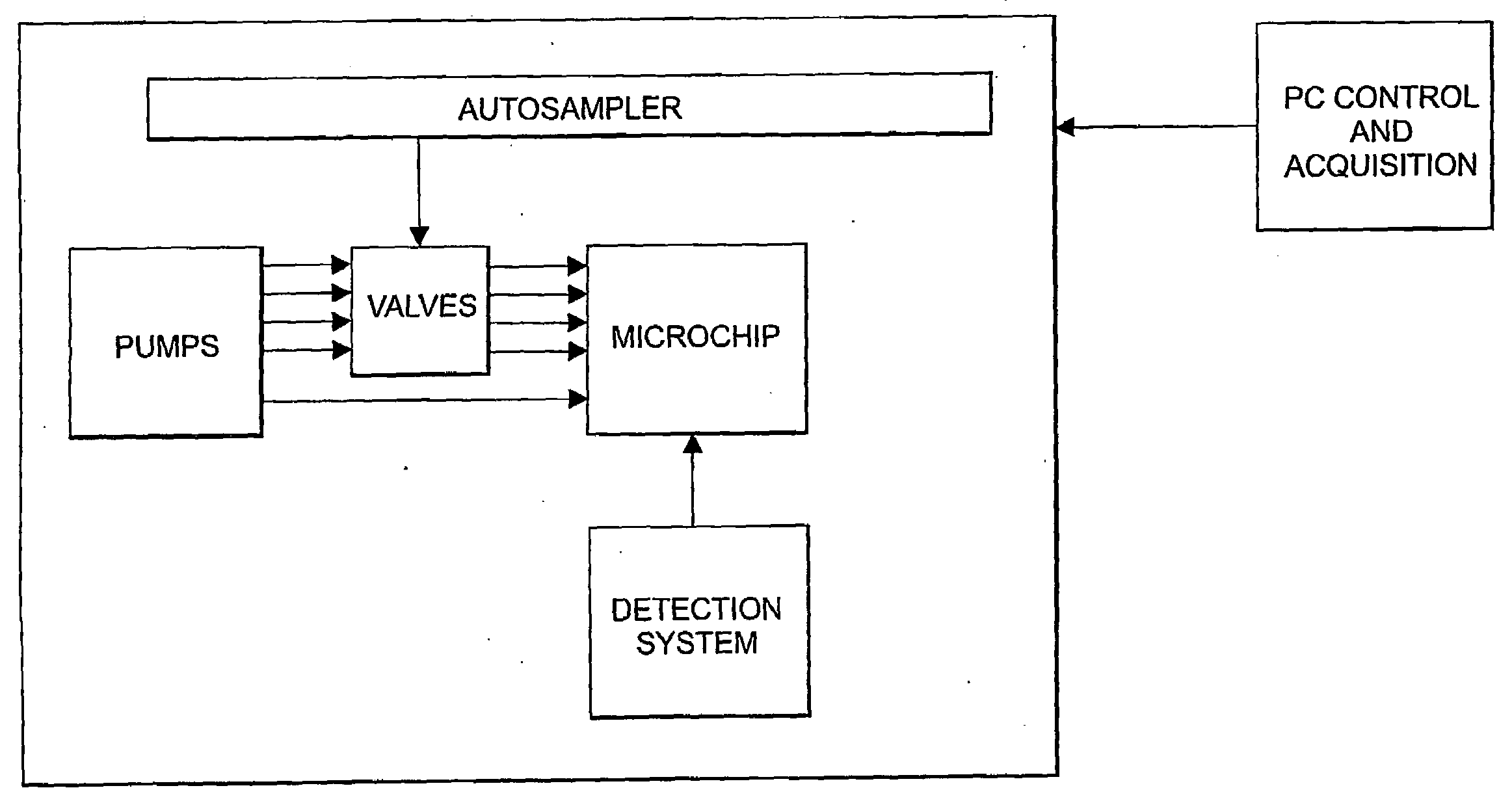
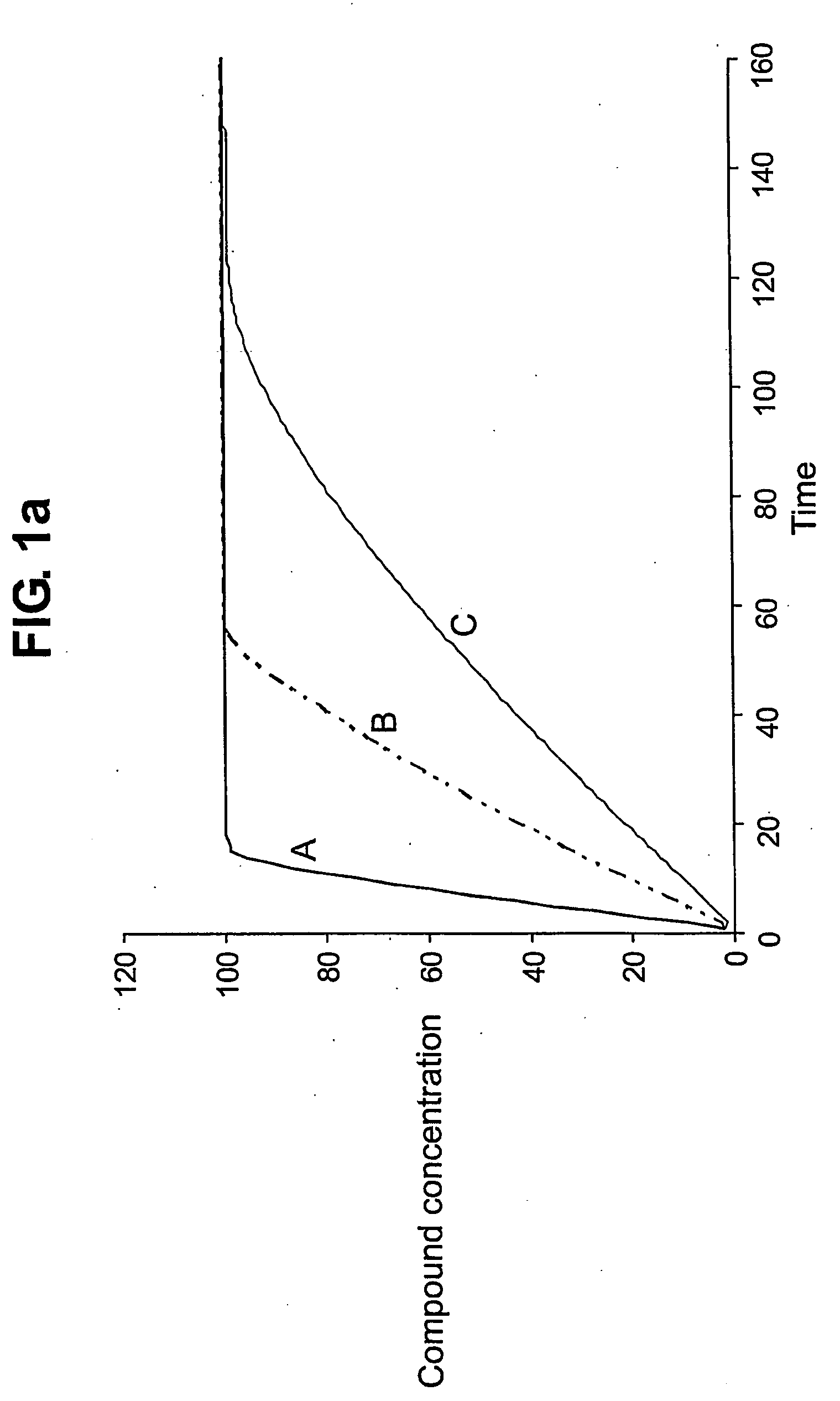
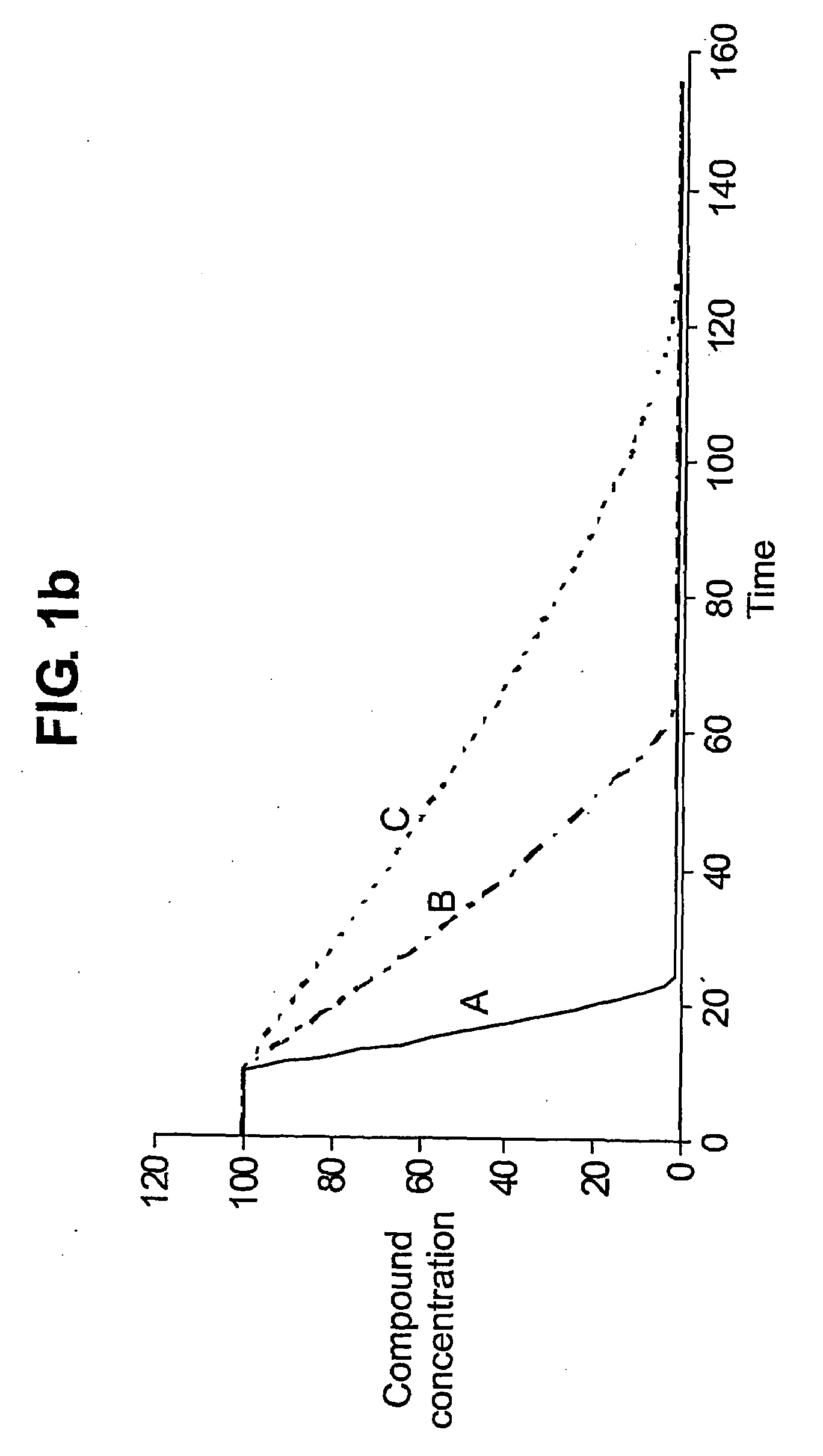
Augmenting Clinical Trials Using Microfluidic Techniques
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Technology Background and Objectives
Microfluidic technology has evolved significantly over the past three decades, transforming from a conceptual laboratory technique to a powerful platform with diverse applications across multiple scientific disciplines. The fundamental principle of microfluidics involves manipulating fluids at the microscale level, typically handling volumes in the range of picoliters to nanoliters within channels measuring tens to hundreds of micrometers. This technology emerged from the convergence of molecular biology, microelectronics, and microsystem engineering in the early 1990s.
The evolution of microfluidic systems has been marked by several key milestones, including the development of soft lithography techniques using polydimethylsiloxane (PDMS) in the late 1990s, which dramatically reduced fabrication costs and increased accessibility. Subsequently, the integration of various functional components such as valves, pumps, and sensors has enabled increasingly sophisticated fluid manipulation capabilities, leading to the concept of "lab-on-a-chip" systems.
In recent years, microfluidic technology has demonstrated particular promise in revolutionizing clinical trials through its ability to provide high-throughput screening, precise control over experimental conditions, and significant reduction in reagent consumption. The miniaturization of traditional laboratory processes offers unprecedented opportunities for accelerating drug development pipelines while simultaneously reducing associated costs.
The primary technical objectives for microfluidic applications in clinical trials include developing platforms capable of mimicking human physiological conditions more accurately than conventional in vitro models. This encompasses the creation of organ-on-chip systems that replicate the structure and function of human tissues, enabling more predictive preclinical testing. Additionally, there is a focus on establishing high-throughput screening capabilities for rapid assessment of drug candidates across multiple parameters simultaneously.
Another critical objective involves enhancing the sensitivity and specificity of diagnostic applications within clinical trial settings. Microfluidic devices capable of detecting biomarkers at extremely low concentrations could enable earlier intervention and more precise patient stratification, potentially improving clinical trial outcomes and efficiency.
The integration of microfluidics with complementary technologies represents an important trend in this field. Combining microfluidic platforms with advanced imaging techniques, artificial intelligence for data analysis, and wireless connectivity for remote monitoring creates powerful systems capable of generating and processing complex biological data in real-time during clinical trials.
Looking forward, the trajectory of microfluidic technology development is increasingly focused on addressing challenges related to standardization, scalability, and regulatory compliance. These factors are essential for the broader adoption of microfluidic techniques in clinical trial settings, where reproducibility and reliability are paramount concerns.
The evolution of microfluidic systems has been marked by several key milestones, including the development of soft lithography techniques using polydimethylsiloxane (PDMS) in the late 1990s, which dramatically reduced fabrication costs and increased accessibility. Subsequently, the integration of various functional components such as valves, pumps, and sensors has enabled increasingly sophisticated fluid manipulation capabilities, leading to the concept of "lab-on-a-chip" systems.
In recent years, microfluidic technology has demonstrated particular promise in revolutionizing clinical trials through its ability to provide high-throughput screening, precise control over experimental conditions, and significant reduction in reagent consumption. The miniaturization of traditional laboratory processes offers unprecedented opportunities for accelerating drug development pipelines while simultaneously reducing associated costs.
The primary technical objectives for microfluidic applications in clinical trials include developing platforms capable of mimicking human physiological conditions more accurately than conventional in vitro models. This encompasses the creation of organ-on-chip systems that replicate the structure and function of human tissues, enabling more predictive preclinical testing. Additionally, there is a focus on establishing high-throughput screening capabilities for rapid assessment of drug candidates across multiple parameters simultaneously.
Another critical objective involves enhancing the sensitivity and specificity of diagnostic applications within clinical trial settings. Microfluidic devices capable of detecting biomarkers at extremely low concentrations could enable earlier intervention and more precise patient stratification, potentially improving clinical trial outcomes and efficiency.
The integration of microfluidics with complementary technologies represents an important trend in this field. Combining microfluidic platforms with advanced imaging techniques, artificial intelligence for data analysis, and wireless connectivity for remote monitoring creates powerful systems capable of generating and processing complex biological data in real-time during clinical trials.
Looking forward, the trajectory of microfluidic technology development is increasingly focused on addressing challenges related to standardization, scalability, and regulatory compliance. These factors are essential for the broader adoption of microfluidic techniques in clinical trial settings, where reproducibility and reliability are paramount concerns.
Clinical Trial Market Needs Analysis
The clinical trial landscape is experiencing significant challenges that microfluidic technologies could potentially address. Current clinical trials face escalating costs, with the average expense for bringing a new drug to market exceeding $2.6 billion, while success rates remain discouragingly low at approximately 12% across all therapeutic areas. This economic pressure creates a substantial market need for technologies that can reduce costs while improving success rates.
Time efficiency represents another critical market demand, as traditional clinical trials typically require 7-10 years from conception to completion. This extended timeline delays patient access to potentially life-saving treatments and reduces the effective patent protection period for pharmaceutical companies. Microfluidic technologies offer the potential to accelerate various stages of the clinical trial process through rapid sample processing, real-time monitoring, and automated analysis.
Patient recruitment and retention continue to be significant hurdles, with over 80% of clinical trials failing to meet enrollment deadlines. This challenge is particularly acute for rare disease studies where patient populations are inherently limited. The market increasingly demands personalized approaches that can maximize data collection from smaller patient cohorts, an area where microfluidic organ-on-chip and patient-derived organoid technologies show particular promise.
Data quality and reproducibility concerns persist throughout the industry, with studies indicating that approximately 50-85% of preclinical research results cannot be replicated in subsequent investigations. This reproducibility crisis undermines confidence in trial outcomes and increases development costs through failed confirmatory studies. The controlled microenvironments provided by microfluidic platforms offer improved standardization and reproducibility compared to conventional methods.
Regulatory bodies worldwide are increasingly emphasizing the need for reduced animal testing while maintaining or improving safety assessments. This regulatory shift creates market demand for alternative testing platforms that can better predict human responses. Microfluidic human-on-chip technologies align with this trend by providing physiologically relevant models that may better recapitulate human biology than traditional animal models.
The growing focus on precision medicine has created market demand for technologies that can support stratified or personalized approaches to clinical trials. Pharmaceutical companies seek methods to identify responder populations earlier in the development process, potentially increasing success rates and reducing costs. Microfluidic technologies enable high-throughput screening of patient-derived samples against multiple treatment conditions, supporting this precision medicine paradigm.
Time efficiency represents another critical market demand, as traditional clinical trials typically require 7-10 years from conception to completion. This extended timeline delays patient access to potentially life-saving treatments and reduces the effective patent protection period for pharmaceutical companies. Microfluidic technologies offer the potential to accelerate various stages of the clinical trial process through rapid sample processing, real-time monitoring, and automated analysis.
Patient recruitment and retention continue to be significant hurdles, with over 80% of clinical trials failing to meet enrollment deadlines. This challenge is particularly acute for rare disease studies where patient populations are inherently limited. The market increasingly demands personalized approaches that can maximize data collection from smaller patient cohorts, an area where microfluidic organ-on-chip and patient-derived organoid technologies show particular promise.
Data quality and reproducibility concerns persist throughout the industry, with studies indicating that approximately 50-85% of preclinical research results cannot be replicated in subsequent investigations. This reproducibility crisis undermines confidence in trial outcomes and increases development costs through failed confirmatory studies. The controlled microenvironments provided by microfluidic platforms offer improved standardization and reproducibility compared to conventional methods.
Regulatory bodies worldwide are increasingly emphasizing the need for reduced animal testing while maintaining or improving safety assessments. This regulatory shift creates market demand for alternative testing platforms that can better predict human responses. Microfluidic human-on-chip technologies align with this trend by providing physiologically relevant models that may better recapitulate human biology than traditional animal models.
The growing focus on precision medicine has created market demand for technologies that can support stratified or personalized approaches to clinical trials. Pharmaceutical companies seek methods to identify responder populations earlier in the development process, potentially increasing success rates and reducing costs. Microfluidic technologies enable high-throughput screening of patient-derived samples against multiple treatment conditions, supporting this precision medicine paradigm.
Current Microfluidic Applications and Barriers
Microfluidic technologies have gained significant traction in clinical trial applications, offering unprecedented capabilities for drug development and testing processes. Current applications span several key areas, with organ-on-a-chip platforms representing one of the most promising developments. These sophisticated systems recreate the physiological microenvironment of human organs, enabling researchers to observe drug responses in conditions closely mimicking in vivo scenarios, thereby potentially reducing animal testing requirements and improving predictive accuracy.
Patient-derived tumor-on-chip models have emerged as valuable tools for personalized medicine approaches in oncology trials. These platforms allow for the testing of multiple drug combinations on patient-specific cancer cells, facilitating more targeted therapeutic strategies and potentially accelerating the identification of effective treatments for individual patients.
High-throughput drug screening represents another significant application, where microfluidic devices enable parallel testing of numerous drug candidates against cellular targets. This capability substantially reduces the time and resources required for preliminary drug efficacy assessments, streamlining the early phases of clinical trials.
Despite these promising applications, several barriers impede widespread adoption of microfluidic techniques in clinical trials. Technical challenges include difficulties in achieving consistent cell culture conditions across microfluidic platforms, which can lead to variability in experimental outcomes. The integration of sensing technologies for real-time monitoring remains complex, limiting the ability to capture dynamic cellular responses to therapeutic interventions.
Standardization issues present significant obstacles, as the lack of universally accepted protocols for microfluidic device fabrication and operation complicates cross-laboratory validation and regulatory compliance. This absence of standardization creates uncertainty in data interpretation and hampers the establishment of microfluidics as a routine tool in clinical trial workflows.
Regulatory hurdles constitute another major barrier, with regulatory bodies still developing frameworks to evaluate and approve microfluidic-based evidence in clinical trial submissions. The novelty of these technologies means that clear pathways for validation and qualification remain underdeveloped, creating uncertainty for pharmaceutical companies considering their implementation.
Scalability concerns also limit adoption, as many current microfluidic applications remain confined to laboratory settings with limited throughput capabilities. The transition from proof-of-concept demonstrations to industrial-scale implementations capable of supporting large clinical trials requires significant technological advancement and investment.
Cost factors further restrict widespread implementation, with specialized equipment, materials, and expertise representing substantial financial barriers, particularly for smaller research organizations and biotechnology startups. The return on investment remains uncertain without established precedents demonstrating clear advantages in clinical trial outcomes.
Patient-derived tumor-on-chip models have emerged as valuable tools for personalized medicine approaches in oncology trials. These platforms allow for the testing of multiple drug combinations on patient-specific cancer cells, facilitating more targeted therapeutic strategies and potentially accelerating the identification of effective treatments for individual patients.
High-throughput drug screening represents another significant application, where microfluidic devices enable parallel testing of numerous drug candidates against cellular targets. This capability substantially reduces the time and resources required for preliminary drug efficacy assessments, streamlining the early phases of clinical trials.
Despite these promising applications, several barriers impede widespread adoption of microfluidic techniques in clinical trials. Technical challenges include difficulties in achieving consistent cell culture conditions across microfluidic platforms, which can lead to variability in experimental outcomes. The integration of sensing technologies for real-time monitoring remains complex, limiting the ability to capture dynamic cellular responses to therapeutic interventions.
Standardization issues present significant obstacles, as the lack of universally accepted protocols for microfluidic device fabrication and operation complicates cross-laboratory validation and regulatory compliance. This absence of standardization creates uncertainty in data interpretation and hampers the establishment of microfluidics as a routine tool in clinical trial workflows.
Regulatory hurdles constitute another major barrier, with regulatory bodies still developing frameworks to evaluate and approve microfluidic-based evidence in clinical trial submissions. The novelty of these technologies means that clear pathways for validation and qualification remain underdeveloped, creating uncertainty for pharmaceutical companies considering their implementation.
Scalability concerns also limit adoption, as many current microfluidic applications remain confined to laboratory settings with limited throughput capabilities. The transition from proof-of-concept demonstrations to industrial-scale implementations capable of supporting large clinical trials requires significant technological advancement and investment.
Cost factors further restrict widespread implementation, with specialized equipment, materials, and expertise representing substantial financial barriers, particularly for smaller research organizations and biotechnology startups. The return on investment remains uncertain without established precedents demonstrating clear advantages in clinical trial outcomes.
Current Microfluidic Approaches for Clinical Trials
01 Microfluidic device fabrication techniques
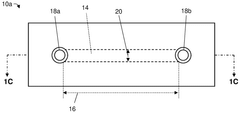
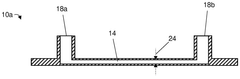
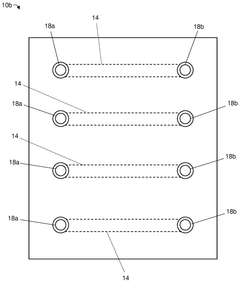
Various fabrication methods are employed to create microfluidic devices with precise channel geometries and surface properties. These techniques include soft lithography, etching, 3D printing, and bonding processes that enable the production of complex microfluidic structures. Advanced fabrication approaches allow for the integration of multiple functional components such as valves, pumps, and sensors within a single microfluidic platform.- Microfluidic device fabrication and design: Various techniques for fabricating microfluidic devices, including materials selection, channel design, and manufacturing processes. These approaches enable precise control over fluid flow at microscale dimensions, allowing for miniaturized laboratory operations. Advanced fabrication methods incorporate features like valves, mixers, and sensors directly into microfluidic platforms, enhancing functionality while maintaining compact form factors.
- Droplet-based microfluidics: Systems that generate, manipulate, and analyze discrete droplets within microfluidic channels. These droplets function as isolated microreactors, enabling high-throughput experimentation and analysis. The technology allows precise control over droplet size, composition, and movement, making it valuable for applications requiring compartmentalization of reactions or samples, such as digital PCR, single-cell analysis, and chemical synthesis.
- Microfluidic systems for biological applications: Specialized microfluidic platforms designed for biological research, diagnostics, and therapeutic applications. These systems enable manipulation of cells, proteins, and other biomolecules in controlled microenvironments. Applications include organ-on-a-chip models, point-of-care diagnostics, cell sorting, and drug screening assays. The microscale environment allows for reduced reagent consumption, faster analysis times, and more physiologically relevant conditions compared to traditional methods.
- Flow control and manipulation in microfluidics: Techniques for precisely controlling fluid movement within microfluidic channels, including pumping mechanisms, valve systems, and flow regulators. These methods enable complex fluid handling operations such as mixing, separation, and gradient generation at the microscale. Advanced flow control systems incorporate active elements like electrokinetic forces, acoustic waves, or magnetic fields to manipulate fluids and suspended particles with high precision.
- Integration of sensing and detection in microfluidic systems: Methods for incorporating analytical capabilities directly into microfluidic platforms, enabling real-time monitoring and detection of analytes. These integrated systems combine microfluidic handling with detection technologies such as optical, electrochemical, or mass spectrometry-based sensing. The integration allows for automated sample processing and analysis within a single device, reducing contamination risks and analysis time while improving sensitivity and reproducibility.
02 Fluid manipulation and control systems
Microfluidic techniques for precise control and manipulation of fluids at the microscale include pressure-driven flow, electrokinetic transport, and centrifugal forces. These systems incorporate specialized components such as micropumps, microvalves, and flow regulators to achieve accurate fluid handling. Advanced control mechanisms enable dynamic adjustment of flow rates, mixing ratios, and reaction conditions within microfluidic channels.Expand Specific Solutions03 Microfluidic analytical and detection methods
Microfluidic platforms integrate various analytical and detection techniques for sensitive and rapid analysis of samples. These methods include optical detection, electrochemical sensing, mass spectrometry coupling, and spectroscopic approaches. The miniaturized format allows for reduced sample volumes, faster analysis times, and improved detection limits compared to conventional analytical techniques, making them valuable tools for diagnostics, environmental monitoring, and chemical analysis.Expand Specific Solutions04 Droplet-based microfluidics
Droplet-based microfluidic systems enable the generation, manipulation, and analysis of discrete droplets within immiscible carrier fluids. These techniques allow for compartmentalization of reactions, high-throughput screening, and digital analysis of biological samples. The ability to create uniform droplets with precise volumes facilitates applications such as single-cell analysis, directed evolution, and digital PCR where isolation of individual reaction components is critical.Expand Specific Solutions05 Microfluidic applications in biological and medical research
Microfluidic techniques have been widely applied in biological and medical research, including organ-on-a-chip systems, point-of-care diagnostics, and drug delivery platforms. These applications leverage the advantages of microfluidics such as controlled microenvironments, physiologically relevant flow conditions, and reduced reagent consumption. The integration of biological components with microfluidic systems enables the development of advanced in vitro models for disease research, drug screening, and personalized medicine approaches.Expand Specific Solutions
Leading Companies in Microfluidic Clinical Solutions
Microfluidic techniques in clinical trials are currently in a growth phase, with the market expanding rapidly due to increasing demand for more efficient, cost-effective trial methodologies. The global market is projected to reach significant scale as pharmaceutical companies seek to reduce development costs and timelines. Technologically, the field shows varying maturity levels across applications, with companies like Roche Diagnostics, Revvity Health Sciences, and Becton, Dickinson & Co. leading commercial implementation. Research institutions such as California Institute of Technology and academic players like Nanjing University are advancing fundamental innovations, while specialized firms like Micronics and Reliant Immune Diagnostics focus on point-of-care applications. The ecosystem demonstrates a healthy mix of established medical technology corporations and emerging specialized players collaborating to overcome remaining technical challenges.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences has developed advanced microfluidic platforms that integrate high-throughput screening capabilities with precision medicine applications for clinical trials. Their technology employs digital microfluidic systems capable of generating and analyzing thousands of discrete nanoliter-sized droplets per second, each functioning as an independent reaction vessel. This approach enables massive parallelization of biochemical assays while consuming minimal reagent volumes (reducing consumption by up to 99% compared to conventional methods). The company's microfluidic chips incorporate sophisticated surface modifications using proprietary polymers that minimize non-specific binding and sample loss, achieving exceptional sensitivity for detecting low-abundance biomarkers. Revvity's platforms feature integrated cell isolation and single-cell analysis capabilities, allowing researchers to characterize rare cell populations within heterogeneous clinical samples with unprecedented resolution. Their systems also incorporate automated quality control measures that continuously monitor assay performance across multiple parameters, ensuring data consistency throughout multi-site clinical trials. Additionally, Revvity has developed specialized microfluidic devices for organ-on-chip applications that can model patient-specific drug responses prior to actual patient dosing.
Strengths: Exceptional throughput capabilities combined with minimal sample requirements make their platforms ideal for large-scale biomarker discovery in clinical trials. Advanced automation reduces operator-dependent variability. Weaknesses: Complex systems require specialized technical expertise, and integration with existing clinical workflows can present implementation challenges.
Roche Sequencing Solutions, Inc.
Technical Solution: Roche Sequencing Solutions has developed microfluidic-based next-generation sequencing (NGS) preparation technologies specifically optimized for clinical trial applications. Their platform utilizes droplet-based digital microfluidics to partition individual DNA molecules into nanoliter-sized reaction chambers, enabling highly sensitive detection of rare genetic variants at frequencies as low as 0.1%. The company's microfluidic chips incorporate integrated valves and pumps fabricated using multi-layer soft lithography techniques, allowing precise control over sample processing steps including DNA fragmentation, end repair, adapter ligation, and amplification. This automation significantly reduces hands-on time from approximately 8 hours to less than 2 hours while minimizing cross-contamination risks. Their technology enables simultaneous processing of multiple patient samples through spatial multiplexing within a single microfluidic device, increasing throughput while maintaining sample integrity. The platform also features real-time quality control monitoring throughout the workflow, ensuring consistent data quality across different clinical trial sites.
Strengths: Exceptional sensitivity for detecting rare genetic variants and biomarkers critical for precision medicine trials. Highly automated workflows reduce technical variability between operators and sites. Weaknesses: Higher per-sample costs compared to conventional bulk processing methods, and specialized equipment requirements may limit accessibility for some research settings.
Key Microfluidic Patents and Research Breakthroughs
Microfluidic system and methods
PatentInactiveUS20090142853A1
Innovation
- A microfluidic system with fluorinated or fluorous channel surfaces and a non-aqueous sheath medium immiscible with the aqueous medium is used to prevent adsorption of components, maintaining their concentration and reducing the need for washing, thereby enhancing assay reliability and throughput.
Methods for performing miniaturized dynamic assays using microfluidics and related systems
PatentWO2025144420A1
Innovation
- The use of microfluidic chips with channels less than 100 microliters in volume, where therapeutic reagents flow over cultured target cells under controlled conditions, mimicking physiological environments with features like shear stress, gas composition, and temperature, allowing for dynamic interaction analysis.
Regulatory Considerations for Microfluidic Clinical Tools
The integration of microfluidic technologies into clinical trials necessitates careful navigation of complex regulatory frameworks across global jurisdictions. In the United States, the FDA has established specific pathways for microfluidic devices through the Center for Devices and Radiological Health (CDRH), with classification depending on risk level and intended use. Most microfluidic tools for clinical trials fall under Class II (moderate risk) or Class III (high risk) categories, requiring either 510(k) clearance or Premarket Approval (PMA).
European regulatory frameworks present additional considerations through the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stringent requirements for clinical evidence, post-market surveillance, and unique device identification. Manufacturers must obtain CE marking by demonstrating compliance with these regulations, a process that typically involves notified body assessment for higher-risk classifications.
Quality management systems conforming to ISO 13485 standards are essential for regulatory compliance across most jurisdictions. This standard specifies requirements for design, development, production, installation, and servicing of medical devices, including microfluidic technologies. Additionally, microfluidic devices used in clinical trials must adhere to Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards.
Data privacy and security regulations present another critical dimension, particularly for microfluidic devices that generate patient data. Compliance with GDPR in Europe, HIPAA in the US, and equivalent regulations in other regions is mandatory. These regulations govern data collection, storage, processing, and transfer, with significant penalties for non-compliance.
Validation and verification protocols represent a substantial regulatory hurdle for microfluidic clinical tools. Manufacturers must demonstrate analytical validity (accuracy, precision, sensitivity, specificity), clinical validity (correlation with clinical outcomes), and clinical utility (improvement in patient management). The FDA's recent Biomarker Qualification Program offers potential pathways for novel biomarkers detected through microfluidic technologies.
Regulatory harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually standardizing requirements across regions, though significant differences remain. Companies developing microfluidic technologies for clinical trials should engage with regulatory authorities early through pre-submission consultations and consider adaptive clinical trial designs that incorporate regulatory feedback throughout development phases.
Emerging regulatory frameworks for combination products (device-drug combinations) and companion diagnostics are particularly relevant for microfluidic technologies that integrate therapeutic monitoring or patient stratification capabilities. These frameworks typically involve coordination between multiple regulatory divisions and require comprehensive evidence of both device performance and clinical benefit.
European regulatory frameworks present additional considerations through the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stringent requirements for clinical evidence, post-market surveillance, and unique device identification. Manufacturers must obtain CE marking by demonstrating compliance with these regulations, a process that typically involves notified body assessment for higher-risk classifications.
Quality management systems conforming to ISO 13485 standards are essential for regulatory compliance across most jurisdictions. This standard specifies requirements for design, development, production, installation, and servicing of medical devices, including microfluidic technologies. Additionally, microfluidic devices used in clinical trials must adhere to Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards.
Data privacy and security regulations present another critical dimension, particularly for microfluidic devices that generate patient data. Compliance with GDPR in Europe, HIPAA in the US, and equivalent regulations in other regions is mandatory. These regulations govern data collection, storage, processing, and transfer, with significant penalties for non-compliance.
Validation and verification protocols represent a substantial regulatory hurdle for microfluidic clinical tools. Manufacturers must demonstrate analytical validity (accuracy, precision, sensitivity, specificity), clinical validity (correlation with clinical outcomes), and clinical utility (improvement in patient management). The FDA's recent Biomarker Qualification Program offers potential pathways for novel biomarkers detected through microfluidic technologies.
Regulatory harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually standardizing requirements across regions, though significant differences remain. Companies developing microfluidic technologies for clinical trials should engage with regulatory authorities early through pre-submission consultations and consider adaptive clinical trial designs that incorporate regulatory feedback throughout development phases.
Emerging regulatory frameworks for combination products (device-drug combinations) and companion diagnostics are particularly relevant for microfluidic technologies that integrate therapeutic monitoring or patient stratification capabilities. These frameworks typically involve coordination between multiple regulatory divisions and require comprehensive evidence of both device performance and clinical benefit.
Cost-Benefit Analysis of Microfluidic Implementation
The implementation of microfluidic technologies in clinical trials represents a significant investment that requires thorough financial analysis. Initial capital expenditures for microfluidic platforms range from $50,000 to $500,000, depending on complexity and scale. This includes costs for specialized equipment, clean room facilities, and precision manufacturing tools necessary for device fabrication. However, these upfront investments must be weighed against substantial long-term operational savings.
Operational cost reductions manifest primarily through reagent conservation, with microfluidic systems typically requiring only 1-5% of traditional reagent volumes. For a mid-sized clinical trial, this can translate to savings of $150,000-$300,000 annually. Labor costs also decrease significantly, as automated microfluidic platforms reduce manual handling time by 60-80%, allowing skilled personnel to focus on data analysis rather than repetitive tasks.
Time-to-result acceleration represents another critical economic benefit. Microfluidic systems can deliver analytical results in minutes to hours, compared to days or weeks with conventional methods. This acceleration can reduce the overall clinical trial duration by 15-30%, potentially saving millions in development costs and accelerating time-to-market for new therapeutics.
Infrastructure requirements present a mixed cost profile. While microfluidic labs require less physical space (reducing facility costs by 30-50%), they demand more sophisticated environmental controls and precision utilities, partially offsetting these savings. Additionally, specialized training for personnel represents a transitional cost that diminishes as institutional expertise develops.
Risk mitigation provides less quantifiable but equally important economic benefits. Microfluidic systems' closed nature reduces contamination risks and experimental failures, decreasing costly trial delays and repeat experiments. Statistical modeling suggests this risk reduction can prevent expenses equivalent to 5-10% of total trial costs.
Return-on-investment calculations indicate that most microfluidic implementations achieve break-even within 18-36 months, with subsequent years delivering substantial positive returns. Organizations conducting multiple or ongoing trials see accelerated ROI timelines due to equipment reusability and knowledge transfer between projects.
Scalability considerations reveal that while initial costs are high, incremental scaling of microfluidic operations incurs relatively modest additional expenses. This creates favorable economics for larger trials or multi-site implementations, where per-sample costs decrease as volume increases, unlike traditional methods where costs scale more linearly with sample numbers.
Operational cost reductions manifest primarily through reagent conservation, with microfluidic systems typically requiring only 1-5% of traditional reagent volumes. For a mid-sized clinical trial, this can translate to savings of $150,000-$300,000 annually. Labor costs also decrease significantly, as automated microfluidic platforms reduce manual handling time by 60-80%, allowing skilled personnel to focus on data analysis rather than repetitive tasks.
Time-to-result acceleration represents another critical economic benefit. Microfluidic systems can deliver analytical results in minutes to hours, compared to days or weeks with conventional methods. This acceleration can reduce the overall clinical trial duration by 15-30%, potentially saving millions in development costs and accelerating time-to-market for new therapeutics.
Infrastructure requirements present a mixed cost profile. While microfluidic labs require less physical space (reducing facility costs by 30-50%), they demand more sophisticated environmental controls and precision utilities, partially offsetting these savings. Additionally, specialized training for personnel represents a transitional cost that diminishes as institutional expertise develops.
Risk mitigation provides less quantifiable but equally important economic benefits. Microfluidic systems' closed nature reduces contamination risks and experimental failures, decreasing costly trial delays and repeat experiments. Statistical modeling suggests this risk reduction can prevent expenses equivalent to 5-10% of total trial costs.
Return-on-investment calculations indicate that most microfluidic implementations achieve break-even within 18-36 months, with subsequent years delivering substantial positive returns. Organizations conducting multiple or ongoing trials see accelerated ROI timelines due to equipment reusability and knowledge transfer between projects.
Scalability considerations reveal that while initial costs are high, incremental scaling of microfluidic operations incurs relatively modest additional expenses. This creates favorable economics for larger trials or multi-site implementations, where per-sample costs decrease as volume increases, unlike traditional methods where costs scale more linearly with sample numbers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!