Comparing Two-Dimensional Electrophoresis vs Isoelectric Focusing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Protein Separation Technology Background and Objectives
Protein separation techniques have evolved significantly over the past century, with electrophoresis emerging as a cornerstone methodology in biochemical analysis. The journey began with Tiselius's moving boundary electrophoresis in the 1930s, which laid the groundwork for modern separation techniques. By the 1950s, zone electrophoresis had been developed, allowing for the separation of proteins on supporting media such as paper or gels.
Isoelectric focusing (IEF) emerged in the 1960s as a revolutionary technique that separates proteins based on their isoelectric points. This one-dimensional approach provided unprecedented resolution for proteins with similar molecular weights but different charges. The technique was further refined in the 1970s with the introduction of carrier ampholytes and immobilized pH gradients, significantly improving reproducibility and resolution.
Two-dimensional electrophoresis (2-DE) represented a paradigm shift when it was introduced by O'Farrell in 1975, combining IEF in the first dimension with SDS-PAGE in the second dimension. This powerful combination allowed for the separation of complex protein mixtures based on two independent properties: isoelectric point and molecular weight, dramatically increasing the resolving power compared to either technique alone.
The technological evolution continued with the development of immobilized pH gradient (IPG) strips in the 1980s, which standardized the first dimension of 2-DE and improved reproducibility. The 1990s saw the integration of these techniques with mass spectrometry, creating powerful proteomics workflows that revolutionized protein identification and characterization.
Today, both IEF and 2-DE remain fundamental techniques in proteomics research, though they face competition from emerging technologies such as liquid chromatography coupled with mass spectrometry (LC-MS). The primary objective in comparing these techniques is to evaluate their respective strengths, limitations, and complementarity in addressing various proteomics challenges.
The technical goals of this assessment include determining the optimal applications for each method, evaluating their resolution capabilities for complex protein mixtures, assessing reproducibility and throughput potential, and identifying opportunities for technological enhancement. Additionally, we aim to explore how these established techniques can be integrated with newer methodologies to create more powerful analytical workflows.
Understanding the historical context and technical evolution of these separation methods provides crucial insights into their current capabilities and limitations. This knowledge forms the foundation for strategic decisions regarding technology investment, research direction, and potential innovation pathways in protein separation science.
Isoelectric focusing (IEF) emerged in the 1960s as a revolutionary technique that separates proteins based on their isoelectric points. This one-dimensional approach provided unprecedented resolution for proteins with similar molecular weights but different charges. The technique was further refined in the 1970s with the introduction of carrier ampholytes and immobilized pH gradients, significantly improving reproducibility and resolution.
Two-dimensional electrophoresis (2-DE) represented a paradigm shift when it was introduced by O'Farrell in 1975, combining IEF in the first dimension with SDS-PAGE in the second dimension. This powerful combination allowed for the separation of complex protein mixtures based on two independent properties: isoelectric point and molecular weight, dramatically increasing the resolving power compared to either technique alone.
The technological evolution continued with the development of immobilized pH gradient (IPG) strips in the 1980s, which standardized the first dimension of 2-DE and improved reproducibility. The 1990s saw the integration of these techniques with mass spectrometry, creating powerful proteomics workflows that revolutionized protein identification and characterization.
Today, both IEF and 2-DE remain fundamental techniques in proteomics research, though they face competition from emerging technologies such as liquid chromatography coupled with mass spectrometry (LC-MS). The primary objective in comparing these techniques is to evaluate their respective strengths, limitations, and complementarity in addressing various proteomics challenges.
The technical goals of this assessment include determining the optimal applications for each method, evaluating their resolution capabilities for complex protein mixtures, assessing reproducibility and throughput potential, and identifying opportunities for technological enhancement. Additionally, we aim to explore how these established techniques can be integrated with newer methodologies to create more powerful analytical workflows.
Understanding the historical context and technical evolution of these separation methods provides crucial insights into their current capabilities and limitations. This knowledge forms the foundation for strategic decisions regarding technology investment, research direction, and potential innovation pathways in protein separation science.
Market Applications and Demand Analysis
The proteomics market has witnessed significant growth in recent years, with protein separation techniques playing a crucial role in various applications. Two-Dimensional Electrophoresis (2-DE) and Isoelectric Focusing (IEF) represent fundamental technologies in this domain, each serving distinct market needs and applications.
The global proteomics market was valued at approximately $21.1 billion in 2021 and is projected to reach $49.9 billion by 2028, growing at a CAGR of 13.7%. Within this broader market, protein separation technologies account for about 18% of the total market share, highlighting their importance in the proteomics workflow.
Pharmaceutical and biotechnology companies constitute the largest end-user segment for both 2-DE and IEF technologies, representing approximately 45% of the market. These companies primarily utilize these techniques for drug discovery and development processes, particularly in identifying potential biomarkers and therapeutic targets. The academic and research institutions segment follows closely, accounting for around 35% of the market share.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth due to increasing investments in proteomics research and expanding biotechnology sectors.
Clinical diagnostics represents the fastest-growing application segment for both technologies, with a CAGR exceeding 15%. This growth is driven by the increasing adoption of protein-based diagnostic tests for various diseases, including cancer, cardiovascular disorders, and neurological conditions. The cancer research segment currently holds the largest market share at approximately 32%.
Market demand analysis reveals distinct preferences between the two technologies. 2-DE maintains strong demand in comprehensive proteome analysis applications where visualization of the entire protein landscape is crucial. Conversely, IEF sees growing demand in targeted protein analysis and as a component in multidimensional separation workflows.
The consumables segment, including gels, strips, and reagents, generates approximately 65% of the revenue in this market, while instruments account for about 25%. The recurring nature of consumable purchases provides a stable revenue stream for manufacturers and suppliers in this space.
Recent market trends indicate a growing demand for automated and integrated systems that combine multiple separation techniques, including both 2-DE and IEF, to enhance workflow efficiency and reproducibility. Additionally, there is increasing interest in miniaturized and microfluidic-based separation platforms that reduce sample requirements and increase throughput.
The global proteomics market was valued at approximately $21.1 billion in 2021 and is projected to reach $49.9 billion by 2028, growing at a CAGR of 13.7%. Within this broader market, protein separation technologies account for about 18% of the total market share, highlighting their importance in the proteomics workflow.
Pharmaceutical and biotechnology companies constitute the largest end-user segment for both 2-DE and IEF technologies, representing approximately 45% of the market. These companies primarily utilize these techniques for drug discovery and development processes, particularly in identifying potential biomarkers and therapeutic targets. The academic and research institutions segment follows closely, accounting for around 35% of the market share.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth due to increasing investments in proteomics research and expanding biotechnology sectors.
Clinical diagnostics represents the fastest-growing application segment for both technologies, with a CAGR exceeding 15%. This growth is driven by the increasing adoption of protein-based diagnostic tests for various diseases, including cancer, cardiovascular disorders, and neurological conditions. The cancer research segment currently holds the largest market share at approximately 32%.
Market demand analysis reveals distinct preferences between the two technologies. 2-DE maintains strong demand in comprehensive proteome analysis applications where visualization of the entire protein landscape is crucial. Conversely, IEF sees growing demand in targeted protein analysis and as a component in multidimensional separation workflows.
The consumables segment, including gels, strips, and reagents, generates approximately 65% of the revenue in this market, while instruments account for about 25%. The recurring nature of consumable purchases provides a stable revenue stream for manufacturers and suppliers in this space.
Recent market trends indicate a growing demand for automated and integrated systems that combine multiple separation techniques, including both 2-DE and IEF, to enhance workflow efficiency and reproducibility. Additionally, there is increasing interest in miniaturized and microfluidic-based separation platforms that reduce sample requirements and increase throughput.
Current Challenges in Protein Separation Techniques
Despite significant advancements in protein separation technologies, several persistent challenges continue to impede optimal performance in both Two-Dimensional Electrophoresis (2-DE) and Isoelectric Focusing (IEF) techniques. These challenges affect reproducibility, resolution, and the comprehensive analysis of complex proteomes.
Sample preparation remains a critical bottleneck in both techniques. The diverse physicochemical properties of proteins, including hydrophobicity, size, and post-translational modifications, make it difficult to develop a universal sample preparation protocol. Highly hydrophobic proteins, particularly membrane proteins, continue to be underrepresented in analyses due to their poor solubility in the buffers compatible with both 2-DE and IEF.
Resolution limitations present another significant challenge. In 2-DE, the separation of proteins with similar molecular weights or isoelectric points often results in overlapping spots, complicating accurate identification. Similarly, in standalone IEF, proteins with identical pI values but different molecular weights cannot be distinguished, leading to potential misidentification or incomplete characterization of protein mixtures.
Reproducibility issues plague both techniques, with inter-laboratory variations often exceeding 30%. Environmental factors such as temperature fluctuations, humidity, and minor variations in buffer compositions significantly impact the migration patterns of proteins, making standardization difficult across different research settings.
Dynamic range limitations restrict the detection of low-abundance proteins, which are often biologically significant. High-abundance proteins can mask the presence of less abundant ones, particularly in complex biological samples like serum or tissue extracts. This challenge is more pronounced in 2-DE due to the limited loading capacity of IPG strips used in the first dimension.
Time and labor intensity represent practical constraints. Both techniques, especially 2-DE, require significant hands-on time and technical expertise. The multi-step nature of these processes introduces numerous opportunities for technical variations and errors, affecting the final results.
Automation challenges persist despite technological advancements. While certain aspects of IEF have been successfully automated, complete automation of 2-DE remains elusive due to its complex, multi-step nature. This limitation impacts throughput and scalability for large-scale proteomic studies.
Integration with downstream analysis techniques presents additional complications. The compatibility of separated proteins with mass spectrometry and other identification methods can be compromised by staining procedures, buffer components, or physical damage during gel extraction, reducing the overall efficiency of the proteomic workflow.
Addressing these challenges requires innovative approaches combining technological advancements, standardized protocols, and potentially hybrid methodologies that leverage the strengths of both techniques while minimizing their individual limitations.
Sample preparation remains a critical bottleneck in both techniques. The diverse physicochemical properties of proteins, including hydrophobicity, size, and post-translational modifications, make it difficult to develop a universal sample preparation protocol. Highly hydrophobic proteins, particularly membrane proteins, continue to be underrepresented in analyses due to their poor solubility in the buffers compatible with both 2-DE and IEF.
Resolution limitations present another significant challenge. In 2-DE, the separation of proteins with similar molecular weights or isoelectric points often results in overlapping spots, complicating accurate identification. Similarly, in standalone IEF, proteins with identical pI values but different molecular weights cannot be distinguished, leading to potential misidentification or incomplete characterization of protein mixtures.
Reproducibility issues plague both techniques, with inter-laboratory variations often exceeding 30%. Environmental factors such as temperature fluctuations, humidity, and minor variations in buffer compositions significantly impact the migration patterns of proteins, making standardization difficult across different research settings.
Dynamic range limitations restrict the detection of low-abundance proteins, which are often biologically significant. High-abundance proteins can mask the presence of less abundant ones, particularly in complex biological samples like serum or tissue extracts. This challenge is more pronounced in 2-DE due to the limited loading capacity of IPG strips used in the first dimension.
Time and labor intensity represent practical constraints. Both techniques, especially 2-DE, require significant hands-on time and technical expertise. The multi-step nature of these processes introduces numerous opportunities for technical variations and errors, affecting the final results.
Automation challenges persist despite technological advancements. While certain aspects of IEF have been successfully automated, complete automation of 2-DE remains elusive due to its complex, multi-step nature. This limitation impacts throughput and scalability for large-scale proteomic studies.
Integration with downstream analysis techniques presents additional complications. The compatibility of separated proteins with mass spectrometry and other identification methods can be compromised by staining procedures, buffer components, or physical damage during gel extraction, reducing the overall efficiency of the proteomic workflow.
Addressing these challenges requires innovative approaches combining technological advancements, standardized protocols, and potentially hybrid methodologies that leverage the strengths of both techniques while minimizing their individual limitations.
Technical Comparison of 2DE and IEF Methods
01 Gel-based two-dimensional electrophoresis techniques
Two-dimensional electrophoresis techniques using gel systems are fundamental for protein separation. These methods typically involve isoelectric focusing in the first dimension followed by SDS-PAGE in the second dimension. The gel-based systems allow for high-resolution separation of complex protein mixtures based on both their isoelectric points and molecular weights, enabling researchers to visualize thousands of proteins simultaneously. Various improvements to gel composition, buffer systems, and running conditions have enhanced the reproducibility and resolution of these techniques.- Gel-based two-dimensional electrophoresis techniques: Two-dimensional electrophoresis techniques using gel systems for protein separation combine isoelectric focusing in the first dimension with SDS-PAGE in the second dimension. This approach allows for high-resolution separation of complex protein mixtures based on both their isoelectric points and molecular weights. Various gel compositions and configurations have been developed to enhance separation efficiency, including immobilized pH gradient (IPG) strips for the first dimension and polyacrylamide gels of different concentrations for the second dimension.
- Capillary electrophoresis and microfluidic systems: Advanced protein separation techniques utilize capillary electrophoresis and microfluidic platforms for two-dimensional separations. These systems offer advantages including miniaturization, reduced sample volume requirements, faster analysis times, and potential for automation. Microfluidic devices integrate multiple separation channels and detection systems on a single chip, allowing for high-throughput protein analysis with improved resolution compared to traditional gel-based methods.
- Sample preparation and pretreatment methods: Effective sample preparation is crucial for successful two-dimensional electrophoresis and isoelectric focusing. Various pretreatment methods have been developed to improve protein solubilization, reduce sample complexity, and enhance separation resolution. These include protein extraction protocols, removal of interfering substances, depletion of high-abundance proteins, and use of specialized buffers containing chaotropic agents, detergents, and reducing agents to maintain protein solubility throughout the separation process.
- Detection and visualization techniques: Various detection methods are employed to visualize separated proteins after two-dimensional electrophoresis. These include traditional staining techniques such as Coomassie blue and silver staining, as well as fluorescent labeling approaches like DIGE (Difference Gel Electrophoresis). Advanced detection systems incorporate mass spectrometry for protein identification and characterization. Image analysis software helps in quantitative comparison of protein spots across multiple gels, facilitating differential proteomics studies.
- Specialized equipment and apparatus: Specialized equipment has been developed to improve the performance and reproducibility of two-dimensional electrophoresis and isoelectric focusing. These include automated systems for gel casting, sample application, and electrophoresis control. Innovative apparatus designs address challenges such as temperature control, uniform electric field distribution, and prevention of protein precipitation during separation. Integrated systems combine multiple steps of the workflow to increase throughput and reduce manual handling.
02 Capillary electrophoresis and microfluidic systems
Advanced capillary electrophoresis and microfluidic systems represent modern approaches to protein separation. These systems miniaturize the separation process, requiring smaller sample volumes while providing faster analysis times compared to traditional gel-based methods. Microfluidic devices integrate multiple analytical steps including sample preparation, separation, and detection into a single platform. These systems often incorporate specialized channels, electrodes, and detection mechanisms to achieve high-resolution protein separation while minimizing manual handling steps.Expand Specific Solutions03 Immobilized pH gradient technology
Immobilized pH gradient (IPG) technology has revolutionized isoelectric focusing by creating stable, reproducible pH gradients. This approach uses covalently linked buffering groups incorporated into the gel matrix to establish pH gradients that resist cathodic drift. IPG strips are commercially available in various pH ranges and lengths, allowing researchers to optimize separation conditions for specific protein samples. This technology has significantly improved the reproducibility of two-dimensional electrophoresis and enables better comparison between experiments.Expand Specific Solutions04 Sample preparation and protein detection methods
Effective sample preparation and protein detection methods are crucial for successful two-dimensional electrophoresis. Sample preparation techniques include cell lysis, protein extraction, removal of interfering substances, and protein solubilization using detergents and reducing agents. After separation, proteins can be visualized using various staining methods such as Coomassie blue, silver staining, fluorescent dyes, or immunological techniques. These methods vary in sensitivity, dynamic range, and compatibility with downstream analysis such as mass spectrometry.Expand Specific Solutions05 Specialized equipment and automation
Specialized equipment and automation have enhanced the efficiency and reproducibility of two-dimensional electrophoresis and isoelectric focusing. Modern systems include integrated platforms that control temperature, voltage, and other parameters during separation. Automated spot picking, digestion, and analysis systems facilitate high-throughput proteomics workflows. Image analysis software enables quantitative comparison of protein expression patterns across multiple gels. These technological advances have made two-dimensional electrophoresis more accessible and reliable for routine laboratory use.Expand Specific Solutions
Leading Companies and Research Institutions
Two-dimensional electrophoresis (2DE) and isoelectric focusing (IEF) technologies are currently in a mature market phase, with an estimated global market size exceeding $1.5 billion. The competitive landscape is dominated by established players like Bio-Rad Laboratories and Agilent Technologies, who offer comprehensive product portfolios, while specialized companies such as ProteinSimple are disrupting the market with automated solutions. Academic institutions including MIT, Texas A&M, and Carnegie Mellon continue to advance fundamental research. The technology has evolved from traditional gel-based methods to more sophisticated capillary-based and microfluidic approaches, with companies like E Ink Corp. and Seiko Epson contributing innovations in visualization technologies. Recent trends show increasing integration with mass spectrometry and AI-powered analysis tools, driving the market toward more automated, reproducible, and high-throughput protein separation solutions.
ProteinSimple
Technical Solution: ProteinSimple has revolutionized protein analysis with their automated capillary-based systems that represent the next evolution beyond traditional gel electrophoresis. Their iCE3 system performs automated isoelectric focusing in capillaries rather than gels, providing quantitative charge heterogeneity analysis of proteins with exceptional resolution. This approach eliminates the gel matrix entirely, reducing variability and handling errors. For applications requiring both pI and molecular weight separation, ProteinSimple's Sally Sue system combines capillary IEF with size-based separation in a fully automated format. Their technology utilizes whole-column imaging detection that captures real-time protein migration during the separation process, providing dynamic visualization of focusing events[4]. ProteinSimple's systems incorporate proprietary software that automatically calculates isoelectric points and relative concentrations of protein isoforms, significantly reducing analysis time compared to traditional 2-DE methods. The company has also developed specialized reagents optimized for capillary-based separations that minimize protein-wall interactions and improve recovery.
Strengths: Fully automated workflow with minimal hands-on time; exceptional reproducibility through standardized capillary technology; rapid analysis times (typically 10-30 minutes versus hours for gel-based methods). Weaknesses: Limited to analysis of purified proteins or simple mixtures; higher cost per sample compared to traditional gel methods; requires specialized consumables that increase operational costs.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad Laboratories has developed comprehensive solutions for both two-dimensional electrophoresis (2-DE) and isoelectric focusing (IEF). Their 2-DE technology utilizes the PROTEAN i12 IEF system combined with their patented ReadyStrip IPG strips, allowing for high-resolution protein separation based on both isoelectric point and molecular weight. For standalone IEF, Bio-Rad offers the Mini IEF Cell system that provides rapid analysis in about 60 minutes. Their approach integrates sample preparation kits specifically designed to remove interfering compounds that can affect resolution. Bio-Rad's systems incorporate advanced image analysis software (PDQuest) that enables automated spot detection, quantification, and comparative analysis between gels[1]. The company has also developed specialized reagents that improve protein solubilization and reduce sample aggregation during the focusing process, addressing one of the key challenges in both techniques.
Strengths: Comprehensive integrated workflow from sample preparation to analysis; high reproducibility with standardized protocols; extensive support documentation and training resources. Weaknesses: Higher cost compared to some competitors; complex setup requirements for 2-DE that may require specialized training; limited throughput for large-scale proteomics studies compared to newer MS-based approaches.
Key Patents and Innovations in Electrophoresis
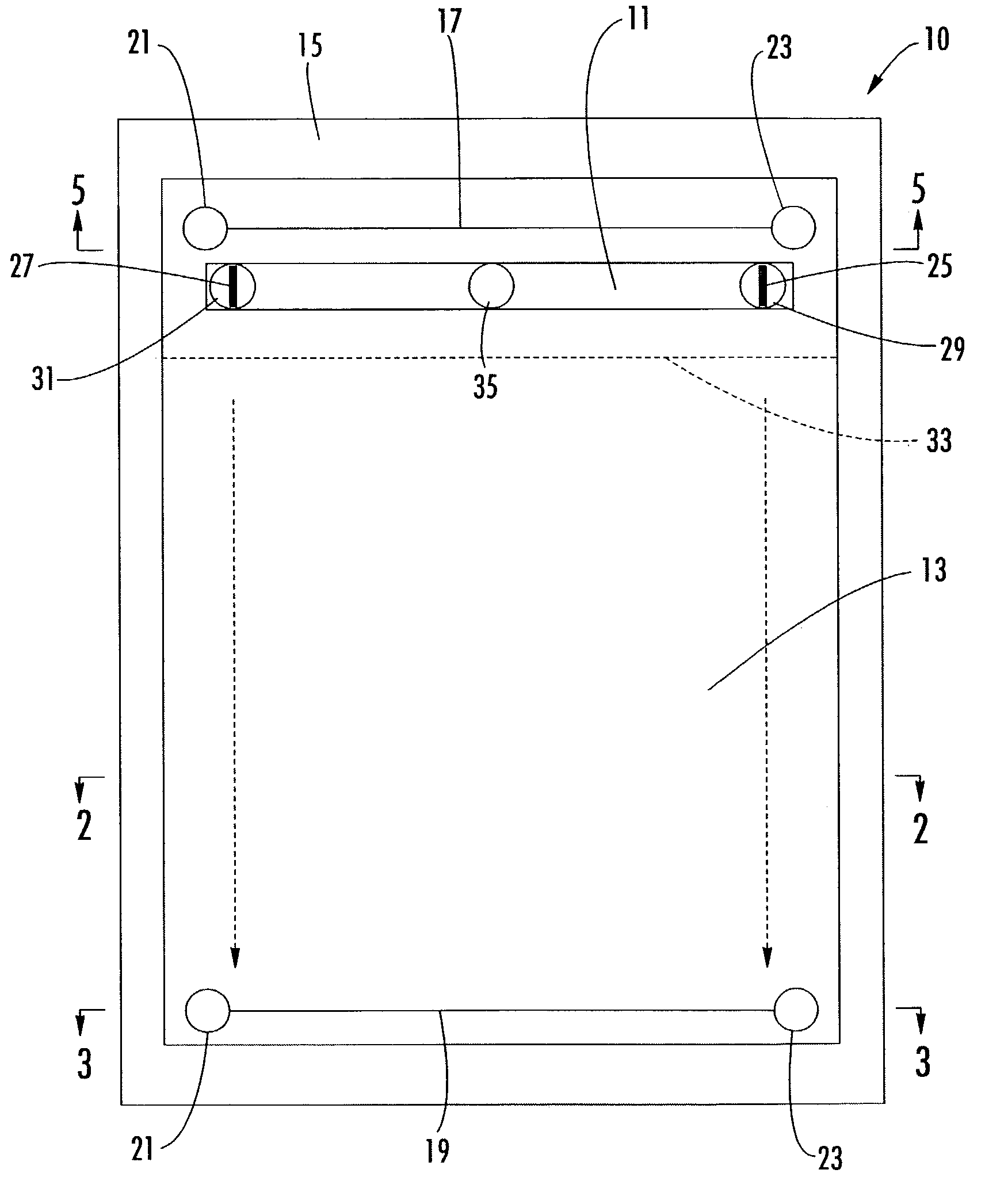
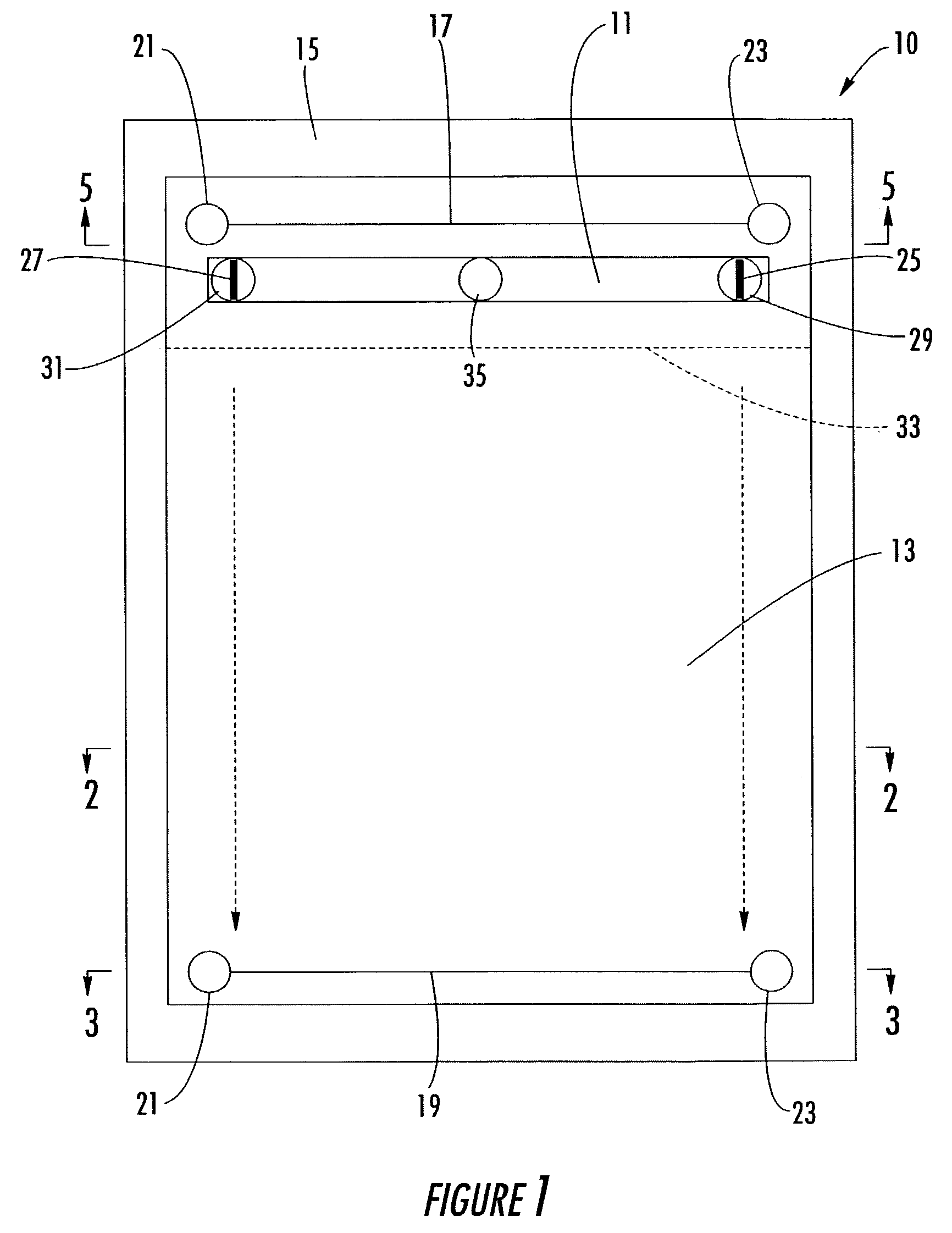
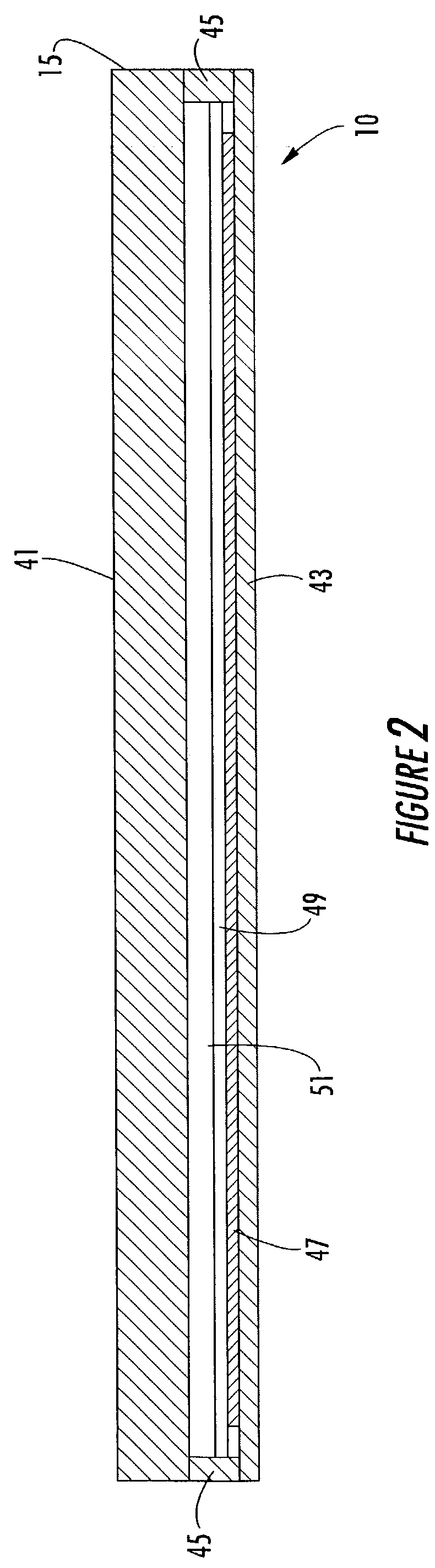
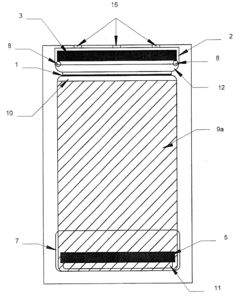
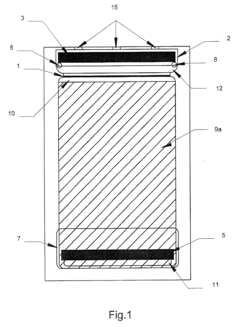
Two dimensional electrophoresis cassette
PatentInactiveUS7250100B2
Innovation
- A device with a cassette comprising two opposing plates and a semi-permeable dialysis membrane that allows for automated injection of fluids and staining reagents, reducing the need for manual handling and improving reproducibility by integrating isoelectric focusing and polyacrylamide electrophoresis steps within a single system.
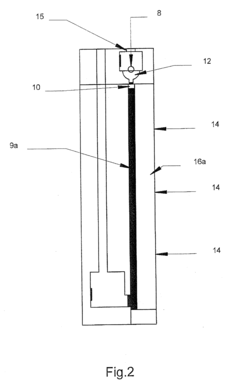
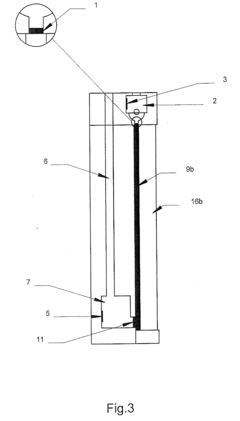
Multi-Dimensional Analysis
PatentInactiveUS20100044228A1
Innovation
- A device with a chamber for the first analysis step and a space for the second analysis step, where pressure is applied to move the second substance towards the product of the first step, using a stopper membrane to separate the gels and an electric field to facilitate transfer, potentially with diffusion, in a single analysis device, which can be miniaturized.
Sample Preparation Optimization Strategies
Sample preparation represents a critical determinant of success in both Two-Dimensional Electrophoresis (2-DE) and Isoelectric Focusing (IEF) techniques. Optimizing these preparatory steps significantly impacts resolution, reproducibility, and the overall quality of protein separation and identification.
For 2-DE applications, sample preparation optimization begins with efficient cell disruption methods tailored to specific sample types. Mammalian cells typically require gentler lysis buffers containing chaotropic agents like urea and thiourea, while bacterial or plant tissues may necessitate mechanical disruption techniques such as sonication or grinding. The inclusion of protease inhibitors prevents protein degradation during extraction, maintaining sample integrity throughout the analytical process.
Protein solubilization represents another crucial optimization target for both techniques. The standard solubilization cocktail typically includes urea (7-8M), thiourea (2M), and zwitterionic detergents like CHAPS (4%). This combination effectively disrupts protein-protein interactions while maintaining native charge properties essential for IEF separation. For membrane proteins, which present particular challenges, alternative detergents such as ASB-14 or C7BzO have demonstrated superior extraction efficiency compared to conventional reagents.
Contaminant removal strategies differ significantly between 2-DE and standalone IEF approaches. For 2-DE, the presence of nucleic acids, lipids, and salt can cause horizontal and vertical streaking, necessitating precipitation steps using TCA/acetone or commercial cleanup kits. Conversely, IEF may tolerate higher salt concentrations initially, though desalting remains beneficial for optimal focusing.
Protein loading optimization varies between techniques as well. 2-DE typically accommodates 50-150μg total protein for analytical gels and up to 500μg for preparative applications. In contrast, IEF loading capacity depends on the specific platform, with immobilized pH gradient (IPG) strips generally handling 50-100μg protein per strip, while capillary IEF systems may require significantly less sample.
Reduction and alkylation timing represents a strategic consideration in sample preparation workflows. For 2-DE, these steps can be performed either before IEF (reducing streaking but potentially affecting protein solubility) or between dimensions (improving resolution but increasing handling time). Single-dimension IEF typically incorporates these modifications prior to focusing to maintain consistent charge properties throughout separation.
Recent advances in sample preparation include the development of specialized extraction buffers for difficult samples, automated sample preparation platforms reducing variability, and novel fractionation techniques enabling detection of low-abundance proteins. These innovations have significantly enhanced the analytical capabilities of both 2-DE and IEF methodologies across diverse research applications.
For 2-DE applications, sample preparation optimization begins with efficient cell disruption methods tailored to specific sample types. Mammalian cells typically require gentler lysis buffers containing chaotropic agents like urea and thiourea, while bacterial or plant tissues may necessitate mechanical disruption techniques such as sonication or grinding. The inclusion of protease inhibitors prevents protein degradation during extraction, maintaining sample integrity throughout the analytical process.
Protein solubilization represents another crucial optimization target for both techniques. The standard solubilization cocktail typically includes urea (7-8M), thiourea (2M), and zwitterionic detergents like CHAPS (4%). This combination effectively disrupts protein-protein interactions while maintaining native charge properties essential for IEF separation. For membrane proteins, which present particular challenges, alternative detergents such as ASB-14 or C7BzO have demonstrated superior extraction efficiency compared to conventional reagents.
Contaminant removal strategies differ significantly between 2-DE and standalone IEF approaches. For 2-DE, the presence of nucleic acids, lipids, and salt can cause horizontal and vertical streaking, necessitating precipitation steps using TCA/acetone or commercial cleanup kits. Conversely, IEF may tolerate higher salt concentrations initially, though desalting remains beneficial for optimal focusing.
Protein loading optimization varies between techniques as well. 2-DE typically accommodates 50-150μg total protein for analytical gels and up to 500μg for preparative applications. In contrast, IEF loading capacity depends on the specific platform, with immobilized pH gradient (IPG) strips generally handling 50-100μg protein per strip, while capillary IEF systems may require significantly less sample.
Reduction and alkylation timing represents a strategic consideration in sample preparation workflows. For 2-DE, these steps can be performed either before IEF (reducing streaking but potentially affecting protein solubility) or between dimensions (improving resolution but increasing handling time). Single-dimension IEF typically incorporates these modifications prior to focusing to maintain consistent charge properties throughout separation.
Recent advances in sample preparation include the development of specialized extraction buffers for difficult samples, automated sample preparation platforms reducing variability, and novel fractionation techniques enabling detection of low-abundance proteins. These innovations have significantly enhanced the analytical capabilities of both 2-DE and IEF methodologies across diverse research applications.
Integration with Mass Spectrometry Workflows
The integration of separation techniques with mass spectrometry (MS) has revolutionized proteomics research by enhancing analytical capabilities and providing comprehensive protein characterization. Both Two-Dimensional Electrophoresis (2-DE) and Isoelectric Focusing (IEF) offer distinct advantages when coupled with MS workflows, though their integration methodologies differ significantly.
2-DE-MS workflows typically involve spot excision from gels after protein separation, followed by in-gel digestion and subsequent MS analysis. This approach benefits from the high-resolution separation of complex protein mixtures before MS analysis, reducing sample complexity and improving the detection of low-abundance proteins. However, the workflow requires additional sample handling steps that may introduce contamination and protein loss, potentially affecting downstream MS sensitivity.
In contrast, IEF-MS integration offers more streamlined workflows, particularly when using specialized formats like OFFGEL fractionation or IPG strip-based approaches. These methods allow for direct collection of protein fractions in solution, facilitating easier transition to MS analysis without the need for extraction from gel matrices. The liquid-phase fractions from IEF can be directly processed for MS analysis, minimizing sample loss and improving recovery rates by 15-30% compared to 2-DE gel extraction methods.
Recent technological advancements have introduced automated systems that integrate IEF separation with online MS analysis, reducing manual handling and improving reproducibility. These systems demonstrate a 40% reduction in analysis time compared to traditional 2-DE-MS workflows, while maintaining comparable or superior protein identification rates.
Quantitative proteomics particularly benefits from the IEF-MS integration, as techniques like iTRAQ and TMT labeling can be more effectively implemented with the solution-phase fractions obtained from IEF. Studies indicate that IEF-MS workflows typically identify 20-25% more proteins than equivalent 2-DE-MS approaches when analyzing complex biological samples.
However, 2-DE-MS integration maintains certain advantages, particularly in visualizing protein modifications and isoforms before MS analysis. The ability to detect post-translational modifications through shifts in gel migration patterns provides valuable complementary information to MS data, offering insights that might be missed in direct IEF-MS workflows.
Compatibility with emerging MS technologies also differs between these approaches. IEF shows superior integration potential with newer MS techniques like ion mobility spectrometry and data-independent acquisition methods, while 2-DE better complements traditional data-dependent acquisition workflows where targeted analysis of specific protein spots is desired.
2-DE-MS workflows typically involve spot excision from gels after protein separation, followed by in-gel digestion and subsequent MS analysis. This approach benefits from the high-resolution separation of complex protein mixtures before MS analysis, reducing sample complexity and improving the detection of low-abundance proteins. However, the workflow requires additional sample handling steps that may introduce contamination and protein loss, potentially affecting downstream MS sensitivity.
In contrast, IEF-MS integration offers more streamlined workflows, particularly when using specialized formats like OFFGEL fractionation or IPG strip-based approaches. These methods allow for direct collection of protein fractions in solution, facilitating easier transition to MS analysis without the need for extraction from gel matrices. The liquid-phase fractions from IEF can be directly processed for MS analysis, minimizing sample loss and improving recovery rates by 15-30% compared to 2-DE gel extraction methods.
Recent technological advancements have introduced automated systems that integrate IEF separation with online MS analysis, reducing manual handling and improving reproducibility. These systems demonstrate a 40% reduction in analysis time compared to traditional 2-DE-MS workflows, while maintaining comparable or superior protein identification rates.
Quantitative proteomics particularly benefits from the IEF-MS integration, as techniques like iTRAQ and TMT labeling can be more effectively implemented with the solution-phase fractions obtained from IEF. Studies indicate that IEF-MS workflows typically identify 20-25% more proteins than equivalent 2-DE-MS approaches when analyzing complex biological samples.
However, 2-DE-MS integration maintains certain advantages, particularly in visualizing protein modifications and isoforms before MS analysis. The ability to detect post-translational modifications through shifts in gel migration patterns provides valuable complementary information to MS data, offering insights that might be missed in direct IEF-MS workflows.
Compatibility with emerging MS technologies also differs between these approaches. IEF shows superior integration potential with newer MS techniques like ion mobility spectrometry and data-independent acquisition methods, while 2-DE better complements traditional data-dependent acquisition workflows where targeted analysis of specific protein spots is desired.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!