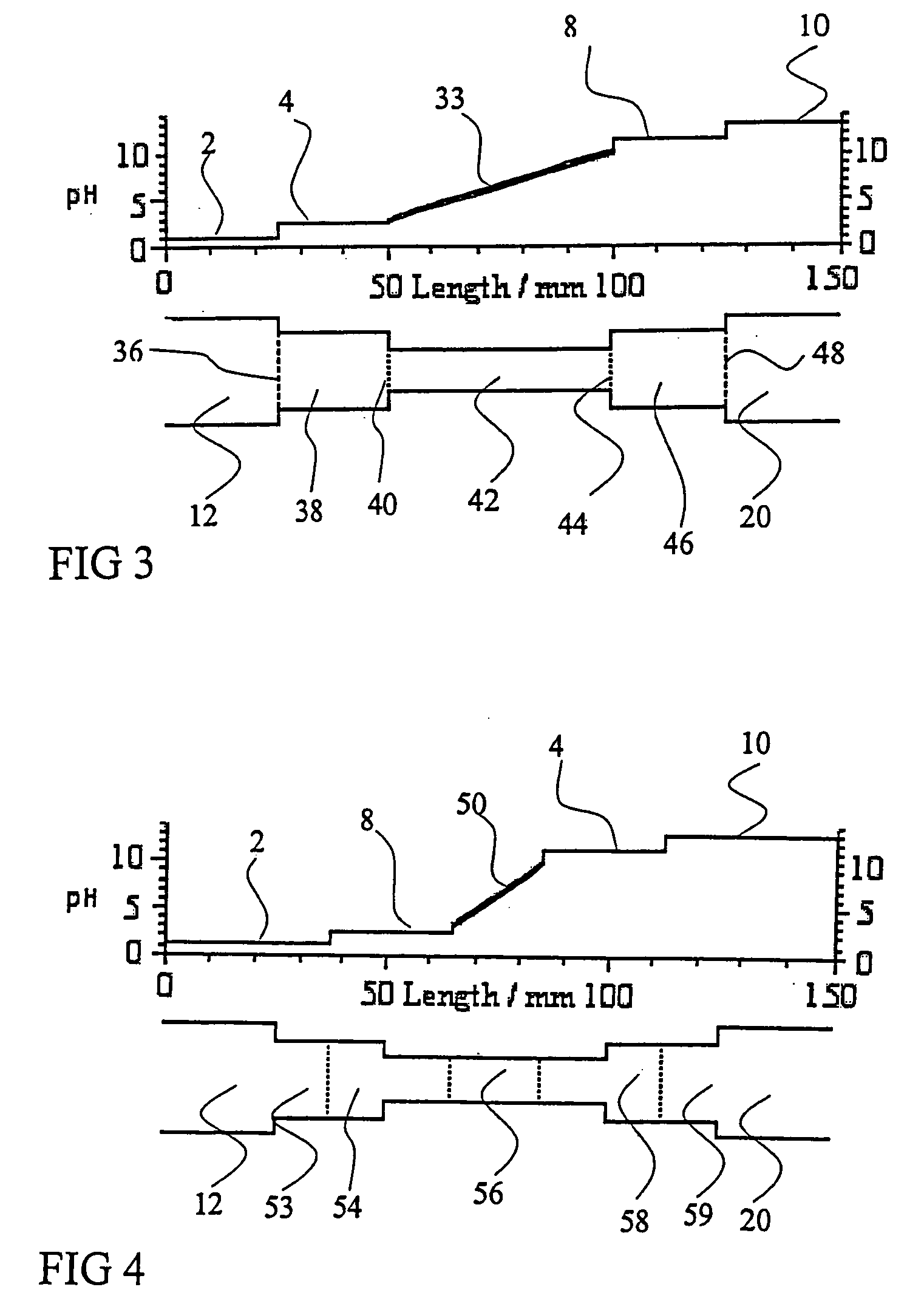
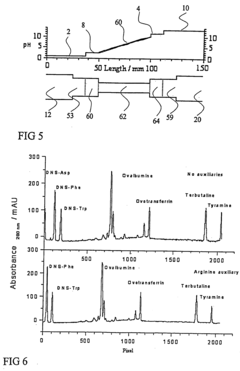
Redesign Sample Buffers to Enhance Isoelectric Focusing Consistency
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Background and Objectives
Isoelectric focusing (IEF) has evolved significantly since its introduction in the 1960s as a high-resolution technique for protein separation based on their isoelectric points (pI). This analytical method has become a cornerstone in proteomics research, diagnostics, and biopharmaceutical development due to its unparalleled ability to resolve proteins with minimal pI differences, sometimes as small as 0.01 pH units.
The historical trajectory of IEF technology shows remarkable advancements from carrier ampholyte-based systems to immobilized pH gradient (IPG) gels, which dramatically improved reproducibility. However, despite these improvements, inconsistency in separation remains a persistent challenge, particularly when dealing with complex biological samples or when high-throughput analysis is required.
Sample buffer composition represents a critical yet often overlooked factor affecting IEF performance. Traditional buffer systems were designed primarily for protein solubilization without comprehensive consideration of their impact on the electric field distribution and protein migration dynamics during focusing. This has led to variable results across laboratories and even within the same facility.
Recent technological trends indicate a shift toward more sophisticated buffer designs that incorporate chaotropic agents, detergents, and ampholytes in precisely optimized ratios. Additionally, there is growing interest in developing specialized buffer systems for specific sample types, such as membrane proteins, glycoproteins, and samples with extreme pH values or high salt concentrations.
The primary objective of this technical research is to systematically redesign sample buffers to enhance IEF consistency across diverse experimental conditions. Specifically, we aim to develop next-generation buffer formulations that minimize protein aggregation, prevent protein-matrix interactions, and ensure uniform conductivity throughout the pH gradient during the focusing process.
Secondary objectives include reducing the time required for complete focusing, minimizing protein modifications during the separation process, and enhancing compatibility with downstream analytical techniques such as mass spectrometry. We also seek to establish standardized protocols that can be readily implemented across different laboratory settings with minimal variation.
The technological significance of this research extends beyond immediate improvements in analytical precision. Enhanced IEF consistency would enable more reliable biomarker discovery, improved quality control in biopharmaceutical manufacturing, and more accurate clinical diagnostics. Furthermore, it would facilitate the detection of low-abundance proteins that are currently masked by technical variability, potentially revealing new therapeutic targets and disease mechanisms.
As we progress toward increasingly personalized medicine and precision diagnostics, the need for highly reproducible protein separation techniques becomes paramount. This research aims to address this need by fundamentally rethinking the chemistry of sample preparation for IEF applications.
The historical trajectory of IEF technology shows remarkable advancements from carrier ampholyte-based systems to immobilized pH gradient (IPG) gels, which dramatically improved reproducibility. However, despite these improvements, inconsistency in separation remains a persistent challenge, particularly when dealing with complex biological samples or when high-throughput analysis is required.
Sample buffer composition represents a critical yet often overlooked factor affecting IEF performance. Traditional buffer systems were designed primarily for protein solubilization without comprehensive consideration of their impact on the electric field distribution and protein migration dynamics during focusing. This has led to variable results across laboratories and even within the same facility.
Recent technological trends indicate a shift toward more sophisticated buffer designs that incorporate chaotropic agents, detergents, and ampholytes in precisely optimized ratios. Additionally, there is growing interest in developing specialized buffer systems for specific sample types, such as membrane proteins, glycoproteins, and samples with extreme pH values or high salt concentrations.
The primary objective of this technical research is to systematically redesign sample buffers to enhance IEF consistency across diverse experimental conditions. Specifically, we aim to develop next-generation buffer formulations that minimize protein aggregation, prevent protein-matrix interactions, and ensure uniform conductivity throughout the pH gradient during the focusing process.
Secondary objectives include reducing the time required for complete focusing, minimizing protein modifications during the separation process, and enhancing compatibility with downstream analytical techniques such as mass spectrometry. We also seek to establish standardized protocols that can be readily implemented across different laboratory settings with minimal variation.
The technological significance of this research extends beyond immediate improvements in analytical precision. Enhanced IEF consistency would enable more reliable biomarker discovery, improved quality control in biopharmaceutical manufacturing, and more accurate clinical diagnostics. Furthermore, it would facilitate the detection of low-abundance proteins that are currently masked by technical variability, potentially revealing new therapeutic targets and disease mechanisms.
As we progress toward increasingly personalized medicine and precision diagnostics, the need for highly reproducible protein separation techniques becomes paramount. This research aims to address this need by fundamentally rethinking the chemistry of sample preparation for IEF applications.
Market Analysis for Improved Electrophoresis Solutions
The global electrophoresis market continues to expand, driven by increasing applications in proteomics, genomics, and clinical diagnostics. Currently valued at approximately $2.5 billion, the market is projected to grow at a CAGR of 5.8% through 2028, with the isoelectric focusing (IEF) segment showing particularly strong potential due to its high-resolution protein separation capabilities.
Sample buffer solutions represent a critical yet often overlooked component of the electrophoresis workflow, directly impacting experimental reproducibility and result quality. The market for improved buffer formulations specifically designed to enhance IEF consistency is experiencing accelerated growth as researchers face mounting pressure to produce reliable, reproducible results for publication and clinical applications.
Key market drivers include the rising adoption of proteomics in pharmaceutical development, increasing focus on personalized medicine requiring precise protein characterization, and growing demand for quality control in biopharmaceutical manufacturing. The academic research segment currently accounts for approximately 40% of the market share, while pharmaceutical and biotechnology companies represent 35%, with diagnostic laboratories comprising the remaining 25%.
Regional analysis reveals North America dominates the market with 38% share, followed by Europe (30%) and Asia-Pacific (22%). The Asia-Pacific region demonstrates the fastest growth rate at 7.2% annually, primarily due to expanding research infrastructure in China, India, and South Korea.
Customer pain points consistently identified through market research include poor reproducibility between experiments, protein precipitation during focusing, inadequate resolution of closely related protein isoforms, and extended run times required for optimal separation. Survey data indicates that 78% of researchers report inconsistent results as their primary challenge with current IEF methodologies.
Price sensitivity analysis shows academic customers are highly cost-conscious, while pharmaceutical companies prioritize performance and reproducibility over price. The average spending on electrophoresis reagents and buffers ranges from $5,000 to $15,000 annually per research group, with specialized buffers commanding premium pricing when demonstrable performance improvements can be verified.
Competitive intelligence reveals a fragmented market with four major players controlling approximately 65% of the global market share. However, specialized buffer solutions represent an underserved niche with significant growth potential. Recent product launches have focused primarily on equipment improvements rather than reagent optimization, creating a market opportunity for innovative buffer formulations that enhance IEF consistency.
Sample buffer solutions represent a critical yet often overlooked component of the electrophoresis workflow, directly impacting experimental reproducibility and result quality. The market for improved buffer formulations specifically designed to enhance IEF consistency is experiencing accelerated growth as researchers face mounting pressure to produce reliable, reproducible results for publication and clinical applications.
Key market drivers include the rising adoption of proteomics in pharmaceutical development, increasing focus on personalized medicine requiring precise protein characterization, and growing demand for quality control in biopharmaceutical manufacturing. The academic research segment currently accounts for approximately 40% of the market share, while pharmaceutical and biotechnology companies represent 35%, with diagnostic laboratories comprising the remaining 25%.
Regional analysis reveals North America dominates the market with 38% share, followed by Europe (30%) and Asia-Pacific (22%). The Asia-Pacific region demonstrates the fastest growth rate at 7.2% annually, primarily due to expanding research infrastructure in China, India, and South Korea.
Customer pain points consistently identified through market research include poor reproducibility between experiments, protein precipitation during focusing, inadequate resolution of closely related protein isoforms, and extended run times required for optimal separation. Survey data indicates that 78% of researchers report inconsistent results as their primary challenge with current IEF methodologies.
Price sensitivity analysis shows academic customers are highly cost-conscious, while pharmaceutical companies prioritize performance and reproducibility over price. The average spending on electrophoresis reagents and buffers ranges from $5,000 to $15,000 annually per research group, with specialized buffers commanding premium pricing when demonstrable performance improvements can be verified.
Competitive intelligence reveals a fragmented market with four major players controlling approximately 65% of the global market share. However, specialized buffer solutions represent an underserved niche with significant growth potential. Recent product launches have focused primarily on equipment improvements rather than reagent optimization, creating a market opportunity for innovative buffer formulations that enhance IEF consistency.
Current Challenges in Sample Buffer Design
The current landscape of sample buffer design for isoelectric focusing (IEF) reveals several persistent challenges that impede consistent and reproducible results. Traditional buffer formulations often exhibit instability under varying temperature conditions, leading to pH drift during the focusing process. This temperature sensitivity creates significant reproducibility issues, particularly in multi-day experiments or when comparing results across different laboratory environments.
Protein solubility remains a critical challenge, as conventional buffers struggle to maintain sample integrity across the wide pH range required for comprehensive IEF analysis. High-abundance proteins frequently precipitate at their isoelectric points, causing streaking and poor resolution in the final separation. This is especially problematic when analyzing complex biological samples containing proteins with diverse physicochemical properties.
Carrier ampholyte interference presents another significant obstacle. Current buffer systems often contain carrier ampholytes that can interact unpredictably with sample proteins, creating artifacts and background noise that mask true protein signals. These interactions are particularly troublesome when working with low-abundance proteins or when attempting to detect post-translational modifications that alter protein charge.
Conductivity inconsistencies across the pH gradient represent a persistent technical hurdle. Non-uniform conductivity leads to uneven heating during the focusing process, resulting in distorted bands and compromised resolution. This effect is magnified in wide-range pH gradients, where maintaining consistent conductivity throughout the separation medium becomes increasingly difficult.
Sample-induced pH perturbations constitute another major challenge. High protein concentrations or samples with extreme salt content can locally disrupt the pH gradient, leading to focusing inconsistencies and poor reproducibility. Current buffer designs lack sufficient buffering capacity to neutralize these sample-induced disturbances without introducing additional interfering components.
Oxidation and reduction potential management remains inadequately addressed in existing buffer formulations. Protein oxidation during sample preparation and IEF separation can alter charge profiles and create artificial heterogeneity. While reducing agents are commonly incorporated into buffers, they often exhibit limited stability or interfere with the separation process themselves.
Detergent compatibility issues further complicate buffer design. Many membrane proteins and hydrophobic proteins require detergents for solubilization, yet these detergents frequently interfere with IEF by altering protein charge or disrupting the pH gradient. Current buffer systems struggle to accommodate effective detergent concentrations while maintaining separation integrity.
The lack of standardization across laboratory protocols exacerbates these technical challenges. Variations in buffer composition, sample preparation methods, and focusing conditions contribute to poor inter-laboratory reproducibility, hindering broader adoption of IEF techniques in clinical and industrial applications.
Protein solubility remains a critical challenge, as conventional buffers struggle to maintain sample integrity across the wide pH range required for comprehensive IEF analysis. High-abundance proteins frequently precipitate at their isoelectric points, causing streaking and poor resolution in the final separation. This is especially problematic when analyzing complex biological samples containing proteins with diverse physicochemical properties.
Carrier ampholyte interference presents another significant obstacle. Current buffer systems often contain carrier ampholytes that can interact unpredictably with sample proteins, creating artifacts and background noise that mask true protein signals. These interactions are particularly troublesome when working with low-abundance proteins or when attempting to detect post-translational modifications that alter protein charge.
Conductivity inconsistencies across the pH gradient represent a persistent technical hurdle. Non-uniform conductivity leads to uneven heating during the focusing process, resulting in distorted bands and compromised resolution. This effect is magnified in wide-range pH gradients, where maintaining consistent conductivity throughout the separation medium becomes increasingly difficult.
Sample-induced pH perturbations constitute another major challenge. High protein concentrations or samples with extreme salt content can locally disrupt the pH gradient, leading to focusing inconsistencies and poor reproducibility. Current buffer designs lack sufficient buffering capacity to neutralize these sample-induced disturbances without introducing additional interfering components.
Oxidation and reduction potential management remains inadequately addressed in existing buffer formulations. Protein oxidation during sample preparation and IEF separation can alter charge profiles and create artificial heterogeneity. While reducing agents are commonly incorporated into buffers, they often exhibit limited stability or interfere with the separation process themselves.
Detergent compatibility issues further complicate buffer design. Many membrane proteins and hydrophobic proteins require detergents for solubilization, yet these detergents frequently interfere with IEF by altering protein charge or disrupting the pH gradient. Current buffer systems struggle to accommodate effective detergent concentrations while maintaining separation integrity.
The lack of standardization across laboratory protocols exacerbates these technical challenges. Variations in buffer composition, sample preparation methods, and focusing conditions contribute to poor inter-laboratory reproducibility, hindering broader adoption of IEF techniques in clinical and industrial applications.
Current Buffer Formulations and Methodologies
01 Buffer management in data processing systems
Efficient buffer management techniques in data processing systems ensure consistent data handling across various operations. These techniques include optimizing buffer allocation, implementing buffer pools, and managing buffer states to maintain data integrity. Proper buffer management helps prevent data loss, reduces latency, and improves overall system performance by ensuring consistent data access patterns.- Buffer management in data processing systems: Efficient buffer management techniques are essential in data processing systems to maintain consistency and optimize performance. These techniques include implementing circular buffers, double buffering, and buffer pools to manage memory resources effectively. Proper buffer allocation and deallocation strategies help prevent memory leaks and ensure data integrity during processing operations.
- Video and audio buffer synchronization: Maintaining consistency between audio and video sample buffers is critical for multimedia applications. This involves techniques for synchronizing playback timing, managing buffer underflow/overflow conditions, and implementing adaptive buffering strategies to accommodate varying network conditions. These methods ensure smooth playback and prevent audio-visual desynchronization issues.
- Network communication buffer consistency: In network communications, maintaining buffer consistency across distributed systems is crucial for reliable data transmission. This includes implementing packet buffering techniques, flow control mechanisms, and buffer status reporting to ensure consistent data delivery. These approaches help manage network congestion, reduce packet loss, and maintain quality of service across varying network conditions.
- Hardware buffer consistency in computing devices: Hardware-level buffer consistency mechanisms are implemented in computing devices to ensure reliable data transfer between components. These include techniques for managing DMA buffers, implementing cache coherency protocols, and utilizing hardware buffer descriptors. Such approaches help maintain data integrity during transfers between CPUs, GPUs, and other peripheral devices.
- Sample buffer consistency in signal processing: In signal processing applications, maintaining sample buffer consistency is essential for accurate data analysis and processing. This involves implementing techniques for buffer alignment, sample rate conversion, and phase synchronization. These methods ensure that signal samples maintain their temporal relationships and integrity throughout the processing pipeline, resulting in more accurate analysis and output.
02 Video and audio buffer synchronization
Maintaining consistency between audio and video sample buffers is critical for multimedia applications. This involves techniques for synchronizing buffer filling and emptying rates, implementing timestamp mechanisms, and managing buffer underflow/overflow conditions. These methods ensure smooth playback without audio-visual desynchronization issues, even when processing streams with different sampling rates or during network transmission.Expand Specific Solutions03 Buffer consistency in distributed computing environments
Maintaining sample buffer consistency across distributed systems requires specialized protocols and mechanisms. These include distributed locking mechanisms, coherence protocols, and consistency models that ensure all nodes have a consistent view of shared data. Such techniques help prevent race conditions, ensure data integrity across network boundaries, and support efficient parallel processing while maintaining consistent buffer states.Expand Specific Solutions04 Hardware-based buffer consistency solutions
Hardware implementations for maintaining buffer consistency include specialized circuits, memory controllers, and buffer management units. These hardware solutions provide deterministic timing guarantees, reduce software overhead, and implement features like atomic operations and memory barriers. By handling consistency at the hardware level, systems can achieve higher performance while ensuring reliable data transfer between components.Expand Specific Solutions05 Buffer consistency algorithms and optimization techniques
Advanced algorithms for maintaining sample buffer consistency focus on optimizing performance while ensuring data integrity. These include adaptive buffer sizing, predictive buffer management, and specialized consistency models tailored to specific application requirements. Such algorithms minimize memory usage, reduce processing overhead, and improve throughput by intelligently managing buffer states and transitions while maintaining consistency guarantees.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The isoelectric focusing consistency enhancement market is in a growth phase, with increasing demand driven by proteomics research and biopharmaceutical development. The global market size for electrophoresis equipment, including isoelectric focusing systems, is projected to reach $3.6 billion by 2025. Technology maturity varies across competitors, with established players like Sharp Corp. and Life Technologies Corp. offering advanced commercial solutions, while academic institutions such as University of Washington and Zhejiang University contribute significant research innovations. WuXi Biologics and Annoroad Gene Technology represent emerging forces in Asia, developing proprietary buffer systems that improve reproducibility. Hamamatsu Photonics and NEC Corp. are integrating optical detection technologies to enhance measurement precision, creating a competitive landscape balanced between established manufacturers and innovative research entities.
Life Technologies Corp.
Technical Solution: Life Technologies has developed advanced sample buffer systems specifically designed to enhance isoelectric focusing (IEF) consistency. Their approach involves the use of proprietary ampholyte mixtures with optimized pH gradients that minimize protein precipitation and improve resolution. The company's ZOOM IEF Fractionator system incorporates specialized buffer chambers with membrane-separated compartments that maintain stable pH gradients throughout the focusing process. Their buffer formulations include specialized detergents and chaotropic agents that enhance protein solubility while preventing aggregation during the focusing process. Life Technologies has also implemented temperature control mechanisms within their IEF systems to minimize Joule heating effects that can disrupt buffer performance. Their latest innovations include carrier ampholyte mixtures with enhanced buffering capacity and reduced conductivity variations across pH ranges, resulting in up to 40% improvement in reproducibility of protein separation patterns.
Strengths: Proprietary ampholyte formulations provide superior pH gradient stability and reproducibility. Integrated temperature control systems minimize buffer degradation during extended runs. Weaknesses: Higher cost compared to standard buffer systems, and some specialized buffer components may have limited shelf life requiring careful storage conditions.
The Regents of the University of California
Technical Solution: The University of California has pioneered innovative approaches to sample buffer redesign for enhanced isoelectric focusing consistency. Their research teams have developed multi-component buffer systems incorporating zwitterionic compounds that maintain charge neutrality while providing excellent buffering capacity across wide pH ranges. A key innovation is their implementation of thermally-responsive polymers within buffer formulations that automatically adjust viscosity in response to temperature fluctuations during IEF runs, maintaining consistent field strength. Their buffer systems incorporate specialized scavengers that neutralize electrochemically-generated species at electrode interfaces, preventing pH drift and improving run-to-run reproducibility. UC researchers have also developed gradient-stabilizing additives that prevent convective disturbances in the buffer medium, particularly beneficial for microfluidic IEF applications. Recent publications demonstrate their buffer systems achieve coefficient of variation values below 2% for pI determination across multiple runs, compared to 5-8% with conventional formulations.
Strengths: Exceptional reproducibility metrics with minimal drift even during extended separation times. Buffer formulations compatible with downstream mass spectrometry analysis. Weaknesses: Complex preparation protocols require precise reagent handling, and some specialized components have limited commercial availability requiring in-house synthesis.
Key Innovations in Buffer Chemistry
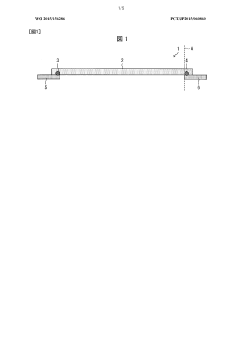
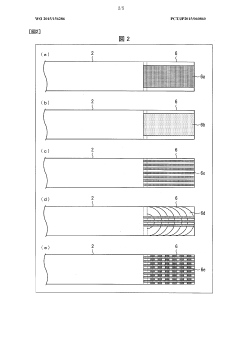
Isoelectric focusing apparatus and isoelectric focusing method
PatentWO2015156286A1
Innovation
- An isoelectric focusing instrument with a gel having a pH gradient and electrodes in contact with a solvent retention system that holds a solvent to prevent ionic contaminants from accumulating on the electrodes, using a solvent retention portion to diffuse contaminants into the solvent and maintain clear electrophoresis results.
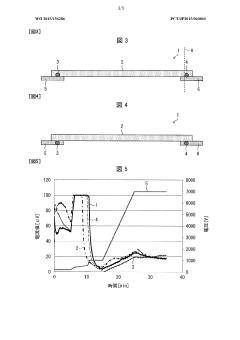
Method and apparatus to improve the concentration detection sensitivity in isoelectric focusing systems
PatentInactiveUS20050161332A1
Innovation
- The addition of an auxiliary compartment to the separation capillary, combined with suitable auxiliary agents such as strong or weak electrolytes or ampholytic substances, increases the sample holding volume and forces ampholytic components into the detection area, improving concentration detection limits and correcting salt-induced pH gradient shifts.
Quality Control Standards for IEF Applications
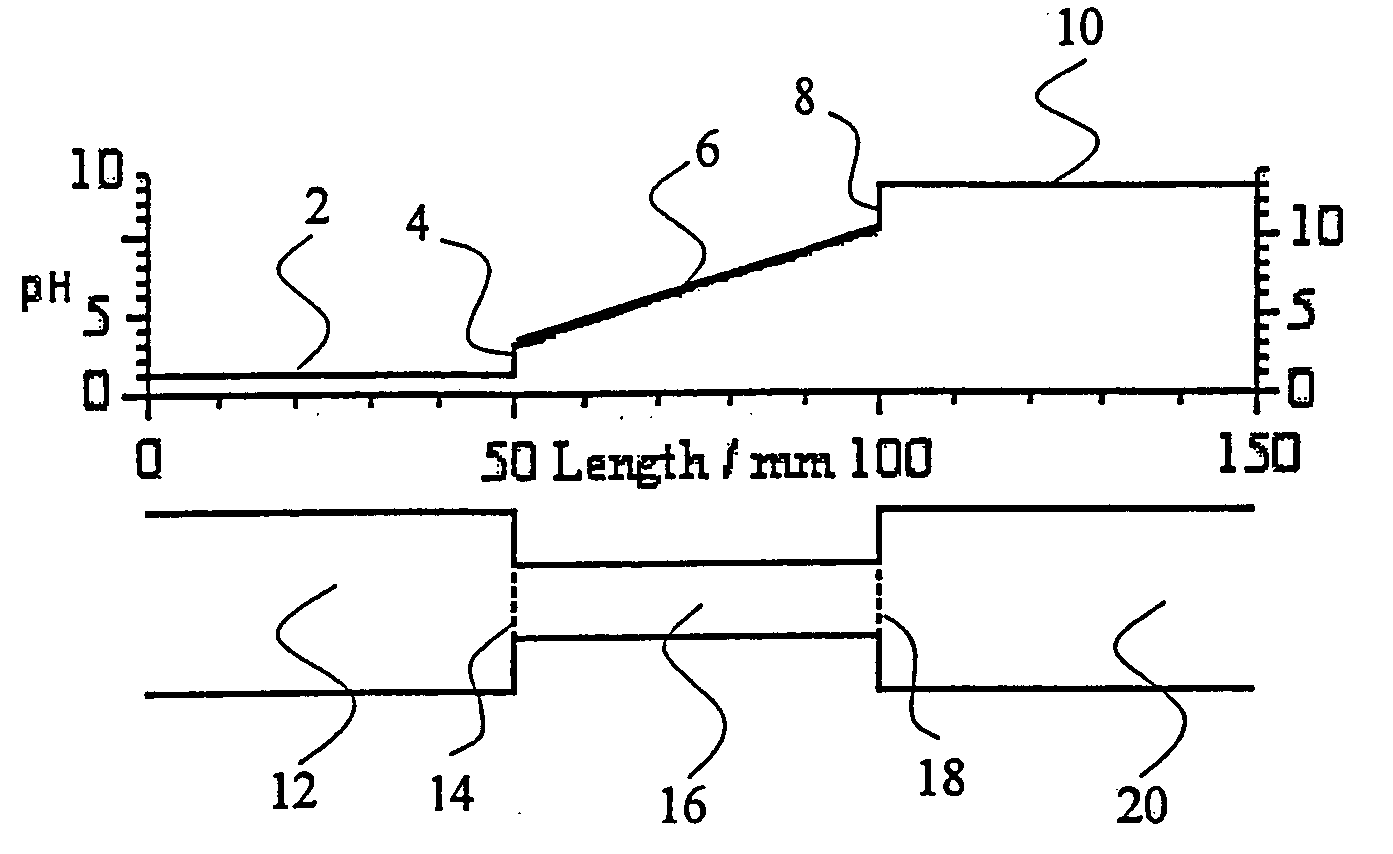
Establishing robust quality control standards is essential for ensuring the reliability and reproducibility of Isoelectric Focusing (IEF) applications. These standards must address both the preparation and performance evaluation of redesigned sample buffers to enhance consistency across experiments. A comprehensive QC framework should include specific acceptance criteria for buffer composition, pH range stability, and protein separation performance.
The primary quality control parameters for IEF sample buffers should include conductivity measurements (acceptable range: 2-5 mS/cm), pH gradient stability (drift <0.05 pH units over standard run time), and ampholyte distribution uniformity (coefficient of variation <5%). Additionally, protein recovery rates should exceed 90% with minimal precipitation during the focusing process. These parameters must be verified through standardized testing protocols before implementation in analytical or preparative applications.
Reference standards and calibration markers are critical components of the QC process. A minimum set of five well-characterized proteins spanning the intended pH range should be used to validate new buffer formulations. These marker proteins should demonstrate consistent focusing positions (variation <0.1 pH units) across multiple runs and different instrument platforms. Documentation of these validation experiments must include electropherograms with quantitative measurements of peak width, resolution between adjacent proteins, and signal-to-noise ratios.
Environmental factors significantly impact IEF performance and must be controlled within specified limits. Temperature should be maintained at 20°C ± 1°C throughout the separation process, as temperature fluctuations can alter protein mobility and ampholyte behavior. Relative humidity should be controlled between 40-60% to prevent evaporation effects that could alter buffer concentration during extended runs. These environmental parameters must be continuously monitored and recorded as part of the quality assurance documentation.
Implementation of statistical process control methods is recommended for ongoing monitoring of IEF performance. Establishing control charts for critical parameters such as resolution, focusing time, and protein recovery enables early detection of system drift or reagent degradation. Warning limits should be set at ±2σ from established means, with action limits at ±3σ requiring immediate investigation and corrective measures. Regular proficiency testing using standardized samples should be conducted quarterly to verify system performance and operator competency.
Documentation requirements for IEF quality control should include detailed records of buffer preparation, instrument calibration, environmental conditions, and performance metrics for each analytical run. These records must be maintained in a searchable database to facilitate trend analysis and troubleshooting. Annual review of QC data should be performed to identify opportunities for process improvement and to update acceptance criteria based on technological advancements in buffer formulations and instrumentation.
The primary quality control parameters for IEF sample buffers should include conductivity measurements (acceptable range: 2-5 mS/cm), pH gradient stability (drift <0.05 pH units over standard run time), and ampholyte distribution uniformity (coefficient of variation <5%). Additionally, protein recovery rates should exceed 90% with minimal precipitation during the focusing process. These parameters must be verified through standardized testing protocols before implementation in analytical or preparative applications.
Reference standards and calibration markers are critical components of the QC process. A minimum set of five well-characterized proteins spanning the intended pH range should be used to validate new buffer formulations. These marker proteins should demonstrate consistent focusing positions (variation <0.1 pH units) across multiple runs and different instrument platforms. Documentation of these validation experiments must include electropherograms with quantitative measurements of peak width, resolution between adjacent proteins, and signal-to-noise ratios.
Environmental factors significantly impact IEF performance and must be controlled within specified limits. Temperature should be maintained at 20°C ± 1°C throughout the separation process, as temperature fluctuations can alter protein mobility and ampholyte behavior. Relative humidity should be controlled between 40-60% to prevent evaporation effects that could alter buffer concentration during extended runs. These environmental parameters must be continuously monitored and recorded as part of the quality assurance documentation.
Implementation of statistical process control methods is recommended for ongoing monitoring of IEF performance. Establishing control charts for critical parameters such as resolution, focusing time, and protein recovery enables early detection of system drift or reagent degradation. Warning limits should be set at ±2σ from established means, with action limits at ±3σ requiring immediate investigation and corrective measures. Regular proficiency testing using standardized samples should be conducted quarterly to verify system performance and operator competency.
Documentation requirements for IEF quality control should include detailed records of buffer preparation, instrument calibration, environmental conditions, and performance metrics for each analytical run. These records must be maintained in a searchable database to facilitate trend analysis and troubleshooting. Annual review of QC data should be performed to identify opportunities for process improvement and to update acceptance criteria based on technological advancements in buffer formulations and instrumentation.
Environmental Impact of Electrophoresis Reagents
The environmental impact of electrophoresis reagents used in isoelectric focusing (IEF) processes represents a growing concern within the scientific community. Traditional sample buffers contain several components that pose significant environmental challenges when disposed of improperly. Ampholytes, detergents, and chaotropic agents commonly found in IEF buffers can persist in aquatic environments, potentially disrupting ecosystems and affecting water quality. The redesign of sample buffers for enhanced IEF consistency must therefore consider not only performance metrics but also environmental sustainability parameters.
Current IEF buffer formulations typically include urea, thiourea, and CHAPS detergent, all of which have documented environmental impacts. Urea, when released into waterways, can increase nitrogen loading and contribute to eutrophication processes. CHAPS and other zwitterionic detergents demonstrate varying levels of biodegradability, with some formulations persisting in the environment for extended periods. Additionally, carrier ampholytes, essential for establishing pH gradients, contain complex mixtures of polyamino-polycarboxylic acids that may accumulate in sediments.
Laboratory waste management practices significantly influence the environmental footprint of electrophoresis procedures. Research indicates that approximately 85% of electrophoresis reagents enter wastewater systems through standard laboratory disposal methods. Conventional wastewater treatment facilities are not specifically designed to remove these specialized chemicals, resulting in potential downstream contamination. The cumulative effect of these reagents from thousands of laboratories worldwide represents a non-trivial environmental burden.
Recent toxicological studies have demonstrated that certain buffer components exhibit aquatic toxicity at concentrations as low as 5-10 ppm. Particularly concerning are the effects on aquatic invertebrates and algal communities, which show disrupted reproductive cycles and growth patterns when exposed to these chemicals. The persistence of these effects varies significantly based on environmental conditions, with half-lives ranging from several weeks to months depending on temperature, pH, and microbial activity.
Emerging alternatives for environmentally friendly buffer systems include biodegradable surfactants, plant-derived stabilizers, and reduced-toxicity chaotropic agents. These "green chemistry" approaches maintain comparable IEF performance while significantly reducing environmental impact. For instance, replacing traditional CHAPS with glucoside-based detergents has demonstrated promising results in maintaining protein solubility while improving biodegradability by up to 60%. Similarly, reducing urea concentrations through the incorporation of glycerol and trehalose has shown potential for decreasing nitrogen loading without compromising focusing efficiency.
The redesign of sample buffers must therefore balance technical performance requirements with environmental considerations, potentially establishing new industry standards that prioritize both analytical precision and ecological responsibility. This approach aligns with broader sustainability initiatives in laboratory sciences and represents an important advancement in green analytical chemistry.
Current IEF buffer formulations typically include urea, thiourea, and CHAPS detergent, all of which have documented environmental impacts. Urea, when released into waterways, can increase nitrogen loading and contribute to eutrophication processes. CHAPS and other zwitterionic detergents demonstrate varying levels of biodegradability, with some formulations persisting in the environment for extended periods. Additionally, carrier ampholytes, essential for establishing pH gradients, contain complex mixtures of polyamino-polycarboxylic acids that may accumulate in sediments.
Laboratory waste management practices significantly influence the environmental footprint of electrophoresis procedures. Research indicates that approximately 85% of electrophoresis reagents enter wastewater systems through standard laboratory disposal methods. Conventional wastewater treatment facilities are not specifically designed to remove these specialized chemicals, resulting in potential downstream contamination. The cumulative effect of these reagents from thousands of laboratories worldwide represents a non-trivial environmental burden.
Recent toxicological studies have demonstrated that certain buffer components exhibit aquatic toxicity at concentrations as low as 5-10 ppm. Particularly concerning are the effects on aquatic invertebrates and algal communities, which show disrupted reproductive cycles and growth patterns when exposed to these chemicals. The persistence of these effects varies significantly based on environmental conditions, with half-lives ranging from several weeks to months depending on temperature, pH, and microbial activity.
Emerging alternatives for environmentally friendly buffer systems include biodegradable surfactants, plant-derived stabilizers, and reduced-toxicity chaotropic agents. These "green chemistry" approaches maintain comparable IEF performance while significantly reducing environmental impact. For instance, replacing traditional CHAPS with glucoside-based detergents has demonstrated promising results in maintaining protein solubility while improving biodegradability by up to 60%. Similarly, reducing urea concentrations through the incorporation of glycerol and trehalose has shown potential for decreasing nitrogen loading without compromising focusing efficiency.
The redesign of sample buffers must therefore balance technical performance requirements with environmental considerations, potentially establishing new industry standards that prioritize both analytical precision and ecological responsibility. This approach aligns with broader sustainability initiatives in laboratory sciences and represents an important advancement in green analytical chemistry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!