How Transient Electronics Revolutionize Point-of-Care Tests?
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Transient Electronics in POCT: Background and Objectives
Transient electronics represent a revolutionary paradigm in electronic device design, characterized by their ability to dissolve, disintegrate, or degrade in a controlled manner after serving their intended function. This emerging field has evolved significantly over the past decade, transitioning from academic curiosity to practical applications with substantial commercial potential. The integration of transient electronics with Point-of-Care Testing (POCT) marks a particularly promising convergence, addressing critical challenges in healthcare accessibility, environmental sustainability, and patient privacy.
The historical trajectory of transient electronics began with fundamental research into biodegradable materials and dissolvable electronic components in the early 2000s. By 2010, researchers had demonstrated functional electronic circuits capable of controlled dissolution in aqueous environments. The field gained momentum through interdisciplinary collaboration between materials scientists, electrical engineers, and biomedical researchers, leading to increasingly sophisticated devices with programmable lifespans and enhanced functionality.
In parallel, POCT has emerged as a cornerstone of modern healthcare delivery, enabling rapid diagnostic testing at or near the patient's location. Traditional POCT devices, however, face limitations regarding disposal, environmental impact, and data security—challenges that transient electronics are uniquely positioned to address.
The primary technical objectives for transient electronics in POCT applications encompass several dimensions. First, achieving precise control over dissolution timing and mechanisms to ensure device functionality during the testing period while guaranteeing complete degradation afterward. Second, developing biocompatible transient materials that maintain performance standards comparable to conventional electronics while ensuring safety for both patients and the environment. Third, integrating sensing, processing, and communication capabilities within transient platforms to enable comprehensive diagnostic functionality.
Current technological trends indicate accelerating progress toward fully integrated transient electronic systems for POCT. These include advancements in water-soluble electronic materials, bioresorbable sensors, and degradable power sources. The convergence of these technologies with microfluidics and biomarker detection methodologies presents opportunities for creating next-generation diagnostic platforms that combine accuracy, convenience, and sustainability.
The ultimate goal of this technological integration is to develop POCT devices that deliver precise diagnostic information, automatically transmit relevant data to healthcare providers, and then harmlessly disappear—eliminating biohazardous waste, reducing environmental impact, and protecting patient privacy through physical data destruction. This vision aligns with broader healthcare trends toward personalized medicine, remote monitoring, and sustainable healthcare practices.
The historical trajectory of transient electronics began with fundamental research into biodegradable materials and dissolvable electronic components in the early 2000s. By 2010, researchers had demonstrated functional electronic circuits capable of controlled dissolution in aqueous environments. The field gained momentum through interdisciplinary collaboration between materials scientists, electrical engineers, and biomedical researchers, leading to increasingly sophisticated devices with programmable lifespans and enhanced functionality.
In parallel, POCT has emerged as a cornerstone of modern healthcare delivery, enabling rapid diagnostic testing at or near the patient's location. Traditional POCT devices, however, face limitations regarding disposal, environmental impact, and data security—challenges that transient electronics are uniquely positioned to address.
The primary technical objectives for transient electronics in POCT applications encompass several dimensions. First, achieving precise control over dissolution timing and mechanisms to ensure device functionality during the testing period while guaranteeing complete degradation afterward. Second, developing biocompatible transient materials that maintain performance standards comparable to conventional electronics while ensuring safety for both patients and the environment. Third, integrating sensing, processing, and communication capabilities within transient platforms to enable comprehensive diagnostic functionality.
Current technological trends indicate accelerating progress toward fully integrated transient electronic systems for POCT. These include advancements in water-soluble electronic materials, bioresorbable sensors, and degradable power sources. The convergence of these technologies with microfluidics and biomarker detection methodologies presents opportunities for creating next-generation diagnostic platforms that combine accuracy, convenience, and sustainability.
The ultimate goal of this technological integration is to develop POCT devices that deliver precise diagnostic information, automatically transmit relevant data to healthcare providers, and then harmlessly disappear—eliminating biohazardous waste, reducing environmental impact, and protecting patient privacy through physical data destruction. This vision aligns with broader healthcare trends toward personalized medicine, remote monitoring, and sustainable healthcare practices.
Market Analysis for Transient POCT Solutions
The global market for transient point-of-care testing (POCT) solutions is experiencing significant growth, driven by increasing healthcare costs, rising prevalence of chronic diseases, and growing demand for rapid diagnostic tools. The transient electronics POCT market was valued at approximately $3.2 billion in 2022 and is projected to reach $7.5 billion by 2028, representing a compound annual growth rate (CAGR) of 15.3%.
North America currently dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (21%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 18.7% during the forecast period, primarily due to improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about early disease diagnosis in countries like China and India.
The transient POCT market can be segmented by application into infectious disease testing, cardiac markers, coagulation testing, glucose monitoring, and others. Infectious disease testing holds the largest market share (35%), followed by cardiac markers (25%) and glucose monitoring (20%). The COVID-19 pandemic significantly accelerated market growth, with a 27% increase in demand for rapid diagnostic solutions in 2020-2021.
By end-user, hospitals and clinics represent the largest segment (45%), followed by diagnostic laboratories (25%), home care settings (20%), and others (10%). The home care segment is projected to grow at the highest rate of 19.2% due to increasing preference for self-testing and remote patient monitoring.
Key market drivers include technological advancements in biosensors and microfluidics, growing preference for decentralized testing, increasing prevalence of chronic and infectious diseases, and supportive government initiatives. The integration of artificial intelligence and machine learning with transient POCT devices is creating new opportunities for market expansion.
However, the market faces challenges such as stringent regulatory requirements, concerns regarding test accuracy and reliability, limited reimbursement policies in developing regions, and high development costs. Additionally, the environmental impact of disposable testing devices is becoming a growing concern among healthcare providers and patients.
Consumer behavior analysis indicates a strong preference for non-invasive or minimally invasive testing methods, with 78% of patients preferring self-testing options when available. Healthcare providers value rapid results, ease of use, and integration capabilities with electronic health records, with 65% citing these factors as critical in adoption decisions.
North America currently dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (21%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 18.7% during the forecast period, primarily due to improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about early disease diagnosis in countries like China and India.
The transient POCT market can be segmented by application into infectious disease testing, cardiac markers, coagulation testing, glucose monitoring, and others. Infectious disease testing holds the largest market share (35%), followed by cardiac markers (25%) and glucose monitoring (20%). The COVID-19 pandemic significantly accelerated market growth, with a 27% increase in demand for rapid diagnostic solutions in 2020-2021.
By end-user, hospitals and clinics represent the largest segment (45%), followed by diagnostic laboratories (25%), home care settings (20%), and others (10%). The home care segment is projected to grow at the highest rate of 19.2% due to increasing preference for self-testing and remote patient monitoring.
Key market drivers include technological advancements in biosensors and microfluidics, growing preference for decentralized testing, increasing prevalence of chronic and infectious diseases, and supportive government initiatives. The integration of artificial intelligence and machine learning with transient POCT devices is creating new opportunities for market expansion.
However, the market faces challenges such as stringent regulatory requirements, concerns regarding test accuracy and reliability, limited reimbursement policies in developing regions, and high development costs. Additionally, the environmental impact of disposable testing devices is becoming a growing concern among healthcare providers and patients.
Consumer behavior analysis indicates a strong preference for non-invasive or minimally invasive testing methods, with 78% of patients preferring self-testing options when available. Healthcare providers value rapid results, ease of use, and integration capabilities with electronic health records, with 65% citing these factors as critical in adoption decisions.
Technical Challenges in Transient Electronics for Diagnostics
Transient electronics for point-of-care diagnostics face several significant technical challenges that currently limit their widespread implementation. The primary obstacle lies in achieving the delicate balance between functional performance and controlled degradability. Unlike conventional electronics designed for durability, transient devices must maintain reliable operation for a specific timeframe before safely degrading, requiring novel material science approaches and engineering solutions.
Material selection presents a complex challenge, as biodegradable substrates and conductive elements must simultaneously provide adequate electrical performance while maintaining controlled dissolution rates. Silicon-based semiconductors, magnesium conductors, and silk or poly(lactic-co-glycolic acid) (PLGA) substrates show promise but struggle with inconsistent degradation profiles under variable environmental conditions such as pH, temperature, and moisture levels.
Encapsulation technologies represent another critical hurdle. Protective layers must shield sensitive electronic components during operation yet permit controlled degradation afterward. Current encapsulation materials often demonstrate unpredictable breakdown characteristics, leading to premature device failure or incomplete dissolution. This unpredictability poses significant risks in diagnostic applications where accuracy is paramount.
Power supply integration remains particularly problematic for transient diagnostic devices. Conventional batteries contain toxic materials incompatible with biodegradable systems, while existing transient power sources offer insufficient energy density for many diagnostic applications. Researchers are exploring biodegradable batteries and energy harvesting techniques, but these solutions currently lack the power output and longevity required for complex diagnostic functions.
Signal processing capabilities in transient electronics lag significantly behind conventional systems. The computational requirements for analyzing biological samples in point-of-care tests often exceed what current biodegradable processors can deliver. Limited transistor density and processing speed constrain the complexity of diagnostic algorithms that can be implemented on transient platforms.
Manufacturing scalability presents substantial challenges, as production techniques for transient electronics often involve complex, multi-step processes incompatible with high-volume manufacturing. Current fabrication methods typically require specialized equipment and controlled environments, resulting in high production costs that limit commercial viability.
Reliability and shelf-life concerns further complicate development efforts. Transient diagnostic devices must maintain stable performance characteristics during storage yet activate predictably upon use. Premature degradation during storage or shipping remains a significant issue, with environmental factors like humidity and temperature fluctuations triggering unintended dissolution processes before deployment.
Material selection presents a complex challenge, as biodegradable substrates and conductive elements must simultaneously provide adequate electrical performance while maintaining controlled dissolution rates. Silicon-based semiconductors, magnesium conductors, and silk or poly(lactic-co-glycolic acid) (PLGA) substrates show promise but struggle with inconsistent degradation profiles under variable environmental conditions such as pH, temperature, and moisture levels.
Encapsulation technologies represent another critical hurdle. Protective layers must shield sensitive electronic components during operation yet permit controlled degradation afterward. Current encapsulation materials often demonstrate unpredictable breakdown characteristics, leading to premature device failure or incomplete dissolution. This unpredictability poses significant risks in diagnostic applications where accuracy is paramount.
Power supply integration remains particularly problematic for transient diagnostic devices. Conventional batteries contain toxic materials incompatible with biodegradable systems, while existing transient power sources offer insufficient energy density for many diagnostic applications. Researchers are exploring biodegradable batteries and energy harvesting techniques, but these solutions currently lack the power output and longevity required for complex diagnostic functions.
Signal processing capabilities in transient electronics lag significantly behind conventional systems. The computational requirements for analyzing biological samples in point-of-care tests often exceed what current biodegradable processors can deliver. Limited transistor density and processing speed constrain the complexity of diagnostic algorithms that can be implemented on transient platforms.
Manufacturing scalability presents substantial challenges, as production techniques for transient electronics often involve complex, multi-step processes incompatible with high-volume manufacturing. Current fabrication methods typically require specialized equipment and controlled environments, resulting in high production costs that limit commercial viability.
Reliability and shelf-life concerns further complicate development efforts. Transient diagnostic devices must maintain stable performance characteristics during storage yet activate predictably upon use. Premature degradation during storage or shipping remains a significant issue, with environmental factors like humidity and temperature fluctuations triggering unintended dissolution processes before deployment.
Current Transient Electronics Implementation in POCT
01 Biodegradable and dissolvable electronic components
Transient electronics that are designed to dissolve or degrade after a predetermined period or under specific environmental conditions. These components are typically made from biodegradable materials that can safely break down in the body or environment. This technology is particularly useful for medical implants, environmental sensors, and temporary electronic devices that don't require retrieval after use.- Biodegradable and dissolvable electronic components: Transient electronics that are designed to dissolve or degrade after a predetermined period or under specific environmental conditions. These electronics utilize biodegradable materials and substrates that can safely break down in the body or environment. Applications include implantable medical devices that don't require surgical removal and environmentally friendly consumer electronics that reduce e-waste.
- Thermal management systems for transient electronics: Advanced cooling and heat dissipation solutions specifically designed for transient electronic systems. These thermal management approaches address the unique challenges of temporary electronic devices, including flexible heat sinks, phase-change materials, and specialized thermal interface materials that maintain performance during the operational lifetime while not impeding the transient nature of the device.
- Power supply solutions for transient electronics: Specialized power sources designed for transient electronic applications, including biodegradable batteries, energy harvesting systems, and temporary power storage solutions. These power systems are engineered to provide sufficient energy during the intended operational period while being compatible with the overall transient nature of the device, either degrading along with the electronics or being easily removable.
- Security features in transient electronic systems: Security mechanisms specifically designed for transient electronics that ensure data protection during the operational lifetime while enabling complete data destruction when the device degrades. These include self-destructing memory, encrypted storage that becomes inaccessible upon triggering conditions, and authentication systems that prevent unauthorized access to sensitive information in temporary electronic devices.
- Circuit design for transient electronic applications: Specialized circuit architectures optimized for transient electronic systems, including flexible printed circuits, stretchable interconnects, and novel component integration methods. These designs accommodate the unique requirements of temporary electronic devices, balancing performance needs during operation with controlled degradation or disassembly mechanisms when the intended lifetime expires.
02 Thermal management systems for transient electronics
Advanced cooling and heat dissipation solutions specifically designed for transient electronic devices. These systems help manage the thermal challenges associated with temporary or short-lived electronic components, ensuring optimal performance during their operational lifetime while maintaining the ability to degrade or dissolve afterward. Effective thermal management is crucial for preventing premature failure of transient electronic devices.Expand Specific Solutions03 Power supply solutions for transient electronics
Specialized power sources and energy management systems designed for transient electronic applications. These include biodegradable batteries, energy harvesting technologies, and power management circuits that can function for a predetermined period before degrading. These solutions address the unique power requirements of transient electronics while maintaining their temporary nature and environmental compatibility.Expand Specific Solutions04 Security features in transient electronic systems
Security mechanisms specifically designed for transient electronic devices that can self-destruct or become inoperable after a certain period or trigger event. These features protect sensitive data and intellectual property by ensuring that the device and its stored information cannot be accessed or recovered after its intended use period. Applications include secure military communications, one-time authentication tokens, and data storage with built-in expiration.Expand Specific Solutions05 Diagnostic and monitoring applications of transient electronics
Implementation of transient electronic systems in medical diagnostics, environmental monitoring, and structural health assessment. These applications leverage the temporary nature of transient electronics to create sensors and monitoring devices that can be deployed in hard-to-reach locations or within living organisms, collect data for a predetermined period, and then harmlessly dissolve or degrade without requiring retrieval or causing long-term environmental impact.Expand Specific Solutions
Industry Leaders in Transient Electronics and POCT
Transient electronics is revolutionizing point-of-care testing, currently in an early growth phase with expanding market potential. The technology enables biodegradable, environmentally friendly diagnostic devices that dissolve after use, addressing waste management challenges in healthcare settings. Market size is projected to grow significantly as applications expand beyond traditional medical settings into remote and resource-limited environments. Companies like Abbott Point of Care, Becton Dickinson, and Laboratory Corporation of America are leading commercial development, while research institutions such as Harbin Institute of Technology and CSIC are advancing fundamental technologies. Emerging players like Zendia GmbH and Calmark Sweden are introducing innovative solutions targeting specific diagnostic needs, indicating a competitive landscape balancing established medical device manufacturers with specialized technology startups.
Abbott Point of Care, Inc.
Technical Solution: Abbott Point of Care has developed a revolutionary transient electronics platform for their i-STAT system, integrating dissolvable sensors and circuits that break down after use. Their technology employs water-soluble silicon components and biodegradable polymers that maintain functionality during testing but decompose within predetermined timeframes afterward. The i-STAT transient system features magnesium-based conductors and silicon nanomembranes that dissolve in biofluids, enabling single-use diagnostic cartridges that minimize biohazardous waste. Abbott's approach incorporates silk fibroin as an encapsulation material, controlling dissolution rates based on crystallinity levels, allowing precise timing for component breakdown after test completion. Their platform enables rapid blood analysis for cardiac markers, electrolytes, and blood gases with results in approximately 2-10 minutes, while ensuring the electronic components safely degrade within 24-48 hours after exposure to disposal conditions.
Strengths: Industry-leading integration of transient electronics with established point-of-care infrastructure; exceptional control over dissolution timing; reduced environmental impact through biodegradable components. Weaknesses: Higher initial manufacturing costs compared to conventional electronics; potential reliability challenges in varying environmental conditions; limited shelf life requiring careful storage protocols.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. has pioneered a transient electronics platform for point-of-care diagnostics called BD Veritor™ Eco, which incorporates dissolvable circuit components made from magnesium, zinc, and silicon that maintain functionality during the testing period but degrade safely afterward. Their technology utilizes specialized silk-based substrates with tunable dissolution rates, allowing precise control over when components begin to break down after test completion. BD's approach features water-soluble electronics encapsulated in biodegradable polymers that protect circuits during use but enable controlled dissolution when exposed to specific environmental conditions. The system includes transient power sources using biodegradable batteries with non-toxic electrolytes that power the diagnostic functions before safely decomposing. Their platform enables rapid detection of infectious diseases including COVID-19, influenza, and strep with results in approximately 15 minutes, while the electronic components begin degradation within hours after disposal and complete breakdown within weeks.
Strengths: Extensive distribution network for rapid market penetration; comprehensive integration with existing healthcare IT systems; advanced manufacturing capabilities for scale production. Weaknesses: Higher unit cost compared to traditional lateral flow tests; more complex supply chain for specialized materials; potential sensitivity to extreme storage conditions affecting dissolution timing.
Key Patents and Research in Transient Diagnostic Technologies
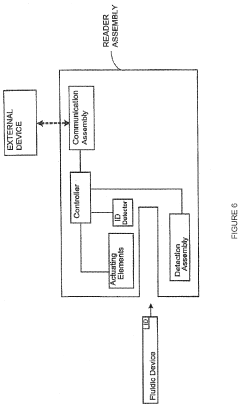
Reducing optical interference in a fluidic device
PatentPendingUS20220196639A1
Innovation
- A fluidic device with a quencher assembly that includes a quenching site and a quenching agent, such as 4-amino-1,11-azobenzene-3,4-disulfonic acid, impregnated in absorbent materials like glass fiber or silica, which reduces optical interference by inactivating reagents and absorbing waste liquids, thereby minimizing interference with the signal of interest.
Potentiometric-sensor chip, potentiometric assay, and assay kit
PatentInactiveUS20110276278A1
Innovation
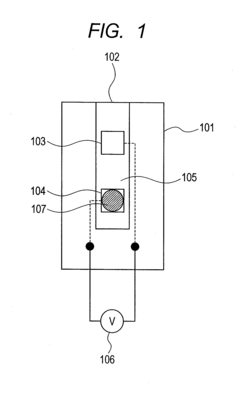
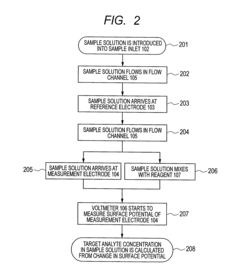
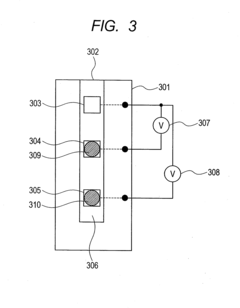
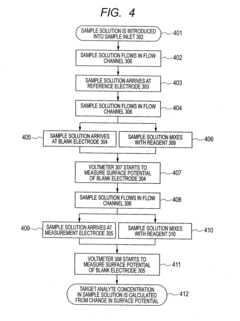
- The electrodes are arranged such that the sample arrives at the reference electrode before the measurement electrode, with a larger distance between them, and the use of blank electrodes in separate flow channels to detect the start of reaction through discontinuous potential changes, allowing for accurate measurement with minimal sample volume.
Environmental Impact and Sustainability Advantages
Transient electronics represent a paradigm shift in medical device sustainability, offering significant environmental advantages over conventional electronic systems used in point-of-care testing. Traditional medical electronics contribute substantially to electronic waste (e-waste), with an estimated 53.6 million metric tons generated globally in 2019, of which medical devices constitute a growing proportion. Disposable diagnostic tools, including single-use point-of-care tests, exacerbate this environmental burden through their persistent materials and potentially hazardous components.
The fundamental environmental benefit of transient electronics lies in their ability to decompose naturally after their intended use period, dramatically reducing waste accumulation. These devices incorporate biodegradable substrates such as silk fibroin, poly(lactic-co-glycolic acid) (PLGA), and cellulose derivatives that can break down into non-toxic byproducts when exposed to environmental conditions or specific triggers. This controlled degradation minimizes landfill burden and eliminates the need for resource-intensive recycling processes.
Water consumption represents another critical environmental consideration in medical device manufacturing. Conventional electronics production requires approximately 10-15 gallons of water per square inch of silicon wafer processed. Transient electronics manufacturing processes have demonstrated potential water usage reductions of up to 30-45% through simplified fabrication techniques and biodegradable material processing that requires fewer purification steps.
Carbon footprint analysis reveals further sustainability advantages. Life cycle assessments indicate that transient point-of-care devices can reduce greenhouse gas emissions by 40-60% compared to conventional alternatives when accounting for raw material extraction, manufacturing, distribution, use, and end-of-life management. This reduction stems primarily from simplified supply chains, decreased transportation requirements for waste management, and elimination of energy-intensive recycling processes.
In resource-limited settings, the environmental benefits of transient electronics become particularly pronounced. Remote healthcare facilities often lack proper medical waste management infrastructure, leading to improper disposal of conventional electronic medical devices. Transient point-of-care tests that naturally decompose after use help prevent soil and water contamination in these vulnerable environments, protecting local ecosystems and community health.
The sustainability advantages extend to resource conservation as well. Many transient electronic components utilize earth-abundant materials rather than rare or precious metals common in conventional electronics. This shift reduces dependence on environmentally destructive mining practices and mitigates supply chain vulnerabilities associated with geopolitically sensitive materials. Studies estimate that widespread adoption of transient electronics in point-of-care applications could reduce critical metal consumption by 15-25% in this sector.
The fundamental environmental benefit of transient electronics lies in their ability to decompose naturally after their intended use period, dramatically reducing waste accumulation. These devices incorporate biodegradable substrates such as silk fibroin, poly(lactic-co-glycolic acid) (PLGA), and cellulose derivatives that can break down into non-toxic byproducts when exposed to environmental conditions or specific triggers. This controlled degradation minimizes landfill burden and eliminates the need for resource-intensive recycling processes.
Water consumption represents another critical environmental consideration in medical device manufacturing. Conventional electronics production requires approximately 10-15 gallons of water per square inch of silicon wafer processed. Transient electronics manufacturing processes have demonstrated potential water usage reductions of up to 30-45% through simplified fabrication techniques and biodegradable material processing that requires fewer purification steps.
Carbon footprint analysis reveals further sustainability advantages. Life cycle assessments indicate that transient point-of-care devices can reduce greenhouse gas emissions by 40-60% compared to conventional alternatives when accounting for raw material extraction, manufacturing, distribution, use, and end-of-life management. This reduction stems primarily from simplified supply chains, decreased transportation requirements for waste management, and elimination of energy-intensive recycling processes.
In resource-limited settings, the environmental benefits of transient electronics become particularly pronounced. Remote healthcare facilities often lack proper medical waste management infrastructure, leading to improper disposal of conventional electronic medical devices. Transient point-of-care tests that naturally decompose after use help prevent soil and water contamination in these vulnerable environments, protecting local ecosystems and community health.
The sustainability advantages extend to resource conservation as well. Many transient electronic components utilize earth-abundant materials rather than rare or precious metals common in conventional electronics. This shift reduces dependence on environmentally destructive mining practices and mitigates supply chain vulnerabilities associated with geopolitically sensitive materials. Studies estimate that widespread adoption of transient electronics in point-of-care applications could reduce critical metal consumption by 15-25% in this sector.
Regulatory Framework for Transient Medical Devices
The regulatory landscape for transient medical devices represents a complex and evolving framework that significantly impacts the development and deployment of transient electronics in point-of-care testing. Currently, most regulatory bodies, including the FDA in the United States and the European Medicines Agency, lack specific guidelines for transient electronics, creating a regulatory gap that manufacturers must navigate carefully.
Traditional medical device regulations typically assume device permanence and durability, whereas transient electronics are designed to dissolve or degrade after fulfilling their function. This fundamental difference necessitates new approaches to safety assessment, performance standards, and disposal protocols. The FDA's current pathways—510(k), De Novo, or Premarket Approval—require adaptation to accommodate the unique characteristics of transient devices.
Risk classification for transient medical devices presents particular challenges. Regulators must consider not only the immediate functionality but also the degradation products and their potential biological interactions. The controlled dissolution of these devices introduces novel safety considerations regarding material biocompatibility, degradation kinetics, and potential toxicity of breakdown products.
Several regulatory milestones have emerged in recent years. In 2019, the International Medical Device Regulators Forum (IMDRF) initiated discussions on novel materials in medical devices, including transient electronics. By 2021, the FDA established an emerging technology program that provides consultation pathways for innovative technologies like transient electronics, offering manufacturers early engagement opportunities with regulators.
Environmental regulations also intersect with transient medical device governance. While these devices offer potential environmental benefits through reduced electronic waste, their novel materials must comply with existing chemical regulations such as REACH in Europe and similar frameworks globally. The biodegradability claims require standardized testing protocols that are still under development.
Looking forward, regulatory harmonization represents a critical need. International collaboration through organizations like the IMDRF and WHO will be essential to develop consistent global standards for transient medical devices. Industry stakeholders are advocating for a risk-based regulatory approach that balances innovation facilitation with patient safety assurance.
For manufacturers entering this space, proactive regulatory engagement strategies are recommended, including early consultation with authorities, participation in standards development, and investment in comprehensive safety data generation that addresses the unique aspects of transient technologies.
Traditional medical device regulations typically assume device permanence and durability, whereas transient electronics are designed to dissolve or degrade after fulfilling their function. This fundamental difference necessitates new approaches to safety assessment, performance standards, and disposal protocols. The FDA's current pathways—510(k), De Novo, or Premarket Approval—require adaptation to accommodate the unique characteristics of transient devices.
Risk classification for transient medical devices presents particular challenges. Regulators must consider not only the immediate functionality but also the degradation products and their potential biological interactions. The controlled dissolution of these devices introduces novel safety considerations regarding material biocompatibility, degradation kinetics, and potential toxicity of breakdown products.
Several regulatory milestones have emerged in recent years. In 2019, the International Medical Device Regulators Forum (IMDRF) initiated discussions on novel materials in medical devices, including transient electronics. By 2021, the FDA established an emerging technology program that provides consultation pathways for innovative technologies like transient electronics, offering manufacturers early engagement opportunities with regulators.
Environmental regulations also intersect with transient medical device governance. While these devices offer potential environmental benefits through reduced electronic waste, their novel materials must comply with existing chemical regulations such as REACH in Europe and similar frameworks globally. The biodegradability claims require standardized testing protocols that are still under development.
Looking forward, regulatory harmonization represents a critical need. International collaboration through organizations like the IMDRF and WHO will be essential to develop consistent global standards for transient medical devices. Industry stakeholders are advocating for a risk-based regulatory approach that balances innovation facilitation with patient safety assurance.
For manufacturers entering this space, proactive regulatory engagement strategies are recommended, including early consultation with authorities, participation in standards development, and investment in comprehensive safety data generation that addresses the unique aspects of transient technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!