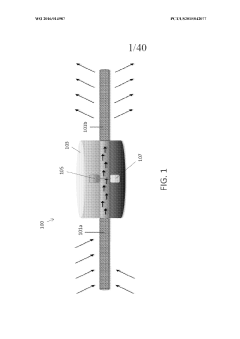
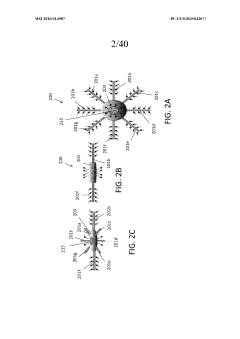
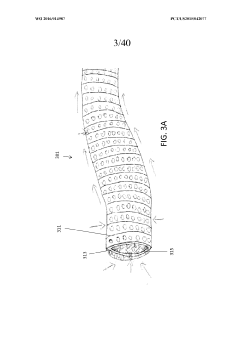
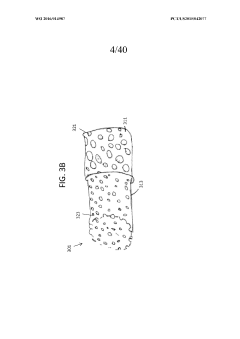
Assess Ultrafiltration Applications in the Pharmaceutical Sector
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pharmaceutical Ultrafiltration Background and Objectives
Ultrafiltration technology has evolved significantly since its inception in the 1960s, transforming from simple laboratory applications to sophisticated industrial processes. In the pharmaceutical sector, ultrafiltration has become increasingly vital for ensuring product purity, consistency, and regulatory compliance. The historical trajectory shows a clear shift from basic separation techniques to highly specialized membrane technologies designed specifically for pharmaceutical applications.
The evolution of ultrafiltration in pharmaceuticals has been driven by increasing regulatory demands, particularly from agencies like the FDA and EMA, which have progressively raised standards for product purity and process validation. This regulatory pressure has catalyzed innovation in membrane materials, module designs, and process control systems over the past three decades.
Current technological trends in pharmaceutical ultrafiltration focus on developing more selective membranes with higher flux rates, improved fouling resistance, and enhanced durability under aggressive cleaning regimes. The integration of ultrafiltration with complementary separation technologies, such as chromatography and nanofiltration, represents another significant trend that enables more comprehensive purification strategies.
The primary objective of this technical assessment is to evaluate the current state and future potential of ultrafiltration applications in pharmaceutical manufacturing. This includes analyzing membrane performance characteristics, process integration approaches, and emerging applications in novel therapeutic modalities such as cell and gene therapies, monoclonal antibodies, and vaccine production.
Additionally, this assessment aims to identify technological gaps that limit the broader adoption of ultrafiltration in pharmaceutical processes, particularly in continuous manufacturing paradigms and single-use systems. Understanding these limitations will help direct future research and development efforts toward solutions that address industry needs.
The assessment will also explore how ultrafiltration technologies can contribute to sustainability goals within pharmaceutical manufacturing by reducing solvent usage, minimizing waste generation, and decreasing energy consumption. These environmental considerations are becoming increasingly important as the industry faces pressure to reduce its ecological footprint.
Finally, this technical evaluation seeks to establish a roadmap for ultrafiltration technology development that aligns with anticipated changes in pharmaceutical manufacturing practices, regulatory requirements, and emerging therapeutic modalities over the next decade. This forward-looking perspective will help guide strategic investments in research, development, and implementation of ultrafiltration technologies.
The evolution of ultrafiltration in pharmaceuticals has been driven by increasing regulatory demands, particularly from agencies like the FDA and EMA, which have progressively raised standards for product purity and process validation. This regulatory pressure has catalyzed innovation in membrane materials, module designs, and process control systems over the past three decades.
Current technological trends in pharmaceutical ultrafiltration focus on developing more selective membranes with higher flux rates, improved fouling resistance, and enhanced durability under aggressive cleaning regimes. The integration of ultrafiltration with complementary separation technologies, such as chromatography and nanofiltration, represents another significant trend that enables more comprehensive purification strategies.
The primary objective of this technical assessment is to evaluate the current state and future potential of ultrafiltration applications in pharmaceutical manufacturing. This includes analyzing membrane performance characteristics, process integration approaches, and emerging applications in novel therapeutic modalities such as cell and gene therapies, monoclonal antibodies, and vaccine production.
Additionally, this assessment aims to identify technological gaps that limit the broader adoption of ultrafiltration in pharmaceutical processes, particularly in continuous manufacturing paradigms and single-use systems. Understanding these limitations will help direct future research and development efforts toward solutions that address industry needs.
The assessment will also explore how ultrafiltration technologies can contribute to sustainability goals within pharmaceutical manufacturing by reducing solvent usage, minimizing waste generation, and decreasing energy consumption. These environmental considerations are becoming increasingly important as the industry faces pressure to reduce its ecological footprint.
Finally, this technical evaluation seeks to establish a roadmap for ultrafiltration technology development that aligns with anticipated changes in pharmaceutical manufacturing practices, regulatory requirements, and emerging therapeutic modalities over the next decade. This forward-looking perspective will help guide strategic investments in research, development, and implementation of ultrafiltration technologies.
Market Analysis of Pharmaceutical Ultrafiltration Demand
The pharmaceutical ultrafiltration market has experienced substantial growth over the past decade, driven primarily by increasing demand for biopharmaceuticals and stringent regulatory requirements for product purity. The global market for pharmaceutical ultrafiltration was valued at approximately 1.5 billion USD in 2022 and is projected to reach 2.7 billion USD by 2028, representing a compound annual growth rate of 10.3%.
Biopharmaceutical manufacturing constitutes the largest application segment, accounting for nearly 55% of the total market share. This dominance stems from ultrafiltration's critical role in downstream processing, particularly in the concentration and purification of therapeutic proteins, monoclonal antibodies, and vaccines. The COVID-19 pandemic significantly accelerated this trend, with manufacturers rapidly scaling up production capacity for vaccine development.
Geographically, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is witnessing the fastest growth rate due to expanding pharmaceutical manufacturing capabilities in China, India, and Singapore, coupled with increasing investments in biotechnology research and development.
The demand for single-use ultrafiltration systems has emerged as a notable trend, growing at 15% annually. This shift reflects pharmaceutical manufacturers' increasing preference for flexible manufacturing solutions that reduce cross-contamination risks and validation requirements while improving operational efficiency.
Continuous manufacturing adoption is creating new opportunities for ultrafiltration technologies. Approximately 35% of pharmaceutical companies are implementing or planning to implement continuous manufacturing processes, which require specialized filtration solutions for real-time processing and quality control.
Environmental sustainability concerns are also influencing market dynamics. Water consumption in pharmaceutical manufacturing has become a critical focus area, with ultrafiltration systems increasingly valued for their ability to recover and recycle process water, reducing overall environmental footprint by up to 30% in some applications.
The contract manufacturing organization (CMO) segment represents another significant growth driver, expanding at 12% annually as pharmaceutical companies increasingly outsource production. This trend has created substantial demand for versatile ultrafiltration systems capable of handling diverse product portfolios and manufacturing scales.
Biopharmaceutical manufacturing constitutes the largest application segment, accounting for nearly 55% of the total market share. This dominance stems from ultrafiltration's critical role in downstream processing, particularly in the concentration and purification of therapeutic proteins, monoclonal antibodies, and vaccines. The COVID-19 pandemic significantly accelerated this trend, with manufacturers rapidly scaling up production capacity for vaccine development.
Geographically, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is witnessing the fastest growth rate due to expanding pharmaceutical manufacturing capabilities in China, India, and Singapore, coupled with increasing investments in biotechnology research and development.
The demand for single-use ultrafiltration systems has emerged as a notable trend, growing at 15% annually. This shift reflects pharmaceutical manufacturers' increasing preference for flexible manufacturing solutions that reduce cross-contamination risks and validation requirements while improving operational efficiency.
Continuous manufacturing adoption is creating new opportunities for ultrafiltration technologies. Approximately 35% of pharmaceutical companies are implementing or planning to implement continuous manufacturing processes, which require specialized filtration solutions for real-time processing and quality control.
Environmental sustainability concerns are also influencing market dynamics. Water consumption in pharmaceutical manufacturing has become a critical focus area, with ultrafiltration systems increasingly valued for their ability to recover and recycle process water, reducing overall environmental footprint by up to 30% in some applications.
The contract manufacturing organization (CMO) segment represents another significant growth driver, expanding at 12% annually as pharmaceutical companies increasingly outsource production. This trend has created substantial demand for versatile ultrafiltration systems capable of handling diverse product portfolios and manufacturing scales.
Current Ultrafiltration Technology Challenges in Pharmaceuticals
Ultrafiltration technology in pharmaceutical applications faces several significant challenges despite its widespread adoption. Membrane fouling remains the most persistent issue, occurring when particles, macromolecules, or biological materials accumulate on membrane surfaces or within pores. This phenomenon reduces filtration efficiency, increases energy consumption, and necessitates frequent cleaning or replacement cycles, substantially impacting operational costs and production schedules.
Scale-up difficulties present another major obstacle. Laboratory-scale ultrafiltration processes often demonstrate excellent performance, but translating these results to industrial production volumes introduces complex hydrodynamic behaviors that can compromise separation efficiency and product quality. The non-linear scaling relationships between membrane area, flow dynamics, and separation performance require sophisticated modeling and extensive validation studies.
Protein aggregation during ultrafiltration represents a critical challenge specific to biopharmaceutical manufacturing. The shear forces generated during the filtration process can trigger protein denaturation and subsequent aggregation, potentially compromising product efficacy and safety. This issue becomes particularly pronounced when processing high-concentration protein solutions for injectable formulations.
Cleaning validation and cross-contamination prevention pose significant regulatory hurdles. Pharmaceutical manufacturers must demonstrate that their ultrafiltration systems can be consistently cleaned to prevent product carryover between batches, especially when using the same equipment for multiple products. The complex geometry of membrane modules makes thorough cleaning difficult to achieve and validate.
Process control limitations further complicate ultrafiltration operations. Real-time monitoring of membrane performance, protein concentration, and product quality remains challenging. Current sensor technologies often lack the sensitivity or specificity required for continuous process verification, making it difficult to implement advanced control strategies or quality-by-design approaches.
Membrane selectivity constraints also impact pharmaceutical applications. Existing ultrafiltration membranes sometimes lack the precise molecular weight cut-off profiles needed for specific separation tasks, particularly when dealing with closely related biomolecules or impurities with similar molecular characteristics to the target product.
Environmental sustainability concerns are increasingly relevant as pharmaceutical companies face pressure to reduce water consumption and waste generation. Ultrafiltration processes typically require significant volumes of water for cleaning and buffer preparation, while spent membranes and cleaning solutions contribute to waste streams requiring specialized disposal.
Scale-up difficulties present another major obstacle. Laboratory-scale ultrafiltration processes often demonstrate excellent performance, but translating these results to industrial production volumes introduces complex hydrodynamic behaviors that can compromise separation efficiency and product quality. The non-linear scaling relationships between membrane area, flow dynamics, and separation performance require sophisticated modeling and extensive validation studies.
Protein aggregation during ultrafiltration represents a critical challenge specific to biopharmaceutical manufacturing. The shear forces generated during the filtration process can trigger protein denaturation and subsequent aggregation, potentially compromising product efficacy and safety. This issue becomes particularly pronounced when processing high-concentration protein solutions for injectable formulations.
Cleaning validation and cross-contamination prevention pose significant regulatory hurdles. Pharmaceutical manufacturers must demonstrate that their ultrafiltration systems can be consistently cleaned to prevent product carryover between batches, especially when using the same equipment for multiple products. The complex geometry of membrane modules makes thorough cleaning difficult to achieve and validate.
Process control limitations further complicate ultrafiltration operations. Real-time monitoring of membrane performance, protein concentration, and product quality remains challenging. Current sensor technologies often lack the sensitivity or specificity required for continuous process verification, making it difficult to implement advanced control strategies or quality-by-design approaches.
Membrane selectivity constraints also impact pharmaceutical applications. Existing ultrafiltration membranes sometimes lack the precise molecular weight cut-off profiles needed for specific separation tasks, particularly when dealing with closely related biomolecules or impurities with similar molecular characteristics to the target product.
Environmental sustainability concerns are increasingly relevant as pharmaceutical companies face pressure to reduce water consumption and waste generation. Ultrafiltration processes typically require significant volumes of water for cleaning and buffer preparation, while spent membranes and cleaning solutions contribute to waste streams requiring specialized disposal.
Current Ultrafiltration Solutions for Pharmaceutical Applications
01 Ultrafiltration membrane technology and materials
Ultrafiltration membranes are key components in filtration systems, designed with specific materials and structures to achieve optimal separation performance. These membranes can be made from various polymeric materials or ceramic compounds with controlled pore sizes to selectively filter particles and molecules. Advanced membrane designs incorporate features to minimize fouling, enhance durability, and improve flux rates while maintaining high rejection capabilities for target contaminants.- Membrane technology for ultrafiltration systems: Ultrafiltration systems utilize specialized membrane technologies to separate particles and molecules based on size. These membranes are designed with specific pore sizes and materials to optimize filtration efficiency and selectivity. Advanced membrane designs incorporate features that minimize fouling, increase flux rates, and improve durability under various operating conditions. The membrane technology is fundamental to the performance of ultrafiltration systems across different applications.
- Industrial wastewater treatment applications: Ultrafiltration is widely employed in industrial wastewater treatment to remove suspended solids, colloids, bacteria, and high molecular weight contaminants. These systems can be configured as standalone units or integrated into larger treatment trains to achieve specific water quality objectives. The technology enables industries to meet discharge regulations, recover valuable materials from waste streams, and reduce environmental impact while optimizing water reuse opportunities.
- Biological and pharmaceutical applications: Ultrafiltration plays a crucial role in biological and pharmaceutical processes, including protein concentration, virus removal, and purification of biopharmaceuticals. The technology allows for gentle separation of biomolecules without denaturation, making it suitable for handling sensitive biological materials. These systems can be designed to operate under aseptic conditions, with precise control over separation parameters to ensure product quality and consistency in bioprocessing applications.
- Process optimization and control systems: Advanced control systems for ultrafiltration processes incorporate real-time monitoring, automated cleaning cycles, and adaptive operating parameters to optimize performance. These systems utilize sensors to monitor transmembrane pressure, flux rates, and permeate quality, allowing for dynamic adjustments to maintain efficiency. Process optimization strategies include feed pretreatment, backwashing protocols, and chemical cleaning regimens that extend membrane life while ensuring consistent filtration performance across varying feed conditions.
- Novel module designs and system configurations: Innovative ultrafiltration module designs and system configurations enhance process efficiency and application versatility. These include spiral-wound elements, hollow fiber modules, tubular systems, and plate-and-frame arrangements, each offering specific advantages for different applications. Novel configurations incorporate energy recovery devices, reduced footprint designs, and hybrid systems that combine ultrafiltration with other separation technologies to achieve comprehensive treatment objectives while minimizing operational costs.
02 Industrial wastewater treatment applications
Ultrafiltration systems are widely employed in industrial wastewater treatment processes to remove suspended solids, colloids, bacteria, and high molecular weight compounds. These systems can be integrated into treatment trains to handle various industrial effluents including those from chemical processing, textile manufacturing, and food production facilities. The technology enables water reuse opportunities while ensuring compliance with discharge regulations through effective removal of contaminants.Expand Specific Solutions03 Biological and pharmaceutical applications
Ultrafiltration plays a crucial role in biological and pharmaceutical processes, including protein purification, enzyme recovery, and vaccine production. The technology enables concentration and fractionation of biomolecules based on molecular size while preserving their biological activity. Specialized ultrafiltration systems are designed to handle sensitive biological materials under controlled conditions, ensuring product quality and sterility requirements are met for pharmaceutical and biotechnology applications.Expand Specific Solutions04 Process optimization and control systems
Advanced control systems and optimization techniques enhance ultrafiltration performance by monitoring and adjusting operational parameters in real-time. These systems incorporate sensors, automated valves, and programmable logic controllers to maintain optimal flux rates, transmembrane pressure, and cleaning cycles. Process optimization strategies include feed pretreatment, cross-flow velocity adjustment, and backwashing sequences to maximize membrane lifespan and filtration efficiency while minimizing energy consumption.Expand Specific Solutions05 Hybrid and integrated filtration systems
Hybrid ultrafiltration systems combine ultrafiltration with other separation technologies such as reverse osmosis, nanofiltration, or conventional treatment methods to achieve comprehensive water purification. These integrated approaches leverage the strengths of each technology to address complex treatment challenges. Multi-stage filtration trains can be designed to progressively remove contaminants of decreasing size, from suspended solids to dissolved salts, providing versatile solutions for drinking water production, industrial process water, and specialized applications.Expand Specific Solutions
Key Industry Players in Pharmaceutical Ultrafiltration
Ultrafiltration in the pharmaceutical sector is experiencing robust growth, currently in a mature expansion phase with an estimated market size of $1.5-2 billion and projected CAGR of 7-9% through 2028. The technology has reached high maturity levels with established applications in downstream processing, protein purification, and vaccine manufacturing. Key players demonstrate varying technological sophistication: EMD Millipore (MilliporeSigma) and Merck Patent GmbH lead with comprehensive filtration portfolios; major pharmaceutical manufacturers like Novartis, AbbVie, Amgen, and Genentech have integrated advanced ultrafiltration systems into production; while emerging players such as FloDesign Sonics and Challenge IM are introducing innovative acoustic-based and specialized membrane technologies, disrupting traditional approaches to biopharmaceutical filtration.
EMD Millipore Corp.
Technical Solution: EMD Millipore has developed comprehensive ultrafiltration solutions specifically designed for pharmaceutical applications, including their Pellicon and Cogent Process Scale systems. Their technology employs tangential flow filtration (TFF) with specialized membranes that achieve high-performance separation based on molecular size. Their systems feature automated process control with integrated sensors for real-time monitoring of transmembrane pressure, flow rates, and filtration parameters. EMD Millipore's pharmaceutical-grade ultrafiltration technology enables efficient concentration and diafiltration of therapeutic proteins, vaccines, and other biologics while maintaining product integrity. Their systems are designed with scalability in mind, allowing seamless transition from laboratory development to commercial manufacturing scales, which is crucial for pharmaceutical process development. Additionally, they've incorporated single-use technology options to reduce cross-contamination risks and cleaning validation requirements.
Strengths: Industry-leading membrane technology with exceptional selectivity and reproducibility; comprehensive validation documentation supporting regulatory compliance; integrated process control systems. Weaknesses: Higher capital investment compared to some competitors; proprietary consumables can increase operational costs; systems may require specialized training for optimal operation.
Genentech, Inc.
Technical Solution: Genentech has developed sophisticated ultrafiltration applications specifically optimized for therapeutic protein manufacturing. Their approach incorporates high-performance tangential flow filtration (TFF) systems with specialized membrane configurations designed to maintain protein structural integrity while achieving efficient concentration and buffer exchange. Genentech's ultrafiltration technology features advanced process control systems that continuously monitor and adjust critical parameters including transmembrane pressure, crossflow velocity, and concentration factors to optimize filtration performance. Their implementation includes innovative diafiltration strategies that minimize buffer consumption while ensuring complete exchange, significantly reducing processing costs for high-value therapeutics. Genentech has also pioneered the integration of single-use ultrafiltration components into their manufacturing processes, reducing cleaning validation requirements and cross-contamination risks. Their systems incorporate comprehensive process analytical technology (PAT) tools that provide real-time monitoring of protein concentration, aggregation levels, and membrane performance. Additionally, Genentech has developed scalable ultrafiltration approaches that maintain consistent performance from laboratory development through commercial manufacturing scales, facilitating seamless technology transfer.
Strengths: Exceptional protein quality preservation during processing; comprehensive process understanding supported by extensive characterization data; demonstrated success with diverse therapeutic modalities including antibodies and fusion proteins. Weaknesses: Complex implementation requiring specialized expertise; higher capital investment compared to conventional filtration; significant process development resources required for optimization.
Critical Patents and Innovations in Pharmaceutical Ultrafiltration
Long-term implantable monitoring system & methods of use
PatentWO2016014987A2
Innovation
- A long-term implantable ultra-filtrate monitoring system using micro-porous membranes to produce an ultra-filtrate of interstitial fluid or blood plasma, which is then transported through a flow-through sensor for continuous monitoring of glucose and other analytes, with a micro-processor controlled pump system and pressure transducers to maintain optimal fluid flow and absorption into capillary and lymphatic vessels.
Process for concentration of antibodies and therapeutic products thereof
PatentInactiveEP1786830A2
Innovation
- A process involving sequential ultrafiltration and diafiltration steps at elevated temperatures (e.g., 30°C to 50°C) to concentrate proteins, including a first ultrafiltration, diafiltration, and a second ultrafiltration, which enhances flux and throughput while minimizing process time and membrane area requirements.
Regulatory Compliance and Validation Requirements
Pharmaceutical ultrafiltration processes must adhere to stringent regulatory frameworks established by global health authorities. The FDA's Current Good Manufacturing Practices (cGMP) and the European Medicines Agency (EMA) guidelines form the cornerstone of these requirements, mandating comprehensive validation protocols for all filtration systems used in drug production. These regulations emphasize the need for consistent product quality, process reproducibility, and contamination control throughout the manufacturing lifecycle.
Validation requirements for ultrafiltration systems in pharmaceutical applications follow a structured approach encompassing Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that equipment specifications match design requirements and that installation complies with manufacturer recommendations. OQ confirms that the ultrafiltration system operates within established parameters across normal and stress conditions. PQ demonstrates consistent performance during actual production conditions over extended periods.
Process Analytical Technology (PAT) integration has become increasingly important for real-time monitoring of ultrafiltration processes. Regulatory bodies now encourage implementation of PAT tools to enhance process understanding and control, allowing for continuous verification rather than solely relying on end-product testing. This approach aligns with Quality by Design (QbD) principles, which regulatory agencies have embraced to ensure built-in quality throughout pharmaceutical manufacturing.
Documentation requirements present significant challenges for pharmaceutical manufacturers utilizing ultrafiltration technology. Companies must maintain comprehensive records of membrane integrity testing, cleaning validation, sterilization procedures, and process parameters. These records must demonstrate that the ultrafiltration system consistently produces results within predetermined specifications and must be readily available during regulatory inspections.
Cross-border regulatory harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have attempted to standardize validation approaches globally. However, regional variations in regulatory interpretation still necessitate tailored compliance strategies for multinational pharmaceutical operations employing ultrafiltration technologies.
Environmental considerations have gained prominence in recent regulatory updates. Sustainable practices in ultrafiltration processes, including water conservation and waste management, are increasingly scrutinized by regulatory bodies. Manufacturers must demonstrate responsible environmental stewardship alongside traditional quality and safety compliance measures.
Risk-based validation approaches have been endorsed by major regulatory agencies, allowing companies to focus validation efforts proportionally to identified risks. This methodology requires thorough risk assessment of ultrafiltration processes, with enhanced validation activities directed toward higher-risk aspects while maintaining appropriate oversight of lower-risk elements.
Validation requirements for ultrafiltration systems in pharmaceutical applications follow a structured approach encompassing Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ verifies that equipment specifications match design requirements and that installation complies with manufacturer recommendations. OQ confirms that the ultrafiltration system operates within established parameters across normal and stress conditions. PQ demonstrates consistent performance during actual production conditions over extended periods.
Process Analytical Technology (PAT) integration has become increasingly important for real-time monitoring of ultrafiltration processes. Regulatory bodies now encourage implementation of PAT tools to enhance process understanding and control, allowing for continuous verification rather than solely relying on end-product testing. This approach aligns with Quality by Design (QbD) principles, which regulatory agencies have embraced to ensure built-in quality throughout pharmaceutical manufacturing.
Documentation requirements present significant challenges for pharmaceutical manufacturers utilizing ultrafiltration technology. Companies must maintain comprehensive records of membrane integrity testing, cleaning validation, sterilization procedures, and process parameters. These records must demonstrate that the ultrafiltration system consistently produces results within predetermined specifications and must be readily available during regulatory inspections.
Cross-border regulatory harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), have attempted to standardize validation approaches globally. However, regional variations in regulatory interpretation still necessitate tailored compliance strategies for multinational pharmaceutical operations employing ultrafiltration technologies.
Environmental considerations have gained prominence in recent regulatory updates. Sustainable practices in ultrafiltration processes, including water conservation and waste management, are increasingly scrutinized by regulatory bodies. Manufacturers must demonstrate responsible environmental stewardship alongside traditional quality and safety compliance measures.
Risk-based validation approaches have been endorsed by major regulatory agencies, allowing companies to focus validation efforts proportionally to identified risks. This methodology requires thorough risk assessment of ultrafiltration processes, with enhanced validation activities directed toward higher-risk aspects while maintaining appropriate oversight of lower-risk elements.
Cost-Benefit Analysis of Ultrafiltration Implementation
Implementing ultrafiltration systems in pharmaceutical manufacturing requires substantial initial capital investment, ranging from $500,000 to $3 million depending on scale and complexity. This includes equipment costs, installation, validation protocols, and facility modifications. However, these systems typically demonstrate positive return on investment within 2-4 years through multiple cost reduction mechanisms.
Operational cost savings represent a significant benefit, with ultrafiltration reducing downstream processing costs by 15-30% compared to conventional separation methods. Energy consumption decreases by approximately 40-60% versus thermal concentration techniques, while water usage can be reduced by up to 75% through recycling capabilities. Additionally, ultrafiltration systems require 30-50% less floor space than traditional separation equipment, optimizing facility utilization.
Product yield improvements constitute another major economic advantage. Studies across multiple pharmaceutical applications demonstrate 5-15% higher API recovery rates using ultrafiltration compared to conventional methods. For high-value biopharmaceuticals, this yield improvement can translate to millions in additional revenue annually. The technology also reduces batch rejection rates by approximately 25% through more consistent separation performance.
Regulatory compliance represents both a cost and benefit consideration. While validation expenses for ultrafiltration systems average $100,000-$250,000 initially, these systems typically experience fewer compliance issues long-term. FDA and EMA data indicate facilities utilizing membrane-based separation technologies face approximately 30% fewer observations during inspections related to product consistency and purity.
Maintenance requirements must be factored into cost-benefit calculations. Membrane replacement constitutes the primary recurring expense, with replacement intervals ranging from 6-24 months depending on application. However, modern ultrafiltration systems feature improved membrane durability, reducing lifetime operational costs by approximately 20% compared to systems from a decade ago.
Environmental impact considerations increasingly influence cost-benefit analyses. Pharmaceutical companies implementing ultrafiltration report 30-50% reduction in waste disposal costs and carbon footprint improvements that support ESG objectives. Several manufacturers have documented cases where ultrafiltration implementation contributed significantly to achieving sustainability targets, enhancing corporate reputation and stakeholder relations.
Operational cost savings represent a significant benefit, with ultrafiltration reducing downstream processing costs by 15-30% compared to conventional separation methods. Energy consumption decreases by approximately 40-60% versus thermal concentration techniques, while water usage can be reduced by up to 75% through recycling capabilities. Additionally, ultrafiltration systems require 30-50% less floor space than traditional separation equipment, optimizing facility utilization.
Product yield improvements constitute another major economic advantage. Studies across multiple pharmaceutical applications demonstrate 5-15% higher API recovery rates using ultrafiltration compared to conventional methods. For high-value biopharmaceuticals, this yield improvement can translate to millions in additional revenue annually. The technology also reduces batch rejection rates by approximately 25% through more consistent separation performance.
Regulatory compliance represents both a cost and benefit consideration. While validation expenses for ultrafiltration systems average $100,000-$250,000 initially, these systems typically experience fewer compliance issues long-term. FDA and EMA data indicate facilities utilizing membrane-based separation technologies face approximately 30% fewer observations during inspections related to product consistency and purity.
Maintenance requirements must be factored into cost-benefit calculations. Membrane replacement constitutes the primary recurring expense, with replacement intervals ranging from 6-24 months depending on application. However, modern ultrafiltration systems feature improved membrane durability, reducing lifetime operational costs by approximately 20% compared to systems from a decade ago.
Environmental impact considerations increasingly influence cost-benefit analyses. Pharmaceutical companies implementing ultrafiltration report 30-50% reduction in waste disposal costs and carbon footprint improvements that support ESG objectives. Several manufacturers have documented cases where ultrafiltration implementation contributed significantly to achieving sustainability targets, enhancing corporate reputation and stakeholder relations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!