How to Use Microfluidics to Enhance Diagnostic Speed and Accuracy
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics Diagnostic Evolution and Objectives
Microfluidics technology has evolved significantly over the past three decades, transforming from a theoretical concept to a practical tool with substantial impact on diagnostic capabilities. The journey began in the 1990s with pioneering work on miniaturized total analysis systems (μTAS), which laid the foundation for manipulating fluids at the microscale level. By the early 2000s, researchers had developed fundamental microfluidic components such as valves, pumps, and mixers, enabling more complex fluid handling operations.
The evolution accelerated in the 2010s with the integration of microfluidics into point-of-care testing platforms, marking a significant shift toward practical clinical applications. This period saw the emergence of paper-based microfluidics and digital microfluidics, expanding the technology's accessibility and versatility. Recent advancements have focused on integrating microfluidics with complementary technologies such as biosensors, artificial intelligence, and smartphone-based detection systems.
Current technological trends in microfluidic diagnostics include the development of organ-on-a-chip platforms for personalized medicine, droplet-based digital PCR for ultrasensitive detection, and integrated sample-to-answer systems that minimize human intervention. The COVID-19 pandemic has further accelerated innovation in this field, highlighting the critical need for rapid, accurate, and accessible diagnostic tools during public health emergencies.
The primary objective of microfluidic diagnostic technology is to revolutionize traditional diagnostic approaches by offering faster results, higher sensitivity, and greater accuracy while using smaller sample volumes. Specific goals include reducing the time-to-result from hours or days to minutes, achieving detection limits comparable to or better than conventional laboratory methods, and enabling multiplexed testing for simultaneous analysis of multiple biomarkers.
Another crucial objective is democratizing access to advanced diagnostics through cost-effective, portable systems that can function in resource-limited settings. This includes developing platforms that require minimal infrastructure and technical expertise to operate, potentially transforming healthcare delivery in underserved regions.
Looking forward, the field aims to create fully integrated diagnostic ecosystems that seamlessly connect sample collection, processing, analysis, and data interpretation. The ultimate vision encompasses real-time monitoring capabilities for continuous health assessment, personalized treatment guidance based on individual biomarker profiles, and integration with telehealth systems for comprehensive remote healthcare solutions.
These technological objectives align with broader healthcare goals of improving patient outcomes through earlier disease detection, more precise diagnosis, and more effective treatment monitoring, while simultaneously reducing healthcare costs and expanding access to quality diagnostic services globally.
The evolution accelerated in the 2010s with the integration of microfluidics into point-of-care testing platforms, marking a significant shift toward practical clinical applications. This period saw the emergence of paper-based microfluidics and digital microfluidics, expanding the technology's accessibility and versatility. Recent advancements have focused on integrating microfluidics with complementary technologies such as biosensors, artificial intelligence, and smartphone-based detection systems.
Current technological trends in microfluidic diagnostics include the development of organ-on-a-chip platforms for personalized medicine, droplet-based digital PCR for ultrasensitive detection, and integrated sample-to-answer systems that minimize human intervention. The COVID-19 pandemic has further accelerated innovation in this field, highlighting the critical need for rapid, accurate, and accessible diagnostic tools during public health emergencies.
The primary objective of microfluidic diagnostic technology is to revolutionize traditional diagnostic approaches by offering faster results, higher sensitivity, and greater accuracy while using smaller sample volumes. Specific goals include reducing the time-to-result from hours or days to minutes, achieving detection limits comparable to or better than conventional laboratory methods, and enabling multiplexed testing for simultaneous analysis of multiple biomarkers.
Another crucial objective is democratizing access to advanced diagnostics through cost-effective, portable systems that can function in resource-limited settings. This includes developing platforms that require minimal infrastructure and technical expertise to operate, potentially transforming healthcare delivery in underserved regions.
Looking forward, the field aims to create fully integrated diagnostic ecosystems that seamlessly connect sample collection, processing, analysis, and data interpretation. The ultimate vision encompasses real-time monitoring capabilities for continuous health assessment, personalized treatment guidance based on individual biomarker profiles, and integration with telehealth systems for comprehensive remote healthcare solutions.
These technological objectives align with broader healthcare goals of improving patient outcomes through earlier disease detection, more precise diagnosis, and more effective treatment monitoring, while simultaneously reducing healthcare costs and expanding access to quality diagnostic services globally.
Market Analysis for Rapid Diagnostic Solutions
The global market for rapid diagnostic solutions is experiencing unprecedented growth, driven by increasing demand for point-of-care testing and early disease detection. The microfluidics-based diagnostic market was valued at approximately $13.5 billion in 2020 and is projected to reach $32.7 billion by 2026, growing at a CAGR of 16.2%. This remarkable expansion is fueled by the convergence of several factors, including the rising prevalence of infectious diseases, growing geriatric population, and increasing awareness about personalized medicine.
Healthcare providers worldwide are seeking diagnostic solutions that deliver faster results without compromising accuracy. Traditional laboratory-based testing methods typically require 24-72 hours for results, creating significant delays in treatment decisions. Microfluidics technology addresses this gap by enabling rapid diagnostics with turnaround times of minutes rather than days, while maintaining or even improving diagnostic accuracy through precise sample handling and analysis.
The COVID-19 pandemic has served as a catalyst for market growth, highlighting the critical importance of rapid and accurate diagnostic capabilities during public health emergencies. This has accelerated investment in microfluidic diagnostic platforms, with venture capital funding in this sector increasing by 215% between 2019 and 2021.
Regional analysis reveals that North America currently dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the highest growth rate of 19.8% during the forecast period, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about early disease detection.
By application segment, infectious disease diagnostics represents the largest market share at 38%, followed by cardiac markers (21%), coagulation testing (15%), and oncology diagnostics (12%). The infectious disease segment is projected to maintain its dominant position due to the continuous emergence of new pathogens and the need for rapid containment strategies.
End-user analysis indicates that hospitals and clinical laboratories remain the primary adopters of microfluidic diagnostic technologies (56%), followed by point-of-care settings (24%) and research institutions (14%). However, the point-of-care segment is growing at the fastest rate of 22.3% annually, reflecting the global shift toward decentralized healthcare delivery models.
Consumer preferences are increasingly favoring diagnostic solutions that offer convenience, speed, and reliability. A recent survey of healthcare providers revealed that 78% consider turnaround time as the most critical factor when selecting diagnostic platforms, followed by accuracy (76%) and cost-effectiveness (65%).
Healthcare providers worldwide are seeking diagnostic solutions that deliver faster results without compromising accuracy. Traditional laboratory-based testing methods typically require 24-72 hours for results, creating significant delays in treatment decisions. Microfluidics technology addresses this gap by enabling rapid diagnostics with turnaround times of minutes rather than days, while maintaining or even improving diagnostic accuracy through precise sample handling and analysis.
The COVID-19 pandemic has served as a catalyst for market growth, highlighting the critical importance of rapid and accurate diagnostic capabilities during public health emergencies. This has accelerated investment in microfluidic diagnostic platforms, with venture capital funding in this sector increasing by 215% between 2019 and 2021.
Regional analysis reveals that North America currently dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the highest growth rate of 19.8% during the forecast period, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about early disease detection.
By application segment, infectious disease diagnostics represents the largest market share at 38%, followed by cardiac markers (21%), coagulation testing (15%), and oncology diagnostics (12%). The infectious disease segment is projected to maintain its dominant position due to the continuous emergence of new pathogens and the need for rapid containment strategies.
End-user analysis indicates that hospitals and clinical laboratories remain the primary adopters of microfluidic diagnostic technologies (56%), followed by point-of-care settings (24%) and research institutions (14%). However, the point-of-care segment is growing at the fastest rate of 22.3% annually, reflecting the global shift toward decentralized healthcare delivery models.
Consumer preferences are increasingly favoring diagnostic solutions that offer convenience, speed, and reliability. A recent survey of healthcare providers revealed that 78% consider turnaround time as the most critical factor when selecting diagnostic platforms, followed by accuracy (76%) and cost-effectiveness (65%).
Current Microfluidic Technologies and Barriers
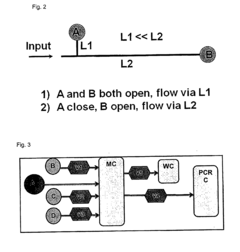
Microfluidic technologies have evolved significantly over the past decade, with several platforms now available for diagnostic applications. Lab-on-a-chip (LOC) systems represent one of the most mature microfluidic technologies, integrating multiple laboratory functions on a single chip measuring only millimeters to a few square centimeters. These systems typically incorporate sample preparation, reagent handling, mixing, separation, and detection components within a unified platform, enabling comprehensive analysis from raw samples to results.
Digital microfluidics (DMF) has emerged as another promising approach, manipulating discrete droplets on an array of electrodes through electrowetting principles. This technology offers unprecedented flexibility in reconfiguring assay protocols without physical redesign of the device, making it particularly valuable for multiplex testing and adaptive diagnostic procedures. DMF systems have demonstrated significant advantages in terms of reagent consumption reduction and parallelization capabilities.
Paper-based microfluidics presents a cost-effective alternative, utilizing capillary action to drive fluid flow through hydrophilic paper channels. These devices require no external power source and can be manufactured at extremely low costs, positioning them as ideal candidates for point-of-care diagnostics in resource-limited settings. Recent advances in paper microfluidics have incorporated sophisticated detection mechanisms while maintaining simplicity of operation.
Despite these technological advances, several barriers impede widespread adoption of microfluidic diagnostics. Surface fouling remains a persistent challenge, as biomolecules tend to adsorb onto microfluidic channel surfaces, leading to altered flow characteristics and potential false readings. This issue becomes particularly problematic when working with complex biological samples like whole blood or serum.
Scaling production from laboratory prototypes to commercial manufacturing presents significant hurdles. Many microfluidic devices employ complex fabrication techniques that are difficult to translate to high-volume production while maintaining quality and performance consistency. The lack of standardization across platforms further complicates integration with existing laboratory infrastructure and regulatory approval processes.
Bubble formation and control represents another technical barrier, as air bubbles can disrupt fluid flow, block channels, and interfere with detection mechanisms. In nanoliter-scale channels, even microscopic bubbles can completely obstruct flow and compromise assay reliability. Current bubble-trapping designs add complexity to device fabrication and operation.
Sample preparation integration remains challenging, as many diagnostic applications require complex pre-processing steps that are difficult to miniaturize and automate within microfluidic platforms. This often necessitates external sample preparation, diminishing the advantages of a fully integrated system and increasing the risk of contamination or user error.
Digital microfluidics (DMF) has emerged as another promising approach, manipulating discrete droplets on an array of electrodes through electrowetting principles. This technology offers unprecedented flexibility in reconfiguring assay protocols without physical redesign of the device, making it particularly valuable for multiplex testing and adaptive diagnostic procedures. DMF systems have demonstrated significant advantages in terms of reagent consumption reduction and parallelization capabilities.
Paper-based microfluidics presents a cost-effective alternative, utilizing capillary action to drive fluid flow through hydrophilic paper channels. These devices require no external power source and can be manufactured at extremely low costs, positioning them as ideal candidates for point-of-care diagnostics in resource-limited settings. Recent advances in paper microfluidics have incorporated sophisticated detection mechanisms while maintaining simplicity of operation.
Despite these technological advances, several barriers impede widespread adoption of microfluidic diagnostics. Surface fouling remains a persistent challenge, as biomolecules tend to adsorb onto microfluidic channel surfaces, leading to altered flow characteristics and potential false readings. This issue becomes particularly problematic when working with complex biological samples like whole blood or serum.
Scaling production from laboratory prototypes to commercial manufacturing presents significant hurdles. Many microfluidic devices employ complex fabrication techniques that are difficult to translate to high-volume production while maintaining quality and performance consistency. The lack of standardization across platforms further complicates integration with existing laboratory infrastructure and regulatory approval processes.
Bubble formation and control represents another technical barrier, as air bubbles can disrupt fluid flow, block channels, and interfere with detection mechanisms. In nanoliter-scale channels, even microscopic bubbles can completely obstruct flow and compromise assay reliability. Current bubble-trapping designs add complexity to device fabrication and operation.
Sample preparation integration remains challenging, as many diagnostic applications require complex pre-processing steps that are difficult to miniaturize and automate within microfluidic platforms. This often necessitates external sample preparation, diminishing the advantages of a fully integrated system and increasing the risk of contamination or user error.
State-of-the-Art Microfluidic Diagnostic Platforms
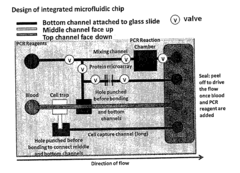
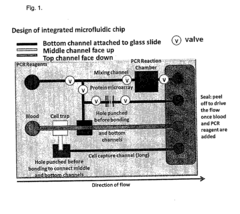
01 Microfluidic device design for rapid diagnostics
Advanced microfluidic device designs can significantly enhance diagnostic speed and accuracy. These designs incorporate specialized channels, chambers, and flow control mechanisms that enable precise sample handling and rapid analysis. By optimizing the physical architecture of microfluidic systems, diagnostic processes can be accelerated while maintaining high levels of accuracy, making them suitable for point-of-care applications where timely results are critical.- Microfluidic device design for rapid diagnostics: Specialized microfluidic device designs can significantly enhance diagnostic speed and accuracy. These designs incorporate features such as optimized channel geometries, integrated reaction chambers, and precise flow control mechanisms. By minimizing sample volumes and reducing diffusion distances, these devices enable faster reaction kinetics and more efficient analyte detection, leading to quicker and more accurate diagnostic results.
- Integration of detection technologies in microfluidic platforms: The integration of advanced detection technologies within microfluidic platforms enhances diagnostic accuracy and speed. These technologies include optical sensors, electrochemical detectors, and spectroscopic methods that can detect biomarkers at low concentrations. By incorporating these detection systems directly into microfluidic chips, sample-to-result times are significantly reduced while maintaining high sensitivity and specificity in diagnostic applications.
- Sample preparation and processing techniques: Advanced sample preparation and processing techniques in microfluidic systems improve diagnostic performance. These techniques include on-chip cell lysis, nucleic acid extraction, and purification steps that can be performed rapidly and with minimal user intervention. By automating and optimizing these pre-analytical steps, microfluidic diagnostics achieve faster processing times and more consistent results, reducing the likelihood of contamination and human error.
- Multiplexed analysis capabilities: Microfluidic platforms with multiplexed analysis capabilities enable simultaneous testing for multiple biomarkers or pathogens, increasing both speed and diagnostic accuracy. These systems utilize parallel processing channels, droplet-based compartmentalization, or array-based detection methods to analyze multiple targets in a single sample. This approach reduces the time required for comprehensive diagnostic testing while providing more complete information for accurate clinical decision-making.
- Automation and integration with data analysis: Automation of microfluidic diagnostic processes and integration with advanced data analysis systems enhances both speed and accuracy. These systems incorporate automated sample handling, precise flow control, and real-time data processing algorithms that can interpret complex diagnostic signals. By reducing manual intervention and applying sophisticated analytical methods such as machine learning, these integrated platforms deliver faster results with improved accuracy and reproducibility.
02 Integration of detection technologies in microfluidic platforms
The integration of advanced detection technologies within microfluidic platforms enhances both the speed and accuracy of diagnostic tests. These technologies include optical, electrochemical, and biosensor-based detection methods that can rapidly identify biomarkers or pathogens in biological samples. By combining these detection systems with microfluidic sample processing, diagnostic results can be obtained more quickly and with greater sensitivity than traditional laboratory methods.Expand Specific Solutions03 Sample preparation and processing techniques
Innovative sample preparation and processing techniques in microfluidic systems can significantly improve diagnostic speed and accuracy. These techniques include automated sample filtration, concentration, and purification steps that can be performed within the microfluidic device. By efficiently preparing samples before analysis, these methods reduce interference from contaminants and enhance the detection of target analytes, leading to faster and more reliable diagnostic results.Expand Specific Solutions04 Multiplexed analysis capabilities
Microfluidic systems with multiplexed analysis capabilities allow for the simultaneous detection of multiple biomarkers or pathogens in a single sample. This approach significantly increases the throughput and efficiency of diagnostic testing while maintaining high accuracy. By performing parallel analyses, these systems can provide comprehensive diagnostic information more rapidly than sequential testing methods, making them valuable tools for complex disease diagnosis and monitoring.Expand Specific Solutions05 Automation and digital integration for enhanced performance
Automation and digital integration in microfluidic diagnostic systems can substantially improve both speed and accuracy. These advancements include automated fluid handling, precise temperature control, and integration with data analysis software. By reducing human intervention and incorporating sophisticated algorithms for result interpretation, these systems minimize errors and accelerate the diagnostic process. Additionally, connectivity features enable rapid data sharing and remote monitoring, further enhancing the utility of microfluidic diagnostics in clinical settings.Expand Specific Solutions
Leading Companies in Microfluidic Diagnostics
Microfluidics for diagnostic enhancement is currently in a growth phase, with the market expected to reach significant expansion due to increasing demand for rapid, accurate point-of-care testing. The competitive landscape features established players like Agilent Technologies and DuPont alongside specialized innovators such as HandyLab and Caliper Life Sciences (acquired by PerkinElmer/Revvity). Academic institutions including Harvard, Tsinghua University, and National University of Singapore are driving fundamental research. The technology is approaching maturity in certain applications, with companies like BOE Technology and HP Development exploring commercial implementations. Integration with AI and IoT technologies by firms such as EFA Engineering and Shenzhen Huamai Xingwei represents the next frontier, promising further improvements in diagnostic capabilities.
Caliper Life Sciences, Inc.
Technical Solution: Caliper Life Sciences has developed LabChip® microfluidic technology that miniaturizes and integrates multiple laboratory processes onto a single chip. Their platform utilizes electrophoretic separation techniques combined with specialized microchannels to perform rapid, high-resolution analysis of proteins, DNA, and RNA. The technology employs precision-fabricated microchannels that control fluid flow at the nanoliter scale, enabling automated sample handling, reagent mixing, separation, and detection within a unified system. Their microfluidic devices incorporate fluorescence detection methods that allow for quantitative analysis with sensitivity comparable to traditional laboratory techniques but with significantly reduced sample volumes (as little as 1-10 nL) and processing times (results in minutes rather than hours)[1][3]. Caliper's systems also feature integrated software for automated data analysis, reducing operator variability and improving reproducibility across diagnostic applications.
Strengths: Exceptional throughput capabilities with ability to process hundreds of samples per hour; dramatic reduction in reagent consumption (up to 99% less than conventional methods); automated workflow minimizes human error. Weaknesses: Higher initial capital investment compared to traditional diagnostic equipment; requires specialized training for operation and maintenance; limited flexibility for certain highly specialized diagnostic applications.
Agilent Technologies, Inc.
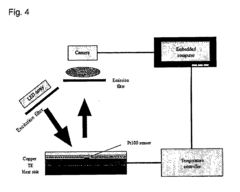
Technical Solution: Agilent Technologies has pioneered advanced microfluidic solutions through their Bioanalyzer and TapeStation systems, which leverage lab-on-a-chip technology for nucleic acid and protein analysis. Their proprietary microfluidic chips contain interconnected microchannels and reservoirs fabricated using precision polymer molding techniques. These systems employ electrokinetic forces and pressure-driven flow to precisely control sample movement through microchannels as small as 50 micrometers. The technology integrates sample preparation, separation, and detection in a single platform, utilizing laser-induced fluorescence detection with sensitivity down to picogram levels[2]. Agilent's microfluidic systems incorporate automated calibration and quality control features that ensure consistent results across different operators and laboratories. Their latest innovations include microfluidic chips with integrated electrodes for impedance-based cell counting and characterization, enabling rapid assessment of cell viability and function for diagnostic applications requiring cellular analysis.
Strengths: Exceptional reproducibility with coefficient of variation typically <5% across runs; rapid analysis times (30-40 minutes versus hours/days for traditional methods); minimal sample consumption (1-5 μL) enabling work with limited clinical specimens. Weaknesses: Limited multiplexing capabilities compared to some newer technologies; relatively fixed protocols with less flexibility for novel assay development; higher cost per test compared to bulk traditional methods for high-volume applications.
Key Patents and Breakthroughs in Microfluidic Detection
Microfluidic based integrated sample analysis system
PatentActiveUS20170001196A1
Innovation
- A portable microfluidic platform integrating a microfluidic chip with on-chip sample separation, PCR, protein, and cell analysis capabilities, utilizing a thermoelectric semiconductor, LED array, and control unit for simultaneous genetic, protein, and cell detection from a small sample volume, allowing for precise temperature control and fluorescence detection.
Microfluidics sensing system
PatentWO2016122705A1
Innovation
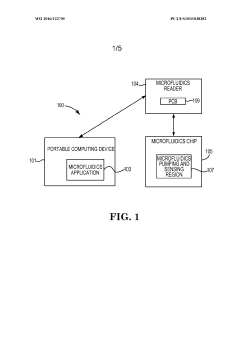
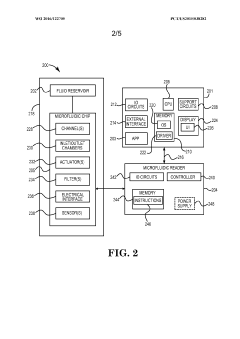
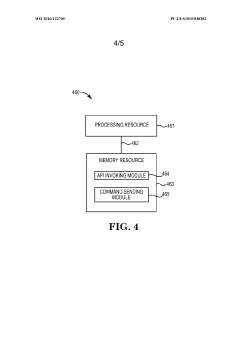
- A microfluidics sensing system comprising a portable computing device and a microfluidic chip with embedded pumps, drop ejectors, impedance sensors, and thermal sensors, capable of performing assays on biologic samples, allowing for digital data capture and analysis, and providing rapid results at a lower cost.
Point-of-Care Implementation Strategies
Successful implementation of microfluidic diagnostic technologies at the point-of-care (POC) requires strategic approaches that address both technical and practical challenges. The transition from laboratory settings to field applications demands careful consideration of device design, user interface, and integration with existing healthcare workflows.
Portable microfluidic devices must be engineered with simplicity and robustness as primary considerations. This includes developing self-contained systems that minimize external equipment requirements while maintaining analytical performance. Recent advances in smartphone-integrated microfluidic platforms demonstrate promising approaches, utilizing built-in cameras and processing power to perform complex diagnostic analyses without specialized laboratory equipment.
Connectivity solutions represent another critical implementation strategy. Modern POC microfluidic devices increasingly incorporate wireless communication capabilities, enabling real-time data transmission to healthcare information systems. This connectivity facilitates immediate clinical decision-making and supports remote monitoring applications, particularly valuable in resource-limited settings where specialist consultation may not be readily available.
Training protocols and user-friendly interfaces significantly impact adoption rates among healthcare professionals. Successful implementation strategies include developing intuitive operational procedures that require minimal technical expertise. Visual guidance systems, color-coded components, and automated sample processing can substantially reduce user error rates while accelerating diagnostic workflows.
Regulatory compliance presents unique challenges for microfluidic POC diagnostics. Implementation strategies must address regional variations in approval requirements while maintaining consistent performance standards. Modular design approaches allow for customization to meet specific regulatory frameworks without necessitating complete system redesign, accelerating deployment across diverse healthcare environments.
Cost-effectiveness remains paramount for widespread adoption, particularly in low-resource settings. Strategic implementation approaches include developing tiered product offerings that balance functionality with affordability. Some manufacturers have successfully implemented subscription-based models for reagents while providing devices at reduced initial cost, improving accessibility while maintaining commercial viability.
Quality control mechanisms must be seamlessly integrated into POC microfluidic systems. Effective strategies incorporate internal controls and automated calibration procedures that verify system performance without requiring additional user intervention. These features ensure diagnostic reliability across varying environmental conditions and operator skill levels, critical for maintaining confidence in results obtained outside traditional laboratory settings.
Portable microfluidic devices must be engineered with simplicity and robustness as primary considerations. This includes developing self-contained systems that minimize external equipment requirements while maintaining analytical performance. Recent advances in smartphone-integrated microfluidic platforms demonstrate promising approaches, utilizing built-in cameras and processing power to perform complex diagnostic analyses without specialized laboratory equipment.
Connectivity solutions represent another critical implementation strategy. Modern POC microfluidic devices increasingly incorporate wireless communication capabilities, enabling real-time data transmission to healthcare information systems. This connectivity facilitates immediate clinical decision-making and supports remote monitoring applications, particularly valuable in resource-limited settings where specialist consultation may not be readily available.
Training protocols and user-friendly interfaces significantly impact adoption rates among healthcare professionals. Successful implementation strategies include developing intuitive operational procedures that require minimal technical expertise. Visual guidance systems, color-coded components, and automated sample processing can substantially reduce user error rates while accelerating diagnostic workflows.
Regulatory compliance presents unique challenges for microfluidic POC diagnostics. Implementation strategies must address regional variations in approval requirements while maintaining consistent performance standards. Modular design approaches allow for customization to meet specific regulatory frameworks without necessitating complete system redesign, accelerating deployment across diverse healthcare environments.
Cost-effectiveness remains paramount for widespread adoption, particularly in low-resource settings. Strategic implementation approaches include developing tiered product offerings that balance functionality with affordability. Some manufacturers have successfully implemented subscription-based models for reagents while providing devices at reduced initial cost, improving accessibility while maintaining commercial viability.
Quality control mechanisms must be seamlessly integrated into POC microfluidic systems. Effective strategies incorporate internal controls and automated calibration procedures that verify system performance without requiring additional user intervention. These features ensure diagnostic reliability across varying environmental conditions and operator skill levels, critical for maintaining confidence in results obtained outside traditional laboratory settings.
Regulatory Pathways for Microfluidic Diagnostic Approval
The regulatory landscape for microfluidic diagnostic devices presents a complex pathway that developers must navigate to bring innovative solutions to market. In the United States, the Food and Drug Administration (FDA) classifies most microfluidic diagnostic devices as in vitro diagnostic (IVD) devices, which typically fall under Class II or Class III medical device categories depending on their intended use and risk profile. The regulatory pathway generally involves either a 510(k) premarket notification, which requires demonstrating substantial equivalence to a legally marketed device, or a Premarket Approval (PMA) application for higher-risk devices.
For novel microfluidic technologies with no predicate devices, the De Novo classification process offers an alternative pathway, allowing innovative diagnostics to enter the market as Class I or Class II devices rather than the more stringent Class III category. Additionally, the FDA's Breakthrough Devices Program provides expedited review for qualifying microfluidic diagnostics that address unmet medical needs for life-threatening conditions.
In the European Union, the regulatory framework has undergone significant transformation with the implementation of the In Vitro Diagnostic Regulation (IVDR), replacing the previous In Vitro Diagnostic Directive (IVDD). The IVDR introduces a risk-based classification system with four classes (A, B, C, and D), with most microfluidic diagnostics falling into classes B through D, requiring conformity assessment by notified bodies.
Clinical validation requirements represent a critical component of the approval process across jurisdictions. Developers must demonstrate both analytical and clinical performance, including sensitivity, specificity, accuracy, and reproducibility. For microfluidic diagnostics, special considerations often apply regarding sample preparation, flow dynamics, and integration with detection systems.
Regulatory bodies increasingly recognize the unique characteristics of microfluidic technologies, with some jurisdictions developing specific guidance documents. The FDA's approach to microfluidic-based devices includes considerations for manufacturing consistency, quality control, and shelf-life stability under various environmental conditions. Similarly, regulatory agencies in Japan, China, and other major markets have established pathways that acknowledge the distinctive aspects of microfluidic diagnostics.
Post-market surveillance requirements have also become more stringent globally, requiring manufacturers to implement robust systems for monitoring device performance and reporting adverse events. For microfluidic diagnostics, this often includes monitoring for issues related to fluid handling, reagent stability, and integration with analytical instruments.
Understanding these regulatory pathways is essential for strategic planning, as regulatory considerations should inform design decisions from the earliest stages of development. Companies developing microfluidic diagnostic technologies must build regulatory expertise or partner with regulatory specialists to navigate these complex approval processes efficiently.
For novel microfluidic technologies with no predicate devices, the De Novo classification process offers an alternative pathway, allowing innovative diagnostics to enter the market as Class I or Class II devices rather than the more stringent Class III category. Additionally, the FDA's Breakthrough Devices Program provides expedited review for qualifying microfluidic diagnostics that address unmet medical needs for life-threatening conditions.
In the European Union, the regulatory framework has undergone significant transformation with the implementation of the In Vitro Diagnostic Regulation (IVDR), replacing the previous In Vitro Diagnostic Directive (IVDD). The IVDR introduces a risk-based classification system with four classes (A, B, C, and D), with most microfluidic diagnostics falling into classes B through D, requiring conformity assessment by notified bodies.
Clinical validation requirements represent a critical component of the approval process across jurisdictions. Developers must demonstrate both analytical and clinical performance, including sensitivity, specificity, accuracy, and reproducibility. For microfluidic diagnostics, special considerations often apply regarding sample preparation, flow dynamics, and integration with detection systems.
Regulatory bodies increasingly recognize the unique characteristics of microfluidic technologies, with some jurisdictions developing specific guidance documents. The FDA's approach to microfluidic-based devices includes considerations for manufacturing consistency, quality control, and shelf-life stability under various environmental conditions. Similarly, regulatory agencies in Japan, China, and other major markets have established pathways that acknowledge the distinctive aspects of microfluidic diagnostics.
Post-market surveillance requirements have also become more stringent globally, requiring manufacturers to implement robust systems for monitoring device performance and reporting adverse events. For microfluidic diagnostics, this often includes monitoring for issues related to fluid handling, reagent stability, and integration with analytical instruments.
Understanding these regulatory pathways is essential for strategic planning, as regulatory considerations should inform design decisions from the earliest stages of development. Companies developing microfluidic diagnostic technologies must build regulatory expertise or partner with regulatory specialists to navigate these complex approval processes efficiently.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!