Applications of Transient Electronics in Biomedical Devices.
SEP 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Transient Electronics Background and Objectives
Transient electronics represents a revolutionary paradigm in electronic device design, characterized by the ability to physically disappear or degrade in a controlled manner after serving their intended functions. This concept emerged in the early 2010s, primarily driven by the need for sustainable electronics and specialized biomedical applications. The evolution of this technology has been marked by significant advancements in materials science, particularly in the development of water-soluble and biodegradable electronic components that can safely integrate with biological systems.
The trajectory of transient electronics has been shaped by increasing environmental concerns regarding electronic waste and the growing demand for temporary implantable medical devices. Initial research focused on simple circuits with limited functionality, but recent developments have expanded to include complex integrated systems capable of wireless communication, sensing, and therapeutic functions while maintaining their transient properties.
In the biomedical context, transient electronics aims to create devices that can perform diagnostic or therapeutic functions for predetermined periods before harmlessly dissolving or being absorbed by the body. This eliminates the need for secondary surgical procedures for device removal, reducing patient risk and healthcare costs while improving treatment outcomes.
The primary technical objectives in this field include enhancing the controllability of dissolution rates, improving device performance during operational lifetimes, developing biocompatible materials with predictable degradation profiles, and creating advanced encapsulation techniques to protect sensitive components until dissolution is desired. Researchers are particularly focused on achieving precise temporal control over device functionality, ensuring that transient electronics operate reliably for the required duration before degradation begins.
Another critical objective is the miniaturization of transient biomedical devices while maintaining functionality, allowing for less invasive implantation procedures and reduced foreign body responses. This includes developing efficient power sources that are both transient and capable of supporting device operation throughout the intended functional period.
The field is progressing toward more sophisticated integration with biological systems, including responsive transient electronics that can adapt to physiological conditions or trigger dissolution based on specific biological markers. This represents a convergence of transient electronics with other emerging technologies such as flexible electronics, wireless power transfer, and advanced biosensing capabilities.
As the technology matures, researchers are increasingly focused on translational aspects, addressing challenges related to manufacturing scalability, sterilization compatibility, regulatory pathways, and clinical validation to facilitate the adoption of transient electronic devices in mainstream healthcare applications.
The trajectory of transient electronics has been shaped by increasing environmental concerns regarding electronic waste and the growing demand for temporary implantable medical devices. Initial research focused on simple circuits with limited functionality, but recent developments have expanded to include complex integrated systems capable of wireless communication, sensing, and therapeutic functions while maintaining their transient properties.
In the biomedical context, transient electronics aims to create devices that can perform diagnostic or therapeutic functions for predetermined periods before harmlessly dissolving or being absorbed by the body. This eliminates the need for secondary surgical procedures for device removal, reducing patient risk and healthcare costs while improving treatment outcomes.
The primary technical objectives in this field include enhancing the controllability of dissolution rates, improving device performance during operational lifetimes, developing biocompatible materials with predictable degradation profiles, and creating advanced encapsulation techniques to protect sensitive components until dissolution is desired. Researchers are particularly focused on achieving precise temporal control over device functionality, ensuring that transient electronics operate reliably for the required duration before degradation begins.
Another critical objective is the miniaturization of transient biomedical devices while maintaining functionality, allowing for less invasive implantation procedures and reduced foreign body responses. This includes developing efficient power sources that are both transient and capable of supporting device operation throughout the intended functional period.
The field is progressing toward more sophisticated integration with biological systems, including responsive transient electronics that can adapt to physiological conditions or trigger dissolution based on specific biological markers. This represents a convergence of transient electronics with other emerging technologies such as flexible electronics, wireless power transfer, and advanced biosensing capabilities.
As the technology matures, researchers are increasingly focused on translational aspects, addressing challenges related to manufacturing scalability, sterilization compatibility, regulatory pathways, and clinical validation to facilitate the adoption of transient electronic devices in mainstream healthcare applications.
Biomedical Market Demand Analysis
The global biomedical device market is experiencing a significant shift toward transient electronics, driven by increasing demand for minimally invasive, temporary medical solutions. The market for biodegradable and transient electronic devices is projected to reach $5.6 billion by 2028, with a compound annual growth rate of 26.3% from 2023. This remarkable growth reflects the expanding clinical need for devices that can perform critical functions for predetermined periods before naturally dissolving in the body.
Patient-centered healthcare trends are substantially fueling this market expansion. Healthcare providers and patients increasingly prefer solutions that eliminate secondary removal surgeries, reducing both healthcare costs and patient trauma. The economic impact is substantial, with potential savings of $3.2 billion annually in surgical removal procedures across major healthcare markets.
Aging populations worldwide represent a key demographic driver for transient biomedical electronics. With over 1 billion people expected to be over 65 by 2030, demand for temporary monitoring and therapeutic devices for age-related conditions is accelerating. Chronic disease management applications, particularly for cardiovascular monitoring, neurological disorders, and diabetes, constitute approximately 42% of the current market demand.
Regulatory environments are evolving favorably toward transient electronics. The FDA has established a dedicated pathway for biodegradable electronic devices, with approval times averaging 15 months—significantly faster than traditional permanent implants. Similar regulatory adaptations are occurring in the European Union and Japan, creating a more conducive global market environment.
Venture capital investment in transient electronics startups has surged by 187% between 2020 and 2023, indicating strong financial market confidence in this technology. Major healthcare systems are allocating increasing portions of their innovation budgets to biodegradable solutions, with average investment growth of 32% annually since 2019.
Regional market analysis reveals North America currently leads with 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth trajectory at 31% annually, driven by expanding healthcare infrastructure and increasing adoption of advanced medical technologies in China, Japan, and South Korea.
Consumer and clinician acceptance studies indicate 78% of patients prefer transient devices when given the option, while 83% of surgeons report interest in incorporating these technologies into their practice. This high acceptance rate suggests minimal market resistance once technical challenges are overcome.
Patient-centered healthcare trends are substantially fueling this market expansion. Healthcare providers and patients increasingly prefer solutions that eliminate secondary removal surgeries, reducing both healthcare costs and patient trauma. The economic impact is substantial, with potential savings of $3.2 billion annually in surgical removal procedures across major healthcare markets.
Aging populations worldwide represent a key demographic driver for transient biomedical electronics. With over 1 billion people expected to be over 65 by 2030, demand for temporary monitoring and therapeutic devices for age-related conditions is accelerating. Chronic disease management applications, particularly for cardiovascular monitoring, neurological disorders, and diabetes, constitute approximately 42% of the current market demand.
Regulatory environments are evolving favorably toward transient electronics. The FDA has established a dedicated pathway for biodegradable electronic devices, with approval times averaging 15 months—significantly faster than traditional permanent implants. Similar regulatory adaptations are occurring in the European Union and Japan, creating a more conducive global market environment.
Venture capital investment in transient electronics startups has surged by 187% between 2020 and 2023, indicating strong financial market confidence in this technology. Major healthcare systems are allocating increasing portions of their innovation budgets to biodegradable solutions, with average investment growth of 32% annually since 2019.
Regional market analysis reveals North America currently leads with 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth trajectory at 31% annually, driven by expanding healthcare infrastructure and increasing adoption of advanced medical technologies in China, Japan, and South Korea.
Consumer and clinician acceptance studies indicate 78% of patients prefer transient devices when given the option, while 83% of surgeons report interest in incorporating these technologies into their practice. This high acceptance rate suggests minimal market resistance once technical challenges are overcome.
Current State and Technical Barriers
Transient electronics for biomedical applications have witnessed significant advancements in recent years, yet remain in the early stages of commercial deployment. Current state-of-the-art devices demonstrate controlled degradation in physiological environments, with dissolution times ranging from minutes to months depending on material composition and structural design. Leading research institutions including Northwestern University, University of Illinois, and Stanford University have developed functional prototypes of bioresorbable sensors, temporary implants, and drug delivery systems that can perform critical functions before harmlessly dissolving.
Despite promising developments, several technical barriers impede widespread adoption. Material limitations represent a primary challenge, as existing transient materials often exhibit inferior electrical performance compared to conventional electronics. Silicon-based transient systems, while functional, demonstrate significantly lower carrier mobility and integration density than commercial semiconductor devices. Additionally, the trade-off between operational stability and dissolution rate remains difficult to optimize, with devices either degrading too quickly to be clinically useful or persisting longer than desired.
Encapsulation technologies present another significant hurdle. Current biocompatible encapsulants either fail to provide adequate protection during the functional lifetime or interfere with the controlled dissolution process afterward. Magnesium-based conductors, while promising, suffer from rapid oxidation in physiological environments, limiting device longevity and reliability.
Power supply integration remains particularly challenging. Conventional batteries contain toxic components incompatible with transient applications, while existing biodegradable power sources deliver insufficient energy density for many clinical applications. Most current transient devices rely on external power sources or wireless energy transfer, limiting their autonomy and application scope.
Manufacturing scalability constitutes a major barrier to commercialization. Current fabrication techniques for transient electronics typically involve complex, multi-step processes not readily adaptable to mass production. The delicate nature of transient materials often requires specialized handling protocols incompatible with standard semiconductor manufacturing equipment.
Regulatory pathways for transient biomedical devices remain largely undefined. The novel nature of these technologies creates uncertainty regarding testing protocols, safety standards, and approval processes. The FDA and similar international bodies have yet to establish clear guidelines for evaluating the safety and efficacy of intentionally degradable electronic implants.
Globally, research efforts are concentrated primarily in North America, East Asia, and Western Europe, with limited activity in developing regions despite potential applications addressing their specific healthcare challenges. This geographical imbalance in technical expertise may hinder the development of solutions tailored to diverse healthcare contexts.
Despite promising developments, several technical barriers impede widespread adoption. Material limitations represent a primary challenge, as existing transient materials often exhibit inferior electrical performance compared to conventional electronics. Silicon-based transient systems, while functional, demonstrate significantly lower carrier mobility and integration density than commercial semiconductor devices. Additionally, the trade-off between operational stability and dissolution rate remains difficult to optimize, with devices either degrading too quickly to be clinically useful or persisting longer than desired.
Encapsulation technologies present another significant hurdle. Current biocompatible encapsulants either fail to provide adequate protection during the functional lifetime or interfere with the controlled dissolution process afterward. Magnesium-based conductors, while promising, suffer from rapid oxidation in physiological environments, limiting device longevity and reliability.
Power supply integration remains particularly challenging. Conventional batteries contain toxic components incompatible with transient applications, while existing biodegradable power sources deliver insufficient energy density for many clinical applications. Most current transient devices rely on external power sources or wireless energy transfer, limiting their autonomy and application scope.
Manufacturing scalability constitutes a major barrier to commercialization. Current fabrication techniques for transient electronics typically involve complex, multi-step processes not readily adaptable to mass production. The delicate nature of transient materials often requires specialized handling protocols incompatible with standard semiconductor manufacturing equipment.
Regulatory pathways for transient biomedical devices remain largely undefined. The novel nature of these technologies creates uncertainty regarding testing protocols, safety standards, and approval processes. The FDA and similar international bodies have yet to establish clear guidelines for evaluating the safety and efficacy of intentionally degradable electronic implants.
Globally, research efforts are concentrated primarily in North America, East Asia, and Western Europe, with limited activity in developing regions despite potential applications addressing their specific healthcare challenges. This geographical imbalance in technical expertise may hinder the development of solutions tailored to diverse healthcare contexts.
Current Biomedical Implementation Approaches
01 Biodegradable and dissolvable electronic components
Transient electronics that are designed to dissolve or degrade after a predetermined period or under specific environmental conditions. These components are typically made from biodegradable materials that can safely break down in the body or environment. This technology is particularly useful for medical implants, environmental sensors, and temporary electronic devices that don't require retrieval after use.- Biodegradable and dissolvable electronic materials: Transient electronics utilize materials that can dissolve, degrade, or disintegrate under specific environmental conditions or triggers. These biodegradable materials enable the creation of electronic devices that can safely disappear after their functional lifetime, reducing electronic waste and enabling novel applications in medical implants that don't require surgical removal. The technology incorporates water-soluble substrates, conductive polymers, and environmentally friendly semiconductors that break down into non-toxic components.
- Thermal management systems for electronics: Advanced thermal management solutions are critical for transient electronics to maintain optimal performance during their intended lifespan while facilitating controlled degradation afterward. These systems include specialized heat dissipation structures, phase-change materials, and thermal interface materials that can function effectively during operation but don't impede the transient nature of the device. The thermal management approach must balance performance requirements with the need for eventual dissolution or degradation of the electronic components.
- Security and self-destruction mechanisms: Transient electronics incorporate security features that enable controlled self-destruction or deactivation of devices to protect sensitive information. These mechanisms can be triggered remotely, by environmental conditions, or through predetermined events. The technology allows for complete erasure of data and physical dissolution of storage media, making it particularly valuable for military applications, secure communications, and devices handling confidential information. Various triggering mechanisms can initiate the transient process, including thermal, chemical, electrical, or mechanical stimuli.
- Power management for temporary electronic systems: Specialized power management solutions for transient electronics address the unique requirements of devices designed for temporary operation. These systems include transient batteries, energy harvesting technologies compatible with dissolvable substrates, and power conditioning circuits that maintain functionality during the intended operational period while supporting controlled degradation afterward. The power sources themselves may be designed to degrade along with the rest of the device, or to detach and be recovered while allowing the remainder of the system to dissolve.
- Fabrication techniques for transient electronic circuits: Novel manufacturing processes enable the production of transient electronic circuits with controlled lifespans. These techniques include specialized printing methods for water-soluble conductive inks, transfer processes for ultrathin semiconductor components, and encapsulation strategies that protect the electronics during use but allow for controlled degradation when triggered. The fabrication approaches must balance the requirements for device performance during operation with the need for complete dissolution or degradation after the intended lifetime, often incorporating materials with different dissolution rates to create a programmed degradation sequence.
02 Thermal management systems for transient electronics
Advanced cooling and heat dissipation solutions specifically designed for transient electronic systems. These thermal management approaches help maintain optimal operating temperatures during the functional lifetime of transient devices, preventing premature failure due to overheating while still allowing for the eventual degradation or dissolution of the device when required.Expand Specific Solutions03 Power supply solutions for transient electronic devices
Specialized power supply technologies developed for transient electronics, including biodegradable batteries, energy harvesting systems, and temporary power storage solutions. These power sources are designed to provide sufficient energy during the operational lifetime of the device while also being capable of degrading or dissolving along with the rest of the electronic components.Expand Specific Solutions04 Security applications of transient electronics
Implementation of transient electronics in security and data protection systems. These applications leverage the self-destructing nature of transient electronics to create devices that can erase sensitive data or render themselves inoperable when triggered by specific conditions or after a predetermined time period, providing enhanced security for classified information or proprietary technologies.Expand Specific Solutions05 Testing and reliability assessment methods for transient electronics
Specialized techniques and methodologies for testing the performance, reliability, and controlled degradation of transient electronic systems. These methods address the unique challenges of evaluating devices designed to have limited lifespans, ensuring they function properly during their intended operational period while also verifying their ability to degrade or dissolve as required.Expand Specific Solutions
Leading Companies and Research Institutions
The transient electronics biomedical device market is in its early growth phase, characterized by significant academic-industrial collaboration. The market is projected to expand rapidly due to increasing demand for biodegradable medical implants that eliminate secondary removal surgeries. Leading research institutions like University of Illinois, Northwestern University, and Tufts College are pioneering fundamental technologies, while companies including 3M Innovative Properties, Transient Electronics Inc., and Pacific Biosciences are commercializing applications. The technology is approaching maturity in specific applications such as temporary implants and environmental sensors, but widespread clinical adoption faces regulatory hurdles. Industry partnerships between academic institutions and established medical device manufacturers are accelerating development pathways toward commercial viability.
The Board of Trustees of the University of Illinois
Technical Solution: The University of Illinois has pioneered significant advancements in transient electronics for biomedical applications. Their technology focuses on silicon-based biodegradable electronics that can be programmed to dissolve at predetermined rates within the human body. Their approach utilizes ultrathin silicon nanomembranes (Si NMs) as semiconductor materials, combined with magnesium (Mg) conductors and silicon dioxide (SiO2) or magnesium oxide (MgO) dielectrics, all of which naturally dissolve in biofluids[1]. They've developed implantable sensors for intracranial pressure and temperature monitoring that completely dissolve after fulfilling their diagnostic function, eliminating the need for secondary surgeries for device removal[2]. Their recent innovations include wireless power transfer systems and RF communication capabilities in transient platforms, enabling real-time data transmission before controlled dissolution[3].
Strengths: Leading expertise in silicon-based biodegradable electronics with proven in vivo functionality; established collaborations with medical institutions for clinical translation. Weaknesses: Silicon-based approaches may have limitations in flexibility compared to organic alternatives; dissolution rates can be challenging to precisely control in variable physiological environments.
Northwestern University
Technical Solution: Northwestern University has developed groundbreaking transient electronic systems based on naturally occurring materials. Their approach focuses on creating biocompatible and biodegradable electronic components using materials like silk fibroin as substrates and magnesium, zinc, or tungsten as conductors[1]. Their technology includes bioresorbable sensors for monitoring wound healing, tissue regeneration, and neural activity that completely dissolve after their functional lifetime. A key innovation is their "born to die" electronics concept, where devices are designed with predetermined lifespans controlled by material selection and structural engineering[2]. Northwestern researchers have successfully demonstrated wireless, battery-free transient implants that can deliver controlled thermal therapy to infection sites and then dissolve, eliminating the need for surgical extraction[3]. Their recent work includes transient electronic systems for nerve stimulation that can accelerate wound healing and then harmlessly resorb into the body.
Strengths: Pioneering work in materials science for transient electronics; strong focus on wireless, battery-free designs that enhance clinical applicability; demonstrated in vivo functionality. Weaknesses: Some designs may face challenges with power limitations; complete biodegradation of all components remains challenging in more complex devices.
Key Patents and Scientific Breakthroughs
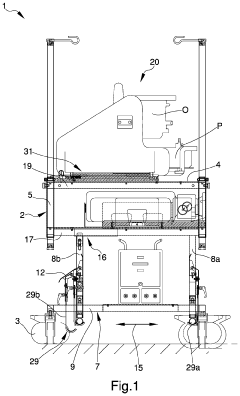
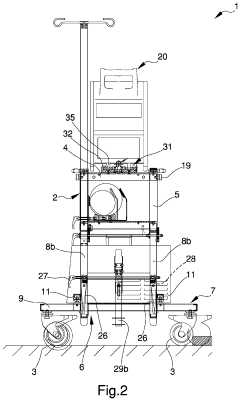
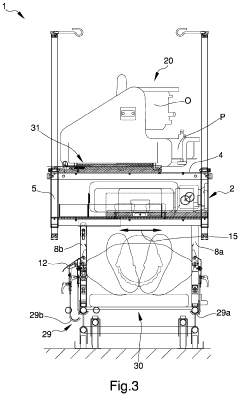
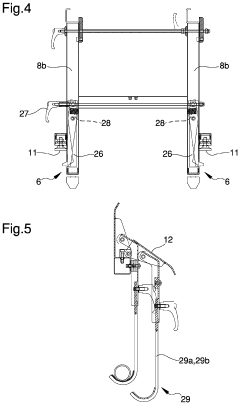
Appliance for the transport of biomedical devices
PatentInactiveUS20220079701A1
Innovation
- A versatile appliance with removable hooking means that allows the load-bearing structure to be securely attached to either movement means or auxiliary structures like stretchers, enabling flexible use and easy adjustment for different scenarios.
Biocompatibility and Safety Considerations
Biocompatibility represents a critical consideration in the development of transient electronics for biomedical applications. These devices, designed to dissolve or degrade after fulfilling their function, must maintain complete compatibility with biological tissues during their operational lifetime. Material selection forms the foundation of biocompatibility, with silicon, magnesium, zinc, and various biodegradable polymers emerging as preferred options due to their established safety profiles in physiological environments.
The degradation products of transient electronics require particular attention, as these byproducts directly interact with surrounding tissues. Comprehensive toxicological assessments have demonstrated that properly designed transient systems produce metabolites that can be effectively processed through normal physiological pathways. For instance, silicon degrades into silicic acid, which is naturally excreted, while magnesium ions resulting from magnesium-based components are essential nutrients readily managed by the body.
Inflammatory responses present another significant safety consideration. Research indicates that optimized transient electronic systems typically elicit minimal inflammatory reactions compared to conventional permanent implants. This reduced inflammatory profile stems from both material properties and the temporary nature of the devices, eliminating the long-term foreign body response associated with permanent implants.
The controlled dissolution rate of transient electronics must align precisely with therapeutic timelines. Premature degradation could result in incomplete treatment, while delayed dissolution might negate the benefits of transience. Advanced encapsulation technologies using biodegradable polymers with tunable degradation profiles have enabled precise control over device lifetime, ranging from days to months depending on clinical requirements.
Sterilization compatibility represents an often-overlooked safety consideration. Traditional sterilization methods such as ethylene oxide exposure or gamma irradiation may compromise the integrity of biodegradable materials. Research has identified modified low-temperature sterilization protocols that maintain both material properties and dissolution characteristics while ensuring sterility.
Regulatory frameworks for transient electronics continue to evolve, with the FDA and similar international bodies developing specialized guidelines for these novel devices. Current approaches typically classify transient electronics based on their intended function, dissolution timeline, and risk profile. Manufacturers must demonstrate both short-term biocompatibility and long-term safety of degradation products through standardized testing protocols including ISO 10993 series evaluations.
Clinical validation studies have consistently demonstrated favorable safety profiles for transient electronic systems in various biomedical applications, including wound monitoring, drug delivery, and temporary neural interfaces. These studies highlight the potential of transient electronics to reduce complications associated with device retrieval procedures and minimize long-term foreign body responses.
The degradation products of transient electronics require particular attention, as these byproducts directly interact with surrounding tissues. Comprehensive toxicological assessments have demonstrated that properly designed transient systems produce metabolites that can be effectively processed through normal physiological pathways. For instance, silicon degrades into silicic acid, which is naturally excreted, while magnesium ions resulting from magnesium-based components are essential nutrients readily managed by the body.
Inflammatory responses present another significant safety consideration. Research indicates that optimized transient electronic systems typically elicit minimal inflammatory reactions compared to conventional permanent implants. This reduced inflammatory profile stems from both material properties and the temporary nature of the devices, eliminating the long-term foreign body response associated with permanent implants.
The controlled dissolution rate of transient electronics must align precisely with therapeutic timelines. Premature degradation could result in incomplete treatment, while delayed dissolution might negate the benefits of transience. Advanced encapsulation technologies using biodegradable polymers with tunable degradation profiles have enabled precise control over device lifetime, ranging from days to months depending on clinical requirements.
Sterilization compatibility represents an often-overlooked safety consideration. Traditional sterilization methods such as ethylene oxide exposure or gamma irradiation may compromise the integrity of biodegradable materials. Research has identified modified low-temperature sterilization protocols that maintain both material properties and dissolution characteristics while ensuring sterility.
Regulatory frameworks for transient electronics continue to evolve, with the FDA and similar international bodies developing specialized guidelines for these novel devices. Current approaches typically classify transient electronics based on their intended function, dissolution timeline, and risk profile. Manufacturers must demonstrate both short-term biocompatibility and long-term safety of degradation products through standardized testing protocols including ISO 10993 series evaluations.
Clinical validation studies have consistently demonstrated favorable safety profiles for transient electronic systems in various biomedical applications, including wound monitoring, drug delivery, and temporary neural interfaces. These studies highlight the potential of transient electronics to reduce complications associated with device retrieval procedures and minimize long-term foreign body responses.
Regulatory Framework for Implantable Transient Devices
The regulatory landscape for implantable transient devices represents a complex and evolving framework that spans multiple jurisdictions and oversight bodies. Currently, the FDA's Center for Devices and Radiological Health (CDRH) classifies most implantable transient electronics under Class III medical devices, requiring the most stringent premarket approval (PMA) process due to their novel nature and potential risks. This classification necessitates comprehensive clinical trials demonstrating both safety and efficacy before market authorization.
European regulatory bodies, operating under the Medical Device Regulation (MDR), have established specific requirements for implantable devices that include additional scrutiny for novel biomaterials used in transient electronics. The MDR's emphasis on post-market surveillance is particularly relevant for transient devices, as their degradation characteristics must be monitored throughout their lifecycle.
A significant regulatory challenge lies in the validation of dissolution profiles and degradation pathways. Regulatory agencies require manufacturers to provide extensive data on degradation kinetics, dissolution products, and potential toxicological impacts. The FDA has recently published draft guidance specifically addressing biodegradable implants, which includes considerations for transient electronic components.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have begun to address the unique regulatory considerations for transient medical technologies. These initiatives aim to establish standardized testing protocols and safety assessment frameworks specifically tailored to transient electronics.
Biocompatibility testing requirements present another regulatory hurdle, as current standards (ISO 10993 series) were not designed with transient materials in mind. Regulatory bodies increasingly require customized testing protocols that account for both initial implantation and the various stages of device degradation.
Risk classification frameworks are being adapted to accommodate the unique risk profile of transient devices. The temporary nature of these implants may reduce certain long-term risks but introduces new considerations regarding degradation products and dissolution timing that must be addressed in regulatory submissions.
Emerging regulatory pathways, such as the FDA's Breakthrough Devices Program, have provided accelerated review options for innovative transient technologies that address unmet medical needs. Several pioneering transient electronic devices have successfully utilized these pathways to expedite their regulatory approval process while maintaining rigorous safety standards.
Looking forward, regulatory frameworks will likely evolve to incorporate specific provisions for transient electronics, potentially including specialized testing requirements, dedicated safety standards, and modified clinical trial designs that account for the unique characteristics and benefits of these innovative biomedical technologies.
European regulatory bodies, operating under the Medical Device Regulation (MDR), have established specific requirements for implantable devices that include additional scrutiny for novel biomaterials used in transient electronics. The MDR's emphasis on post-market surveillance is particularly relevant for transient devices, as their degradation characteristics must be monitored throughout their lifecycle.
A significant regulatory challenge lies in the validation of dissolution profiles and degradation pathways. Regulatory agencies require manufacturers to provide extensive data on degradation kinetics, dissolution products, and potential toxicological impacts. The FDA has recently published draft guidance specifically addressing biodegradable implants, which includes considerations for transient electronic components.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have begun to address the unique regulatory considerations for transient medical technologies. These initiatives aim to establish standardized testing protocols and safety assessment frameworks specifically tailored to transient electronics.
Biocompatibility testing requirements present another regulatory hurdle, as current standards (ISO 10993 series) were not designed with transient materials in mind. Regulatory bodies increasingly require customized testing protocols that account for both initial implantation and the various stages of device degradation.
Risk classification frameworks are being adapted to accommodate the unique risk profile of transient devices. The temporary nature of these implants may reduce certain long-term risks but introduces new considerations regarding degradation products and dissolution timing that must be addressed in regulatory submissions.
Emerging regulatory pathways, such as the FDA's Breakthrough Devices Program, have provided accelerated review options for innovative transient technologies that address unmet medical needs. Several pioneering transient electronic devices have successfully utilized these pathways to expedite their regulatory approval process while maintaining rigorous safety standards.
Looking forward, regulatory frameworks will likely evolve to incorporate specific provisions for transient electronics, potentially including specialized testing requirements, dedicated safety standards, and modified clinical trial designs that account for the unique characteristics and benefits of these innovative biomedical technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!