How Transient Electronics Enable Temporary Healthcare Monitoring?
SEP 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Transient Electronics Evolution and Objectives
Transient electronics represent a revolutionary paradigm shift in electronic device design, moving away from the traditional emphasis on durability and longevity toward intentionally temporary functionality. This emerging field has evolved significantly over the past decade, transitioning from theoretical concepts to practical applications, particularly in healthcare monitoring systems where temporary deployment offers unique advantages.
The evolution of transient electronics began in the early 2000s with fundamental research into biodegradable materials that could serve as substrates and conductors. By 2010, researchers had demonstrated simple circuits capable of dissolving in controlled environments. The watershed moment came in 2012 when Rogers et al. published groundbreaking work on silicon-based transient electronics that could dissolve completely in biofluids, establishing the foundation for medical applications.
Subsequent developments focused on improving dissolution control mechanisms, enhancing performance metrics, and expanding the range of compatible materials. Between 2015 and 2020, significant advancements occurred in creating transient power sources, including biodegradable batteries and energy harvesting systems, which addressed one of the field's major limitations. Recent innovations have concentrated on wireless capabilities and improved sensor technologies that maintain accuracy while preserving transient properties.
The primary objective of transient electronics in healthcare monitoring is to provide accurate physiological data collection without the drawbacks of permanent implantation. These systems aim to eliminate secondary surgical procedures for device removal, reducing patient discomfort, infection risks, and healthcare costs. Additionally, they seek to minimize biocompatibility concerns and long-term foreign body responses that plague conventional implantable electronics.
Technical objectives include developing materials with precisely controllable dissolution rates that can be triggered by specific environmental conditions or external stimuli. Engineers strive to achieve performance comparable to conventional electronics during the functional lifetime while ensuring complete dissolution afterward without leaving harmful residues. Power management represents another critical objective, as transient systems must balance energy requirements with size constraints and dissolution capabilities.
From a clinical perspective, transient healthcare monitoring systems target applications where temporary data collection provides sufficient diagnostic value, such as post-surgical recovery monitoring, acute condition assessment, and therapeutic efficacy evaluation. The technology aims to bridge the gap between external wearable devices and permanent implants, offering an intermediate solution that combines the advantages of both approaches while minimizing their respective limitations.
As the field continues to mature, researchers are increasingly focused on scalable manufacturing processes that can transition these technologies from laboratory demonstrations to commercially viable products, representing the next frontier in transient electronics evolution.
The evolution of transient electronics began in the early 2000s with fundamental research into biodegradable materials that could serve as substrates and conductors. By 2010, researchers had demonstrated simple circuits capable of dissolving in controlled environments. The watershed moment came in 2012 when Rogers et al. published groundbreaking work on silicon-based transient electronics that could dissolve completely in biofluids, establishing the foundation for medical applications.
Subsequent developments focused on improving dissolution control mechanisms, enhancing performance metrics, and expanding the range of compatible materials. Between 2015 and 2020, significant advancements occurred in creating transient power sources, including biodegradable batteries and energy harvesting systems, which addressed one of the field's major limitations. Recent innovations have concentrated on wireless capabilities and improved sensor technologies that maintain accuracy while preserving transient properties.
The primary objective of transient electronics in healthcare monitoring is to provide accurate physiological data collection without the drawbacks of permanent implantation. These systems aim to eliminate secondary surgical procedures for device removal, reducing patient discomfort, infection risks, and healthcare costs. Additionally, they seek to minimize biocompatibility concerns and long-term foreign body responses that plague conventional implantable electronics.
Technical objectives include developing materials with precisely controllable dissolution rates that can be triggered by specific environmental conditions or external stimuli. Engineers strive to achieve performance comparable to conventional electronics during the functional lifetime while ensuring complete dissolution afterward without leaving harmful residues. Power management represents another critical objective, as transient systems must balance energy requirements with size constraints and dissolution capabilities.
From a clinical perspective, transient healthcare monitoring systems target applications where temporary data collection provides sufficient diagnostic value, such as post-surgical recovery monitoring, acute condition assessment, and therapeutic efficacy evaluation. The technology aims to bridge the gap between external wearable devices and permanent implants, offering an intermediate solution that combines the advantages of both approaches while minimizing their respective limitations.
As the field continues to mature, researchers are increasingly focused on scalable manufacturing processes that can transition these technologies from laboratory demonstrations to commercially viable products, representing the next frontier in transient electronics evolution.
Healthcare Monitoring Market Demand Analysis
The global healthcare monitoring market is experiencing unprecedented growth, driven by the aging population, increasing prevalence of chronic diseases, and technological advancements. The market for temporary healthcare monitoring solutions specifically has emerged as a rapidly expanding segment, with a projected compound annual growth rate of 8.7% from 2023 to 2030. This growth is particularly evident in developed regions such as North America and Europe, where healthcare infrastructure supports advanced monitoring technologies.
Consumer demand for less invasive, more comfortable monitoring options has created significant market opportunities for transient electronics in healthcare. Traditional monitoring devices often cause discomfort during extended use, leading to patient non-compliance. Market research indicates that approximately 40% of patients report discomfort with conventional monitoring devices, highlighting a substantial need for temporary alternatives that can dissolve or degrade after fulfilling their function.
The post-pandemic healthcare landscape has accelerated the adoption of remote patient monitoring solutions, with hospitals and healthcare providers seeking efficient ways to monitor patients outside traditional clinical settings. This shift has expanded the potential market for transient electronics, as these technologies enable continuous monitoring without requiring frequent hospital visits for device removal or maintenance.
Clinical applications driving market demand include post-surgical monitoring, maternal-fetal health tracking, and temporary monitoring of patients with acute conditions. The pediatric monitoring segment shows particularly strong growth potential, as transient electronics offer significant advantages for monitoring infants and children who may be prone to removing conventional devices or experiencing skin irritation from long-term adhesives.
Insurance reimbursement trends are increasingly favorable toward temporary monitoring solutions that demonstrate cost-effectiveness through reduced hospital readmissions and complications. Market analysis reveals that healthcare providers can achieve cost savings of up to 30% by implementing appropriate temporary monitoring solutions for eligible patients, creating strong economic incentives for adoption.
Regulatory pathways for temporary healthcare monitoring devices are becoming more defined, reducing market entry barriers. The FDA's breakthrough device designation program has accelerated approval for several innovative monitoring technologies, signaling regulatory recognition of the clinical importance of this market segment.
Consumer willingness to pay for enhanced comfort and convenience in healthcare monitoring has created premium market opportunities, particularly in direct-to-consumer applications such as fitness tracking and preventive health monitoring. Market surveys indicate that consumers are willing to pay 15-25% more for monitoring solutions that offer superior comfort and eliminate the need for device removal procedures.
Consumer demand for less invasive, more comfortable monitoring options has created significant market opportunities for transient electronics in healthcare. Traditional monitoring devices often cause discomfort during extended use, leading to patient non-compliance. Market research indicates that approximately 40% of patients report discomfort with conventional monitoring devices, highlighting a substantial need for temporary alternatives that can dissolve or degrade after fulfilling their function.
The post-pandemic healthcare landscape has accelerated the adoption of remote patient monitoring solutions, with hospitals and healthcare providers seeking efficient ways to monitor patients outside traditional clinical settings. This shift has expanded the potential market for transient electronics, as these technologies enable continuous monitoring without requiring frequent hospital visits for device removal or maintenance.
Clinical applications driving market demand include post-surgical monitoring, maternal-fetal health tracking, and temporary monitoring of patients with acute conditions. The pediatric monitoring segment shows particularly strong growth potential, as transient electronics offer significant advantages for monitoring infants and children who may be prone to removing conventional devices or experiencing skin irritation from long-term adhesives.
Insurance reimbursement trends are increasingly favorable toward temporary monitoring solutions that demonstrate cost-effectiveness through reduced hospital readmissions and complications. Market analysis reveals that healthcare providers can achieve cost savings of up to 30% by implementing appropriate temporary monitoring solutions for eligible patients, creating strong economic incentives for adoption.
Regulatory pathways for temporary healthcare monitoring devices are becoming more defined, reducing market entry barriers. The FDA's breakthrough device designation program has accelerated approval for several innovative monitoring technologies, signaling regulatory recognition of the clinical importance of this market segment.
Consumer willingness to pay for enhanced comfort and convenience in healthcare monitoring has created premium market opportunities, particularly in direct-to-consumer applications such as fitness tracking and preventive health monitoring. Market surveys indicate that consumers are willing to pay 15-25% more for monitoring solutions that offer superior comfort and eliminate the need for device removal procedures.
Current State and Barriers in Transient Electronics
Transient electronics represent a revolutionary paradigm in healthcare monitoring, with significant advancements achieved in recent years. Currently, these systems can successfully integrate biodegradable substrates, conductive materials, and sensing components to create devices that maintain functionality for predetermined periods before harmlessly dissolving. Leading research institutions have demonstrated working prototypes capable of monitoring vital signs, wound healing progress, and neurological activity with clinically relevant accuracy.
Despite these achievements, several critical barriers impede widespread adoption. Material limitations constitute a primary challenge, as existing biodegradable conductors exhibit lower performance compared to conventional electronics. Current transient materials struggle to achieve the optimal balance between functional stability during operation and complete dissolution afterward. Conductivity degradation over time remains problematic, affecting signal quality and measurement reliability in extended monitoring scenarios.
Technical integration challenges persist in combining transient components with power sources and data transmission systems. While progress has been made in biodegradable batteries and energy harvesting solutions, these power systems often represent the weakest link in the dissolution chain, with limited capacity and inconsistent degradation profiles. Similarly, wireless communication modules that can completely dissolve remain in early development stages.
Biocompatibility and safety concerns present another significant hurdle. Though individual components may demonstrate biocompatibility in isolation, the degradation byproducts of complete systems require extensive toxicological evaluation. Regulatory frameworks for transient medical devices remain underdeveloped, creating uncertainty in approval pathways and slowing clinical translation.
Manufacturing scalability represents a substantial barrier to commercialization. Current fabrication techniques for transient electronics primarily rely on laboratory-scale processes that are difficult to scale industrially while maintaining precise control over dissolution timing and functional performance. The absence of standardized manufacturing protocols increases production costs and limits accessibility.
Geographical distribution of research expertise shows concentration in North America, East Asia, and Western Europe, with limited development in other regions. This concentration potentially restricts diverse application perspectives and slows global adoption. Interdisciplinary collaboration between materials scientists, electrical engineers, and medical professionals remains insufficient, creating knowledge silos that impede holistic solutions.
Performance reliability under variable physiological conditions constitutes another significant challenge. Transient systems must maintain consistent functionality across different body locations, activity levels, and environmental factors such as humidity and temperature. Current solutions demonstrate sensitivity to these variables, affecting measurement accuracy and dissolution predictability in real-world healthcare settings.
Despite these achievements, several critical barriers impede widespread adoption. Material limitations constitute a primary challenge, as existing biodegradable conductors exhibit lower performance compared to conventional electronics. Current transient materials struggle to achieve the optimal balance between functional stability during operation and complete dissolution afterward. Conductivity degradation over time remains problematic, affecting signal quality and measurement reliability in extended monitoring scenarios.
Technical integration challenges persist in combining transient components with power sources and data transmission systems. While progress has been made in biodegradable batteries and energy harvesting solutions, these power systems often represent the weakest link in the dissolution chain, with limited capacity and inconsistent degradation profiles. Similarly, wireless communication modules that can completely dissolve remain in early development stages.
Biocompatibility and safety concerns present another significant hurdle. Though individual components may demonstrate biocompatibility in isolation, the degradation byproducts of complete systems require extensive toxicological evaluation. Regulatory frameworks for transient medical devices remain underdeveloped, creating uncertainty in approval pathways and slowing clinical translation.
Manufacturing scalability represents a substantial barrier to commercialization. Current fabrication techniques for transient electronics primarily rely on laboratory-scale processes that are difficult to scale industrially while maintaining precise control over dissolution timing and functional performance. The absence of standardized manufacturing protocols increases production costs and limits accessibility.
Geographical distribution of research expertise shows concentration in North America, East Asia, and Western Europe, with limited development in other regions. This concentration potentially restricts diverse application perspectives and slows global adoption. Interdisciplinary collaboration between materials scientists, electrical engineers, and medical professionals remains insufficient, creating knowledge silos that impede holistic solutions.
Performance reliability under variable physiological conditions constitutes another significant challenge. Transient systems must maintain consistent functionality across different body locations, activity levels, and environmental factors such as humidity and temperature. Current solutions demonstrate sensitivity to these variables, affecting measurement accuracy and dissolution predictability in real-world healthcare settings.
Existing Transient Electronic Solutions for Healthcare
01 Biodegradable electronic components
Transient electronics can be designed with biodegradable components that naturally dissolve or degrade after a predetermined period. These components are typically made from materials such as silk, magnesium, silicon, or zinc oxide that break down in specific environments like water or bodily fluids. This approach enables the creation of temporary electronic devices that can be used for medical implants, environmental monitoring, or security applications without requiring retrieval after their functional lifetime.- Biodegradable and dissolvable electronic components: Transient electronics can be designed with biodegradable or water-soluble materials that naturally break down after a predetermined period. These components are engineered to dissolve or degrade in specific environments, such as bodily fluids or environmental conditions, making them ideal for temporary medical implants or environmental sensors that don't require retrieval after use. The materials used include water-soluble polymers, silk fibroin substrates, and magnesium-based conductors that can safely disappear without leaving harmful residues.
- Controlled degradation mechanisms for temporary electronics: Various mechanisms can be employed to control the degradation timeline of transient electronics. These include engineered trigger systems that respond to specific stimuli such as heat, light, electrical signals, or chemical exposure. By incorporating these mechanisms, electronic devices can be designed to function for a precise duration before self-destructing or becoming inoperable. This controlled degradation is particularly valuable for security applications, one-time use devices, and applications where device retrieval is impractical.
- Substrate technologies for temporary electronic systems: Specialized substrate materials form the foundation of transient electronic systems. These substrates can be made from thin, flexible polymers, bioresorbable materials, or specially treated papers that support electronic components while maintaining the temporary nature of the device. Some substrates are designed to physically deform, crack, or separate under specific conditions, rendering the electronic circuits non-functional after their intended use period. The substrate design often determines the overall flexibility, durability during operational life, and degradation characteristics of the transient device.
- Power sources for transient electronic devices: Temporary electronic systems require specialized power sources that align with their transient nature. These include biodegradable batteries, capacitors with limited charge cycles, or energy harvesting systems designed to function for predetermined periods. Some transient power sources utilize electrolytes that deplete or change composition over time, naturally limiting the device lifespan. Others may incorporate external triggering mechanisms that can disable the power supply when the device's useful life should end, ensuring the entire system becomes inoperative.
- Applications of temporary electronic systems: Transient electronics find applications across multiple fields where temporary functionality is advantageous. In medicine, they serve as dissolvable implants for post-surgical monitoring or controlled drug delivery that eliminate the need for removal procedures. In environmental monitoring, they provide degradable sensors that don't contribute to electronic waste. Military and security applications utilize self-destructing devices that safeguard sensitive information. Consumer electronics benefit from planned obsolescence or recyclable components that reduce electronic waste. These applications leverage the temporary nature of transient electronics to solve problems where permanent devices would be impractical or undesirable.
02 Controlled dissolution mechanisms
Various mechanisms can be employed to control the dissolution rate and timing of transient electronics. These include encapsulation layers with programmable degradation rates, trigger mechanisms that respond to specific stimuli (such as pH changes, temperature, or electrical signals), and structural designs that facilitate controlled breakdown. By engineering these dissolution mechanisms, the lifespan of temporary electronic devices can be precisely tailored to match application requirements.Expand Specific Solutions03 Substrate technologies for temporary electronics
Specialized substrate materials play a crucial role in transient electronics by providing temporary mechanical support while maintaining the ability to disintegrate when needed. These substrates can be made from water-soluble polymers, thermally responsive materials, or mechanically transformable structures. The substrate design influences the overall flexibility, conformability, and dissolution characteristics of the temporary electronic device, making it suitable for applications like wearable health monitors or disposable consumer electronics.Expand Specific Solutions04 Power sources for transient devices
Temporary electronic systems require specialized power sources that can maintain functionality during the operational period while also adhering to the transient nature of the device. These power solutions include biodegradable batteries, energy harvesting systems that collect power from the environment, and temporary storage mechanisms. The power sources are designed to either degrade along with the rest of the device or maintain minimal environmental impact after the device's functional lifetime has ended.Expand Specific Solutions05 Fabrication techniques for temporary electronics
Specialized manufacturing processes have been developed to create transient electronic systems while maintaining performance comparable to conventional electronics. These techniques include transfer printing of thin semiconductor materials, additive manufacturing with dissolvable materials, and hybrid integration approaches that combine permanent and temporary components. The fabrication methods focus on creating ultrathin, flexible circuits that can maintain functionality for the required period before degrading in a controlled manner.Expand Specific Solutions
Leading Companies in Temporary Healthcare Monitoring
Transient electronics for temporary healthcare monitoring is evolving rapidly, currently transitioning from early development to commercial application phases. The market is projected to grow significantly, driven by increasing demand for non-invasive, biodegradable monitoring solutions. While still emerging, the technology shows promising maturity levels with key players advancing different aspects of implementation. Companies like Medtronic and DexCom are leveraging their medical device expertise to develop dissolvable sensors, while Qualcomm and Siemens contribute connectivity solutions. Academic institutions including MIT, Case Western Reserve, and Wisconsin Alumni Research Foundation are pioneering fundamental research. The competitive landscape features established medical technology corporations collaborating with research institutions, creating a dynamic ecosystem that balances innovation with practical healthcare applications.
Medtronic, Inc.
Technical Solution: Medtronic has developed transient electronic systems for temporary cardiac monitoring that utilize bioresorbable materials and advanced miniaturization techniques. Their platform incorporates dissolvable sensors that can be placed directly on cardiac tissue during recovery periods after heart surgery, monitoring parameters such as temperature, pH, and electrical activity before naturally dissolving within 2-3 weeks[1]. The company's proprietary technology employs magnesium-based circuits with silicon dioxide insulation layers of varying thicknesses to control dissolution rates in bodily fluids[2]. Medtronic's systems feature wireless power transfer capabilities, eliminating the need for batteries in some applications, and utilize near-field communication for data transmission to external monitoring devices. Their latest innovations include drug-eluting capabilities combined with monitoring functions, allowing therapeutic agents to be released while simultaneously tracking physiological responses[3]. This approach has shown particular promise for post-operative monitoring of transplant patients, where temporary monitoring is critical but removal surgeries present significant risks.
Strengths: Extensive clinical testing infrastructure; established regulatory expertise; integration capabilities with existing healthcare monitoring systems; strong distribution channels in healthcare settings. Weaknesses: Higher cost compared to traditional temporary monitoring solutions; limited battery life for fully implantable versions; dissolution timing variability depending on individual patient physiology.
Koninklijke Philips NV
Technical Solution: Philips has developed a comprehensive transient electronics platform for temporary patient monitoring called "Dissolve-Sense" that integrates with their broader healthcare ecosystem. Their technology employs ultrathin, flexible circuits fabricated on water-soluble substrates with carefully engineered dissolution rates matched to specific clinical monitoring needs[1]. The system features biodegradable sensors capable of monitoring vital signs, including ECG, temperature, and blood oxygen levels, with a programmable lifespan ranging from 24 hours to 3 weeks depending on the application. Philips' innovation includes a hybrid approach where critical components like wireless transmitters can be easily detached and reused while sensor elements safely dissolve[2]. Their platform incorporates proprietary low-power wireless protocols that extend functional monitoring time while maintaining continuous data transmission to Philips' healthcare informatics systems. Recent advancements include integration with AI-based predictive analytics that can identify deteriorating patient conditions based on subtle changes in monitored parameters[3]. The company has successfully deployed these systems in post-surgical recovery monitoring and for patients transitioning from hospital to home care settings.
Strengths: Seamless integration with existing hospital monitoring infrastructure; scalable manufacturing capabilities; comprehensive data analytics platform; established global distribution network. Weaknesses: Higher initial implementation costs; requires compatible Philips systems for full functionality; more complex than single-purpose monitoring solutions.
Key Patents and Innovations in Biodegradable Electronics
Medical methods and systems incorporating wireless monitoring
PatentInactiveUS7676268B2
Innovation
- The use of wireless transponder-type markers, such as LC resonant circuits, which are implanted in the body and excited by an external signal to generate detectable electromagnetic energy, allowing for the collection of positional information over time, enabling tracking of cardiac dimensions and hemodynamic responses, and aiding in the navigation and positioning of medical devices.
Marker monitoring via a medical device
PatentWO2019051018A1
Innovation
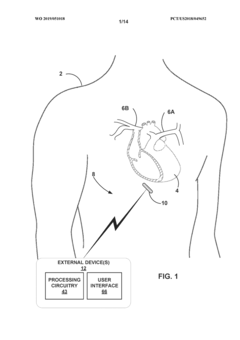
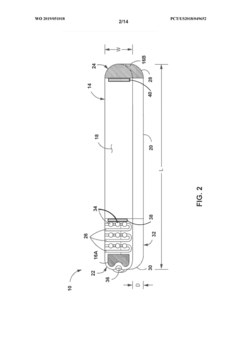
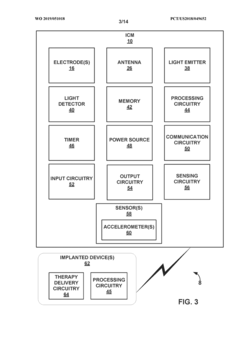
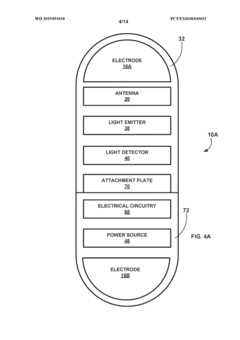
- A medical system comprising an implantable device with a light emitter and detector that tracks fluorescent markers, allowing for real-time monitoring of medication presence and physiological functions, enabling improved patient care and treatment adjustments.
Biocompatibility and Safety Considerations
Biocompatibility represents a critical consideration in the development of transient electronics for healthcare monitoring. These devices, designed to operate for predetermined periods before dissolving or degrading, must interact with human tissue without triggering adverse immune responses or inflammation. Materials selection forms the cornerstone of biocompatibility, with silicon, magnesium, zinc, and various biodegradable polymers emerging as preferred options due to their established safety profiles and controlled degradation characteristics.
The degradation products of transient electronics require thorough toxicological assessment to ensure they do not accumulate in vital organs or disrupt normal physiological functions. Recent studies have demonstrated that silicon-based transient devices produce primarily silicic acid during degradation, a compound naturally present in the human body and safely excreted through renal pathways. Similarly, magnesium-based components generate magnesium ions and hydrogen gas in quantities typically below clinically significant thresholds.
Surface modifications and encapsulation strategies have proven effective in enhancing biocompatibility while maintaining the transient functionality of these devices. Techniques such as atomic layer deposition of biocompatible oxides and application of silk fibroin coatings can modulate the dissolution rate while creating a more favorable interface with biological tissues. These approaches minimize foreign body responses while preserving the intended operational lifetime of the monitoring systems.
Sterilization presents unique challenges for transient electronics, as conventional methods like autoclave sterilization or ethylene oxide exposure may compromise the degradation mechanisms or alter material properties. Research indicates that gamma irradiation and low-temperature plasma sterilization offer viable alternatives that maintain device integrity while ensuring microbial safety for patient use.
Long-term safety monitoring protocols must be established for transient healthcare devices, particularly for those intended for implantation or prolonged skin contact. This includes comprehensive biocompatibility testing according to ISO 10993 standards, with specific attention to cytotoxicity, sensitization, and systemic toxicity evaluations. Additionally, real-time monitoring of local tissue responses through biomarkers can provide valuable safety data during clinical implementation.
Regulatory frameworks for transient electronics continue to evolve, with agencies like the FDA developing specialized guidance for these novel technologies. Current approaches typically require manufacturers to demonstrate both the safety of the intact device and its degradation products, along with validation of the predicted dissolution timeline under physiological conditions. This dual-focus assessment ensures patient safety throughout the entire lifecycle of the transient monitoring system.
The degradation products of transient electronics require thorough toxicological assessment to ensure they do not accumulate in vital organs or disrupt normal physiological functions. Recent studies have demonstrated that silicon-based transient devices produce primarily silicic acid during degradation, a compound naturally present in the human body and safely excreted through renal pathways. Similarly, magnesium-based components generate magnesium ions and hydrogen gas in quantities typically below clinically significant thresholds.
Surface modifications and encapsulation strategies have proven effective in enhancing biocompatibility while maintaining the transient functionality of these devices. Techniques such as atomic layer deposition of biocompatible oxides and application of silk fibroin coatings can modulate the dissolution rate while creating a more favorable interface with biological tissues. These approaches minimize foreign body responses while preserving the intended operational lifetime of the monitoring systems.
Sterilization presents unique challenges for transient electronics, as conventional methods like autoclave sterilization or ethylene oxide exposure may compromise the degradation mechanisms or alter material properties. Research indicates that gamma irradiation and low-temperature plasma sterilization offer viable alternatives that maintain device integrity while ensuring microbial safety for patient use.
Long-term safety monitoring protocols must be established for transient healthcare devices, particularly for those intended for implantation or prolonged skin contact. This includes comprehensive biocompatibility testing according to ISO 10993 standards, with specific attention to cytotoxicity, sensitization, and systemic toxicity evaluations. Additionally, real-time monitoring of local tissue responses through biomarkers can provide valuable safety data during clinical implementation.
Regulatory frameworks for transient electronics continue to evolve, with agencies like the FDA developing specialized guidance for these novel technologies. Current approaches typically require manufacturers to demonstrate both the safety of the intact device and its degradation products, along with validation of the predicted dissolution timeline under physiological conditions. This dual-focus assessment ensures patient safety throughout the entire lifecycle of the transient monitoring system.
Environmental Impact and Sustainability Assessment
Transient electronics represent a paradigm shift in medical device sustainability, offering significant environmental advantages over conventional electronic medical devices. Traditional healthcare monitoring systems contribute substantially to electronic waste (e-waste), with an estimated 53.6 million metric tons generated globally in 2019, of which medical devices constitute a growing segment. Transient electronics, designed to dissolve or degrade after their functional lifetime, dramatically reduce this environmental burden by eliminating the need for device retrieval and disposal.
The materials composition of transient healthcare monitoring devices presents a marked improvement in environmental impact. These devices typically utilize biodegradable substrates such as silk fibroin, poly(lactic-co-glycolic acid) (PLGA), and magnesium-based conductors that naturally decompose into non-toxic byproducts. Life cycle assessment (LCA) studies indicate that transient electronics can reduce the carbon footprint of temporary medical monitoring by up to 60% compared to conventional devices when considering manufacturing, use, and end-of-life phases.
Water consumption represents another critical sustainability metric where transient electronics demonstrate advantages. Manufacturing conventional medical electronics requires approximately 10,000 liters of water per square meter of silicon wafers produced. In contrast, certain transient electronic manufacturing processes have achieved reductions of 30-45% in water usage through optimized fabrication techniques and material selection, particularly those employing water-soluble polymers as structural components.
The controlled degradation mechanisms of transient electronics also minimize the release of harmful substances into ecosystems. Unlike conventional electronics that may leach heavy metals and flame retardants when improperly disposed of, transient devices can be engineered to decompose into environmentally benign compounds. Recent studies have demonstrated complete dissolution of certain transient sensors in physiological conditions within 2-4 weeks, with degradation products falling well below toxicity thresholds for aquatic organisms and soil microbiota.
From a circular economy perspective, transient electronics for healthcare monitoring align with sustainable design principles by intentionally incorporating end-of-life considerations into product development. This "designed degradation" approach represents a fundamental shift from the linear take-make-dispose model toward more environmentally responsible healthcare technologies. However, challenges remain in optimizing the balance between functional lifetime and degradation rate, as premature degradation could lead to increased resource consumption through replacement devices.
Energy efficiency during operation also contributes to the sustainability profile of transient healthcare monitors. Many designs incorporate ultra-low-power operation and energy harvesting capabilities from body heat or motion, reducing or eliminating battery requirements. This approach not only extends functional lifetime but also reduces the environmental impact associated with battery production and disposal, which typically involves energy-intensive processes and potentially toxic materials.
The materials composition of transient healthcare monitoring devices presents a marked improvement in environmental impact. These devices typically utilize biodegradable substrates such as silk fibroin, poly(lactic-co-glycolic acid) (PLGA), and magnesium-based conductors that naturally decompose into non-toxic byproducts. Life cycle assessment (LCA) studies indicate that transient electronics can reduce the carbon footprint of temporary medical monitoring by up to 60% compared to conventional devices when considering manufacturing, use, and end-of-life phases.
Water consumption represents another critical sustainability metric where transient electronics demonstrate advantages. Manufacturing conventional medical electronics requires approximately 10,000 liters of water per square meter of silicon wafers produced. In contrast, certain transient electronic manufacturing processes have achieved reductions of 30-45% in water usage through optimized fabrication techniques and material selection, particularly those employing water-soluble polymers as structural components.
The controlled degradation mechanisms of transient electronics also minimize the release of harmful substances into ecosystems. Unlike conventional electronics that may leach heavy metals and flame retardants when improperly disposed of, transient devices can be engineered to decompose into environmentally benign compounds. Recent studies have demonstrated complete dissolution of certain transient sensors in physiological conditions within 2-4 weeks, with degradation products falling well below toxicity thresholds for aquatic organisms and soil microbiota.
From a circular economy perspective, transient electronics for healthcare monitoring align with sustainable design principles by intentionally incorporating end-of-life considerations into product development. This "designed degradation" approach represents a fundamental shift from the linear take-make-dispose model toward more environmentally responsible healthcare technologies. However, challenges remain in optimizing the balance between functional lifetime and degradation rate, as premature degradation could lead to increased resource consumption through replacement devices.
Energy efficiency during operation also contributes to the sustainability profile of transient healthcare monitors. Many designs incorporate ultra-low-power operation and energy harvesting capabilities from body heat or motion, reducing or eliminating battery requirements. This approach not only extends functional lifetime but also reduces the environmental impact associated with battery production and disposal, which typically involves energy-intensive processes and potentially toxic materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!