Optimizing Electrochemical Detection in Microfluidic Devices
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Microfluidic Detection Background and Objectives
Electrochemical detection in microfluidic devices represents a convergence of analytical chemistry, materials science, and microengineering that has evolved significantly over the past three decades. This technology emerged from the broader field of lab-on-a-chip systems in the early 1990s, with pioneering work by researchers at institutions such as Stanford University and ETH Zurich demonstrating the feasibility of integrating electrochemical sensors within miniaturized fluidic channels.
The evolution of this technology has been characterized by progressive improvements in sensitivity, selectivity, and miniaturization capabilities. Early systems were primarily proof-of-concept demonstrations with limited practical applications, while contemporary platforms can achieve detection limits in the picomolar range with high specificity across diverse analytes. This progression has been enabled by advances in microfabrication techniques, electrode materials, and signal processing methodologies.
Current technological trends point toward increased integration of multiple sensing modalities, enhanced automation, and improved compatibility with portable and point-of-care applications. The integration of nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles has dramatically improved sensor performance, while developments in microfluidic design have enhanced sample handling capabilities and reduced analysis times.
The primary technical objectives for optimizing electrochemical detection in microfluidic devices encompass several critical dimensions. First, improving sensitivity and detection limits to enable analysis of trace analytes in complex matrices remains a paramount goal. Second, enhancing selectivity through novel electrode modifications and sensing strategies to minimize interference from non-target species. Third, increasing throughput capabilities to facilitate high-volume screening applications in pharmaceutical development and clinical diagnostics.
Additional objectives include reducing fabrication costs to enable wider adoption in resource-limited settings, improving device robustness for field applications, and developing standardized interfaces for seamless integration with existing analytical workflows. The ultimate aim is to create platforms that combine the analytical power of sophisticated laboratory instrumentation with the accessibility and ease-of-use of point-of-care devices.
The technological trajectory suggests that future developments will focus on wireless connectivity, integration with artificial intelligence for automated data interpretation, and enhanced multiplexing capabilities to simultaneously detect multiple analytes. These advancements will position electrochemical microfluidic detection as a cornerstone technology in personalized medicine, environmental monitoring, and industrial quality control applications.
The evolution of this technology has been characterized by progressive improvements in sensitivity, selectivity, and miniaturization capabilities. Early systems were primarily proof-of-concept demonstrations with limited practical applications, while contemporary platforms can achieve detection limits in the picomolar range with high specificity across diverse analytes. This progression has been enabled by advances in microfabrication techniques, electrode materials, and signal processing methodologies.
Current technological trends point toward increased integration of multiple sensing modalities, enhanced automation, and improved compatibility with portable and point-of-care applications. The integration of nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles has dramatically improved sensor performance, while developments in microfluidic design have enhanced sample handling capabilities and reduced analysis times.
The primary technical objectives for optimizing electrochemical detection in microfluidic devices encompass several critical dimensions. First, improving sensitivity and detection limits to enable analysis of trace analytes in complex matrices remains a paramount goal. Second, enhancing selectivity through novel electrode modifications and sensing strategies to minimize interference from non-target species. Third, increasing throughput capabilities to facilitate high-volume screening applications in pharmaceutical development and clinical diagnostics.
Additional objectives include reducing fabrication costs to enable wider adoption in resource-limited settings, improving device robustness for field applications, and developing standardized interfaces for seamless integration with existing analytical workflows. The ultimate aim is to create platforms that combine the analytical power of sophisticated laboratory instrumentation with the accessibility and ease-of-use of point-of-care devices.
The technological trajectory suggests that future developments will focus on wireless connectivity, integration with artificial intelligence for automated data interpretation, and enhanced multiplexing capabilities to simultaneously detect multiple analytes. These advancements will position electrochemical microfluidic detection as a cornerstone technology in personalized medicine, environmental monitoring, and industrial quality control applications.
Market Analysis for Microfluidic Electrochemical Sensors
The global market for microfluidic electrochemical sensors is experiencing robust growth, driven by increasing demand for point-of-care diagnostics, environmental monitoring, and pharmaceutical research applications. Current market valuations indicate that the microfluidic sensor segment reached approximately 22 billion USD in 2022, with electrochemical detection systems representing a significant portion of this market at around 5.7 billion USD.
Market research reveals a compound annual growth rate (CAGR) of 11.3% for microfluidic electrochemical sensors between 2023 and 2028, outpacing the broader analytical instrumentation market which grows at 6.8% annually. This accelerated growth is primarily attributed to the expanding applications in healthcare diagnostics, where rapid, accurate, and cost-effective testing solutions are increasingly sought after.
Healthcare applications currently dominate the market landscape, accounting for 63% of total market share. Within this segment, glucose monitoring devices represent the largest application area, followed by infectious disease diagnostics and cardiac biomarker detection. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic capabilities.
Environmental monitoring applications constitute the second-largest market segment at 18%, with water quality testing and pollutant detection driving demand. Industrial applications, including food safety testing and process monitoring, represent 12% of the market, while research applications account for the remaining 7%.
Regionally, North America leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (26%). However, the Asia-Pacific region is projected to witness the highest growth rate of 13.8% through 2028, driven by increasing healthcare infrastructure development, rising environmental concerns, and growing industrial automation in countries like China and India.
Key market drivers include miniaturization trends in analytical devices, increasing prevalence of chronic diseases requiring regular monitoring, stringent environmental regulations, and technological advancements in electrode materials and fabrication techniques. The integration of wireless connectivity and smartphone compatibility is creating new market opportunities, particularly in remote and resource-limited settings.
Market challenges include high initial development costs, technical complexities in achieving reliable mass production, and regulatory hurdles for clinical applications. Additionally, standardization issues and interoperability concerns present barriers to wider adoption in certain sectors.
Customer demand is increasingly focused on multiplexed detection capabilities, improved sensitivity, reduced sample volume requirements, and enhanced user interfaces. The trend toward personalized medicine is expected to further drive market growth for customizable microfluidic electrochemical sensing platforms in the coming years.
Market research reveals a compound annual growth rate (CAGR) of 11.3% for microfluidic electrochemical sensors between 2023 and 2028, outpacing the broader analytical instrumentation market which grows at 6.8% annually. This accelerated growth is primarily attributed to the expanding applications in healthcare diagnostics, where rapid, accurate, and cost-effective testing solutions are increasingly sought after.
Healthcare applications currently dominate the market landscape, accounting for 63% of total market share. Within this segment, glucose monitoring devices represent the largest application area, followed by infectious disease diagnostics and cardiac biomarker detection. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic capabilities.
Environmental monitoring applications constitute the second-largest market segment at 18%, with water quality testing and pollutant detection driving demand. Industrial applications, including food safety testing and process monitoring, represent 12% of the market, while research applications account for the remaining 7%.
Regionally, North America leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (26%). However, the Asia-Pacific region is projected to witness the highest growth rate of 13.8% through 2028, driven by increasing healthcare infrastructure development, rising environmental concerns, and growing industrial automation in countries like China and India.
Key market drivers include miniaturization trends in analytical devices, increasing prevalence of chronic diseases requiring regular monitoring, stringent environmental regulations, and technological advancements in electrode materials and fabrication techniques. The integration of wireless connectivity and smartphone compatibility is creating new market opportunities, particularly in remote and resource-limited settings.
Market challenges include high initial development costs, technical complexities in achieving reliable mass production, and regulatory hurdles for clinical applications. Additionally, standardization issues and interoperability concerns present barriers to wider adoption in certain sectors.
Customer demand is increasingly focused on multiplexed detection capabilities, improved sensitivity, reduced sample volume requirements, and enhanced user interfaces. The trend toward personalized medicine is expected to further drive market growth for customizable microfluidic electrochemical sensing platforms in the coming years.
Current Challenges in Microfluidic Electrochemical Detection
Despite significant advancements in microfluidic electrochemical detection systems, several critical challenges continue to impede optimal performance and widespread adoption. Signal-to-noise ratio remains a fundamental limitation, particularly in complex biological samples where target analytes exist at extremely low concentrations. Background interference from non-target molecules, electrode fouling, and environmental factors frequently mask the electrochemical signals of interest, necessitating sophisticated signal processing algorithms and reference electrode systems.
Miniaturization presents another significant hurdle, as reducing electrode dimensions often leads to decreased sensitivity and increased impedance. The integration of reference electrodes within confined microfluidic channels proves especially challenging, with stability issues arising from the limited electrolyte volume and surface area constraints. Furthermore, the materials used for electrode fabrication must balance conductivity, biocompatibility, and resistance to fouling—a combination that often requires compromises in performance.
Reproducibility and standardization across devices represent persistent challenges in the field. Manufacturing variations in electrode positioning, surface characteristics, and channel dimensions can significantly impact detection performance, making cross-platform comparisons difficult. This lack of standardization has hindered the transition from laboratory prototypes to commercial applications, as reliability and consistency are paramount for clinical and industrial adoption.
The integration of electrochemical detection with other analytical modalities presents both opportunities and challenges. While multi-modal sensing can provide complementary information and improve diagnostic accuracy, the integration of optical, mechanical, or thermal sensing components with electrochemical systems often introduces complexity in fabrication, operation, and data interpretation.
Sample preparation remains a bottleneck for many applications, particularly when dealing with complex biological matrices. Pre-concentration techniques, selective capture mechanisms, and effective filtering systems are needed to isolate target analytes before electrochemical detection, yet these processes must be seamlessly integrated within the microfluidic platform without compromising throughput or sensitivity.
Power requirements and data processing capabilities present additional challenges, especially for point-of-care applications where portable, battery-operated devices are desired. The development of low-power electrochemical detection systems with integrated signal processing capabilities requires innovative circuit design and power management strategies.
Lastly, biofouling and electrode degradation over time significantly impact long-term stability and reliability. Surface modification strategies, self-cleaning mechanisms, and regeneration protocols are being explored, but achieving consistent performance over extended periods remains challenging, particularly for implantable or continuous monitoring applications.
Miniaturization presents another significant hurdle, as reducing electrode dimensions often leads to decreased sensitivity and increased impedance. The integration of reference electrodes within confined microfluidic channels proves especially challenging, with stability issues arising from the limited electrolyte volume and surface area constraints. Furthermore, the materials used for electrode fabrication must balance conductivity, biocompatibility, and resistance to fouling—a combination that often requires compromises in performance.
Reproducibility and standardization across devices represent persistent challenges in the field. Manufacturing variations in electrode positioning, surface characteristics, and channel dimensions can significantly impact detection performance, making cross-platform comparisons difficult. This lack of standardization has hindered the transition from laboratory prototypes to commercial applications, as reliability and consistency are paramount for clinical and industrial adoption.
The integration of electrochemical detection with other analytical modalities presents both opportunities and challenges. While multi-modal sensing can provide complementary information and improve diagnostic accuracy, the integration of optical, mechanical, or thermal sensing components with electrochemical systems often introduces complexity in fabrication, operation, and data interpretation.
Sample preparation remains a bottleneck for many applications, particularly when dealing with complex biological matrices. Pre-concentration techniques, selective capture mechanisms, and effective filtering systems are needed to isolate target analytes before electrochemical detection, yet these processes must be seamlessly integrated within the microfluidic platform without compromising throughput or sensitivity.
Power requirements and data processing capabilities present additional challenges, especially for point-of-care applications where portable, battery-operated devices are desired. The development of low-power electrochemical detection systems with integrated signal processing capabilities requires innovative circuit design and power management strategies.
Lastly, biofouling and electrode degradation over time significantly impact long-term stability and reliability. Surface modification strategies, self-cleaning mechanisms, and regeneration protocols are being explored, but achieving consistent performance over extended periods remains challenging, particularly for implantable or continuous monitoring applications.
Current Optimization Approaches for Electrochemical Microfluidics
01 Electrode design and modification for enhanced sensitivity
Optimizing electrode design and surface modifications can significantly enhance the sensitivity of electrochemical detection in microfluidic devices. This includes using nanomaterials, conductive polymers, or specific coatings to increase the electrode surface area and improve electron transfer kinetics. Modified electrodes can lower detection limits and increase signal-to-noise ratios, making them suitable for detecting trace amounts of analytes in complex samples.- Electrode design and modification for enhanced sensitivity: Optimizing electrode design and surface modifications can significantly enhance the sensitivity of electrochemical detection in microfluidic devices. This includes using nanomaterials, conductive polymers, or specific coatings to increase the electrode surface area and improve electron transfer kinetics. Modified electrodes can lower detection limits and increase signal-to-noise ratios, making them suitable for detecting trace amounts of analytes in complex samples.
- Integration of microfluidic components for improved detection: The integration of various microfluidic components such as mixers, separators, and concentrators with electrochemical detection systems can optimize overall performance. These integrated systems allow for sample preparation, separation, and detection to occur in a single device, reducing analysis time and sample volume requirements. Proper integration also minimizes dead volumes and ensures efficient mass transport to the electrode surface, resulting in more accurate and reliable measurements.
- Signal processing and noise reduction techniques: Advanced signal processing and noise reduction techniques are crucial for optimizing electrochemical detection in microfluidic devices. These include digital filtering, signal averaging, lock-in amplification, and baseline correction algorithms. Implementing these techniques helps to extract meaningful signals from background noise, improving the detection limits and reliability of the measurements, especially in complex biological or environmental samples where interference can be significant.
- Miniaturization and multiplexing strategies: Miniaturization and multiplexing strategies enable simultaneous detection of multiple analytes while maintaining a compact device footprint. These approaches involve designing electrode arrays, microelectrode ensembles, or interdigitated electrode configurations that can perform parallel measurements. Such designs optimize space utilization and increase throughput, making them particularly valuable for point-of-care diagnostics and high-throughput screening applications.
- Flow control and sample handling optimization: Optimizing flow control and sample handling in microfluidic devices is essential for reliable electrochemical detection. This includes precise control of flow rates, sample introduction methods, and residence time at the detection zone. Advanced techniques such as hydrodynamic focusing, droplet-based microfluidics, or stopped-flow analysis can be implemented to enhance mass transport to the electrode surface and improve temporal resolution of measurements, resulting in better detection performance.
02 Integration of microfluidic components with detection systems
Effective integration of microfluidic components with electrochemical detection systems is crucial for optimizing performance. This involves strategic placement of electrodes within microchannels, designing flow patterns that maximize analyte-electrode interactions, and incorporating sample preparation steps on-chip. Proper integration minimizes dead volumes, reduces sample consumption, and enables real-time monitoring of electrochemical reactions in confined spaces.Expand Specific Solutions03 Signal processing and noise reduction techniques
Advanced signal processing and noise reduction techniques are essential for optimizing electrochemical detection in microfluidic devices. These include digital filtering algorithms, lock-in amplification, and impedance-based methods that can extract meaningful signals from background noise. Implementation of these techniques improves detection limits, enhances measurement precision, and enables reliable quantification of target analytes even in complex biological or environmental samples.Expand Specific Solutions04 Novel sensing materials and biorecognition elements
Incorporating novel sensing materials and biorecognition elements can significantly improve the specificity and sensitivity of electrochemical detection in microfluidic platforms. These include functionalized nanomaterials, enzyme-modified electrodes, aptamers, and molecularly imprinted polymers that provide selective binding sites for target analytes. Such materials enable highly specific detection while minimizing interference from non-target molecules present in complex sample matrices.Expand Specific Solutions05 Multiplexed detection and high-throughput analysis
Multiplexed detection systems enable simultaneous analysis of multiple analytes in microfluidic devices, significantly increasing throughput and efficiency. These systems incorporate electrode arrays, multiple detection channels, or addressable sensing elements that can be individually monitored. Advanced multiplexing strategies include spatial separation of detection zones, temporal multiplexing through sequential measurements, and frequency-based approaches that distinguish signals based on their electrochemical properties.Expand Specific Solutions
Leading Companies and Research Institutions in the Field
The electrochemical detection in microfluidic devices market is currently in a growth phase, with increasing adoption across clinical diagnostics and environmental monitoring applications. The global market size is estimated to reach $1.2 billion by 2025, driven by demand for point-of-care testing solutions. Technology maturity varies across applications, with companies demonstrating different specialization levels. Industry leaders like Siemens AG and bioMérieux SA offer comprehensive commercial solutions with established market presence, while academic institutions such as Tsinghua University and Fudan University drive fundamental research innovations. Emerging players like Diagnoswiss SA and Wells Bio focus on specialized microfluidic platforms with enhanced sensitivity. The competitive landscape shows a healthy mix of established medical technology corporations, specialized instrumentation providers like Lumencor and AB Sciex, and research-focused entities developing next-generation detection methodologies.
bioMérieux SA
Technical Solution: bioMérieux has developed advanced electrochemical detection systems for microfluidic devices that integrate miniaturized electrodes with specialized surface modifications to enhance sensitivity. Their technology employs multi-electrode arrays with differential pulse voltammetry techniques to achieve detection limits in the picomolar range. The company's approach includes microfluidic channels designed with optimized geometries to enhance mass transport to electrode surfaces, reducing diffusion limitations. They've implemented on-chip reference electrodes with stable potential characteristics and developed proprietary signal processing algorithms that filter noise and enhance signal quality. Their systems incorporate temperature compensation mechanisms to maintain consistent electrochemical responses across varying environmental conditions, critical for point-of-care diagnostic applications.
Strengths: Exceptional sensitivity for clinical diagnostics with established manufacturing infrastructure and regulatory expertise. Their integrated systems offer robust performance in real-world clinical settings. Weaknesses: Higher production costs compared to simpler detection methods, and their proprietary systems may limit compatibility with third-party microfluidic platforms.
Diagnoswiss SA
Technical Solution: Diagnoswiss has pioneered microfluidic electrochemical detection platforms utilizing nanoporous membranes integrated with microelectrode arrays. Their technology employs a unique dual-channel architecture that separates sample preparation from detection zones, minimizing interference. The company has developed specialized electrode materials with catalytic properties that enhance electron transfer kinetics for specific biomarkers. Their microfluidic chips incorporate reference electrodes with exceptional stability over extended operation periods, addressing drift issues common in portable systems. Diagnoswiss's approach includes impedance-based detection methods alongside amperometric techniques, providing complementary data streams that improve diagnostic accuracy. Their systems feature integrated microfluidic pumps that precisely control flow rates to optimize reaction kinetics at electrode surfaces.
Strengths: Highly specialized in electrochemical microfluidics with excellent sensitivity for protein biomarkers and small molecules. Their dual-detection approach provides superior specificity. Weaknesses: Limited production scale compared to larger competitors, and their specialized technology may require more complex manufacturing processes.
Key Patents and Innovations in Electrode Design and Materials
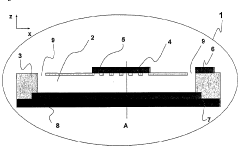
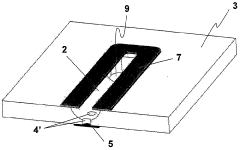
Microfluidic Device with Minimized Ohmic Resistance
PatentInactiveUS20070240986A1
Innovation
- Incorporating an electrically conductive means within the microstructure to reduce ohmic resistance by creating a low-resistance path for current, allowing for improved electrochemical detection without connecting it to the electrochemical detection circuit, thereby minimizing signal perturbation.
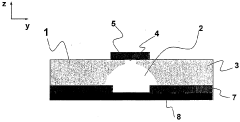
Microfluidic device with minimised ohmic resistance
PatentWO2006050972A1
Innovation
- Incorporating an electrically conductive means within the microstructure to create a low-resistance path for current, reducing overall resistance and maintaining a constant potential along the microstructure, even when not directly connected to the electrochemical detection circuit.
Miniaturization Strategies for Portable Diagnostic Applications
Miniaturization strategies for portable diagnostic applications have become increasingly important in the field of electrochemical detection within microfluidic devices. The drive toward smaller, more efficient diagnostic tools has led to significant innovations in design and fabrication techniques that optimize both performance and portability.
The integration of electrochemical sensors into microfluidic platforms requires careful consideration of electrode miniaturization while maintaining sensitivity and reliability. Recent advances have focused on microfabrication techniques such as photolithography, screen printing, and laser ablation to create electrodes with dimensions in the micrometer range. These approaches enable the production of highly reproducible electrode arrays that can be seamlessly incorporated into microfluidic channels.
Material selection plays a crucial role in miniaturization strategies. Traditional noble metals like gold and platinum remain popular for their excellent conductivity and electrochemical properties, but carbon-based materials including graphene, carbon nanotubes, and conductive polymers have emerged as cost-effective alternatives with enhanced surface area and electrocatalytic activity. These materials facilitate the development of smaller sensing elements without compromising detection capabilities.
Multiplexing represents another key strategy for miniaturization, allowing multiple analytes to be detected simultaneously on a single device. This approach utilizes electrode arrays with different functionalization schemes or microfluidic channel designs that direct samples to discrete sensing regions. Advanced multiplexing techniques have reduced the overall footprint of diagnostic devices while expanding their analytical capabilities.
Power management innovations have addressed one of the most significant challenges in portable diagnostics. The development of low-power electrochemical detection circuits, energy harvesting technologies, and optimized potentiostats has dramatically reduced energy requirements. Some cutting-edge systems now operate on button cell batteries or can even harvest energy from the surrounding environment or the user's body, eliminating bulky power supplies.
Signal processing advancements have further contributed to miniaturization efforts. By implementing on-chip amplification, filtering, and analog-to-digital conversion, the need for external processing equipment has been reduced. Modern microcontrollers and application-specific integrated circuits (ASICs) can perform complex electrochemical measurements while occupying minimal space within portable diagnostic platforms.
Packaging innovations have also played a vital role in creating truly portable systems. Techniques such as 3D printing, injection molding, and lamination allow for the production of compact, sealed devices that protect sensitive components while facilitating sample introduction and result readout. These approaches have enabled the development of complete lab-on-a-chip systems that can be deployed in resource-limited settings.
The integration of electrochemical sensors into microfluidic platforms requires careful consideration of electrode miniaturization while maintaining sensitivity and reliability. Recent advances have focused on microfabrication techniques such as photolithography, screen printing, and laser ablation to create electrodes with dimensions in the micrometer range. These approaches enable the production of highly reproducible electrode arrays that can be seamlessly incorporated into microfluidic channels.
Material selection plays a crucial role in miniaturization strategies. Traditional noble metals like gold and platinum remain popular for their excellent conductivity and electrochemical properties, but carbon-based materials including graphene, carbon nanotubes, and conductive polymers have emerged as cost-effective alternatives with enhanced surface area and electrocatalytic activity. These materials facilitate the development of smaller sensing elements without compromising detection capabilities.
Multiplexing represents another key strategy for miniaturization, allowing multiple analytes to be detected simultaneously on a single device. This approach utilizes electrode arrays with different functionalization schemes or microfluidic channel designs that direct samples to discrete sensing regions. Advanced multiplexing techniques have reduced the overall footprint of diagnostic devices while expanding their analytical capabilities.
Power management innovations have addressed one of the most significant challenges in portable diagnostics. The development of low-power electrochemical detection circuits, energy harvesting technologies, and optimized potentiostats has dramatically reduced energy requirements. Some cutting-edge systems now operate on button cell batteries or can even harvest energy from the surrounding environment or the user's body, eliminating bulky power supplies.
Signal processing advancements have further contributed to miniaturization efforts. By implementing on-chip amplification, filtering, and analog-to-digital conversion, the need for external processing equipment has been reduced. Modern microcontrollers and application-specific integrated circuits (ASICs) can perform complex electrochemical measurements while occupying minimal space within portable diagnostic platforms.
Packaging innovations have also played a vital role in creating truly portable systems. Techniques such as 3D printing, injection molding, and lamination allow for the production of compact, sealed devices that protect sensitive components while facilitating sample introduction and result readout. These approaches have enabled the development of complete lab-on-a-chip systems that can be deployed in resource-limited settings.
Integration with IoT and Data Analytics Platforms
The integration of microfluidic electrochemical detection systems with IoT and data analytics platforms represents a transformative advancement in real-time monitoring capabilities. Current integration frameworks typically employ wireless communication protocols such as Bluetooth Low Energy (BLE), Wi-Fi, or cellular networks to transmit electrochemical sensor data from microfluidic devices to cloud-based platforms. This connectivity enables continuous monitoring and remote access to analytical results, significantly enhancing the utility of these detection systems in various applications including healthcare, environmental monitoring, and industrial process control.
Edge computing architectures have emerged as a critical component in these integrated systems, allowing for preliminary data processing directly at the microfluidic device level. This approach reduces bandwidth requirements and latency while enabling more responsive analytical capabilities. For instance, recent implementations have demonstrated the ability to perform baseline correction, noise filtering, and peak identification algorithms on embedded microcontrollers before transmitting refined data to cloud platforms.
Cloud-based analytics platforms specifically designed for electrochemical data have evolved substantially, incorporating machine learning algorithms that can identify patterns and anomalies across large datasets. These platforms typically offer visualization tools, statistical analysis functions, and predictive modeling capabilities that transform raw electrochemical signals into actionable insights. Notable examples include IBM Watson IoT Platform and Microsoft Azure IoT, which have been adapted for electrochemical sensing applications with specialized analytical modules.
Interoperability remains a significant challenge, with efforts underway to establish standardized data formats and APIs for electrochemical sensing data. The development of MQTT and REST API implementations specifically optimized for electrochemical data transfer has improved cross-platform compatibility. Organizations such as the Chemical Abstracts Service (CAS) and the International Union of Pure and Applied Chemistry (IUPAC) are working toward standardized ontologies for electrochemical measurements to facilitate seamless data exchange.
Security considerations have become increasingly important as these systems find applications in sensitive areas such as clinical diagnostics and industrial quality control. Current best practices include end-to-end encryption of sensor data, secure authentication mechanisms, and compliance with regulations such as HIPAA for healthcare applications and GDPR for systems deployed in Europe. Multi-factor authentication and blockchain-based data integrity verification are emerging as additional security layers in next-generation implementations.
The economic impact of IoT-integrated electrochemical detection systems is substantial, with market analyses projecting a compound annual growth rate of 18.7% for this segment between 2023 and 2028. This growth is driven by increasing demand for remote monitoring solutions in healthcare, environmental compliance requirements, and industrial automation trends that favor real-time analytical capabilities with minimal human intervention.
Edge computing architectures have emerged as a critical component in these integrated systems, allowing for preliminary data processing directly at the microfluidic device level. This approach reduces bandwidth requirements and latency while enabling more responsive analytical capabilities. For instance, recent implementations have demonstrated the ability to perform baseline correction, noise filtering, and peak identification algorithms on embedded microcontrollers before transmitting refined data to cloud platforms.
Cloud-based analytics platforms specifically designed for electrochemical data have evolved substantially, incorporating machine learning algorithms that can identify patterns and anomalies across large datasets. These platforms typically offer visualization tools, statistical analysis functions, and predictive modeling capabilities that transform raw electrochemical signals into actionable insights. Notable examples include IBM Watson IoT Platform and Microsoft Azure IoT, which have been adapted for electrochemical sensing applications with specialized analytical modules.
Interoperability remains a significant challenge, with efforts underway to establish standardized data formats and APIs for electrochemical sensing data. The development of MQTT and REST API implementations specifically optimized for electrochemical data transfer has improved cross-platform compatibility. Organizations such as the Chemical Abstracts Service (CAS) and the International Union of Pure and Applied Chemistry (IUPAC) are working toward standardized ontologies for electrochemical measurements to facilitate seamless data exchange.
Security considerations have become increasingly important as these systems find applications in sensitive areas such as clinical diagnostics and industrial quality control. Current best practices include end-to-end encryption of sensor data, secure authentication mechanisms, and compliance with regulations such as HIPAA for healthcare applications and GDPR for systems deployed in Europe. Multi-factor authentication and blockchain-based data integrity verification are emerging as additional security layers in next-generation implementations.
The economic impact of IoT-integrated electrochemical detection systems is substantial, with market analyses projecting a compound annual growth rate of 18.7% for this segment between 2023 and 2028. This growth is driven by increasing demand for remote monitoring solutions in healthcare, environmental compliance requirements, and industrial automation trends that favor real-time analytical capabilities with minimal human intervention.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!