Microfluidics vs Conventional Labs: Modular Design Benefits
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics Evolution and Design Objectives
Microfluidics technology has evolved significantly over the past three decades, transforming from a conceptual laboratory technique to a revolutionary approach in analytical sciences. The journey began in the early 1990s with rudimentary capillary-based systems, progressing through various developmental phases including the introduction of polydimethylsiloxane (PDMS) fabrication in the late 1990s, which dramatically reduced production costs and increased accessibility.
The 2000s witnessed the integration of multiple functionalities onto single microfluidic platforms, giving rise to the concept of "lab-on-a-chip" systems. This period marked significant advancements in microfabrication techniques, allowing for more complex channel geometries and precise fluid control mechanisms. By the 2010s, the field had matured to incorporate digital microfluidics, droplet-based systems, and organ-on-chip platforms, expanding the application scope beyond traditional analytical chemistry.
Current technological trends indicate a shift toward modular microfluidic designs, which represent a paradigm shift from conventional laboratory setups. These modular systems aim to overcome the limitations of traditional laboratories through reconfigurable components that can be assembled and disassembled according to specific experimental requirements. This approach addresses the inherent inflexibility of conventional laboratory equipment, which often requires substantial space, resources, and specialized training.
The primary technical objectives of modern microfluidic development focus on achieving greater system integration while maintaining operational simplicity. Engineers and scientists are working toward standardized interconnects between modules, improved fluid handling capabilities at microscale, and enhanced detection sensitivities. Additionally, there is a growing emphasis on developing user-friendly interfaces that reduce the technical expertise required for operation, making microfluidic technology more accessible to non-specialists.
Another critical objective is scalability – designing systems that can transition seamlessly from research prototypes to commercial products. This includes addressing manufacturing challenges related to materials selection, production costs, and quality control. Parallel to these efforts is the pursuit of sustainability, with research focused on biodegradable materials and reduced reagent consumption.
The evolution trajectory suggests that future microfluidic systems will increasingly incorporate artificial intelligence for automated operation and data analysis, further reducing human intervention. The ultimate goal remains the development of fully integrated, autonomous analytical platforms that can perform complex laboratory procedures with minimal human oversight, significantly higher throughput, and substantially reduced resource requirements compared to conventional laboratory approaches.
The 2000s witnessed the integration of multiple functionalities onto single microfluidic platforms, giving rise to the concept of "lab-on-a-chip" systems. This period marked significant advancements in microfabrication techniques, allowing for more complex channel geometries and precise fluid control mechanisms. By the 2010s, the field had matured to incorporate digital microfluidics, droplet-based systems, and organ-on-chip platforms, expanding the application scope beyond traditional analytical chemistry.
Current technological trends indicate a shift toward modular microfluidic designs, which represent a paradigm shift from conventional laboratory setups. These modular systems aim to overcome the limitations of traditional laboratories through reconfigurable components that can be assembled and disassembled according to specific experimental requirements. This approach addresses the inherent inflexibility of conventional laboratory equipment, which often requires substantial space, resources, and specialized training.
The primary technical objectives of modern microfluidic development focus on achieving greater system integration while maintaining operational simplicity. Engineers and scientists are working toward standardized interconnects between modules, improved fluid handling capabilities at microscale, and enhanced detection sensitivities. Additionally, there is a growing emphasis on developing user-friendly interfaces that reduce the technical expertise required for operation, making microfluidic technology more accessible to non-specialists.
Another critical objective is scalability – designing systems that can transition seamlessly from research prototypes to commercial products. This includes addressing manufacturing challenges related to materials selection, production costs, and quality control. Parallel to these efforts is the pursuit of sustainability, with research focused on biodegradable materials and reduced reagent consumption.
The evolution trajectory suggests that future microfluidic systems will increasingly incorporate artificial intelligence for automated operation and data analysis, further reducing human intervention. The ultimate goal remains the development of fully integrated, autonomous analytical platforms that can perform complex laboratory procedures with minimal human oversight, significantly higher throughput, and substantially reduced resource requirements compared to conventional laboratory approaches.
Market Analysis for Modular Microfluidic Systems
The global market for modular microfluidic systems is experiencing robust growth, driven by increasing demand for point-of-care diagnostics, personalized medicine, and efficient drug discovery processes. Current market valuations indicate that the microfluidics sector reached approximately 20 billion USD in 2022, with modular systems representing a rapidly expanding segment projected to grow at a compound annual growth rate of 18% through 2028.
Healthcare applications dominate the market landscape, accounting for nearly 60% of current demand. Within this sector, point-of-care testing represents the largest application segment due to the critical need for rapid, decentralized diagnostic capabilities. Pharmaceutical and biotechnology research follows closely, driven by the advantages modular microfluidic systems offer in drug discovery and development processes.
Regional analysis reveals North America currently holds the largest market share at approximately 40%, attributed to substantial research funding, presence of major industry players, and advanced healthcare infrastructure. The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory, fueled by increasing healthcare expenditure, expanding research activities, and government initiatives supporting biomedical innovation.
End-user segmentation shows academic and research institutions currently constitute about 35% of the market, followed by pharmaceutical companies (30%), diagnostic laboratories (20%), and healthcare providers (15%). However, the healthcare provider segment is expected to witness the highest growth rate as point-of-care applications become more widespread.
Key market drivers include increasing demand for miniaturized analytical systems, growing emphasis on personalized medicine, rising prevalence of chronic diseases requiring frequent monitoring, and technological advancements enabling greater functionality in smaller footprints. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid, accessible diagnostic capabilities.
Significant market restraints include high initial development costs, technical challenges in system integration, regulatory hurdles, and limited standardization across platforms. Additionally, the need for specialized expertise to operate these systems presents adoption barriers in resource-limited settings.
Emerging market opportunities exist in developing economies where healthcare infrastructure is expanding rapidly. The integration of artificial intelligence and machine learning with modular microfluidic systems represents another high-potential growth avenue, enabling more sophisticated data analysis and automated decision-making capabilities.
Healthcare applications dominate the market landscape, accounting for nearly 60% of current demand. Within this sector, point-of-care testing represents the largest application segment due to the critical need for rapid, decentralized diagnostic capabilities. Pharmaceutical and biotechnology research follows closely, driven by the advantages modular microfluidic systems offer in drug discovery and development processes.
Regional analysis reveals North America currently holds the largest market share at approximately 40%, attributed to substantial research funding, presence of major industry players, and advanced healthcare infrastructure. The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory, fueled by increasing healthcare expenditure, expanding research activities, and government initiatives supporting biomedical innovation.
End-user segmentation shows academic and research institutions currently constitute about 35% of the market, followed by pharmaceutical companies (30%), diagnostic laboratories (20%), and healthcare providers (15%). However, the healthcare provider segment is expected to witness the highest growth rate as point-of-care applications become more widespread.
Key market drivers include increasing demand for miniaturized analytical systems, growing emphasis on personalized medicine, rising prevalence of chronic diseases requiring frequent monitoring, and technological advancements enabling greater functionality in smaller footprints. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid, accessible diagnostic capabilities.
Significant market restraints include high initial development costs, technical challenges in system integration, regulatory hurdles, and limited standardization across platforms. Additionally, the need for specialized expertise to operate these systems presents adoption barriers in resource-limited settings.
Emerging market opportunities exist in developing economies where healthcare infrastructure is expanding rapidly. The integration of artificial intelligence and machine learning with modular microfluidic systems represents another high-potential growth avenue, enabling more sophisticated data analysis and automated decision-making capabilities.
Current Challenges in Microfluidics vs Conventional Labs
Despite the promising potential of microfluidic technologies, several significant challenges persist when comparing microfluidic systems to conventional laboratory setups. One primary obstacle is the standardization gap. While conventional labs operate with well-established protocols and standardized equipment, microfluidic platforms often suffer from a lack of universal standards for design, fabrication, and operation. This inconsistency creates barriers for cross-laboratory validation and hinders widespread adoption.
Integration complexity represents another substantial challenge. Microfluidic systems frequently require specialized interfaces to connect with existing laboratory infrastructure and analytical instruments. This integration often demands custom solutions, increasing both development time and costs compared to the plug-and-play nature of conventional laboratory equipment.
Material limitations continue to constrain microfluidic advancement. Traditional microfluidic devices predominantly utilize polydimethylsiloxane (PDMS) or glass, which present compatibility issues with certain solvents and biological samples. Additionally, these materials may exhibit surface adsorption properties that affect analytical accuracy. Conventional laboratories, with their diverse material options, typically face fewer such constraints.
Scaling challenges persist in microfluidic technologies. While conventional laboratories can relatively easily scale processes by increasing vessel size or multiplying equipment units, microfluidic systems often require complete redesign when transitioning from prototype to production scale. This redesign necessity significantly impacts development timelines and commercialization potential.
User expertise requirements create adoption barriers for microfluidic systems. Operating microfluidic platforms frequently demands specialized knowledge in fluid dynamics and microfabrication, whereas conventional laboratory techniques benefit from decades of established training protocols and widespread familiarity among laboratory personnel.
Economic considerations further complicate the comparison. Despite the theoretical cost advantages of reduced reagent consumption in microfluidic systems, the initial investment for specialized equipment and design expertise often outweighs these savings in the short term. Conventional laboratories, while reagent-intensive, operate with established economic models and predictable operational costs.
Regulatory and validation challenges represent significant hurdles for microfluidic implementation in regulated environments. Conventional laboratory methods typically have well-established validation protocols and regulatory acceptance, while microfluidic alternatives must navigate complex approval pathways with limited precedent, particularly in clinical and pharmaceutical applications.
Integration complexity represents another substantial challenge. Microfluidic systems frequently require specialized interfaces to connect with existing laboratory infrastructure and analytical instruments. This integration often demands custom solutions, increasing both development time and costs compared to the plug-and-play nature of conventional laboratory equipment.
Material limitations continue to constrain microfluidic advancement. Traditional microfluidic devices predominantly utilize polydimethylsiloxane (PDMS) or glass, which present compatibility issues with certain solvents and biological samples. Additionally, these materials may exhibit surface adsorption properties that affect analytical accuracy. Conventional laboratories, with their diverse material options, typically face fewer such constraints.
Scaling challenges persist in microfluidic technologies. While conventional laboratories can relatively easily scale processes by increasing vessel size or multiplying equipment units, microfluidic systems often require complete redesign when transitioning from prototype to production scale. This redesign necessity significantly impacts development timelines and commercialization potential.
User expertise requirements create adoption barriers for microfluidic systems. Operating microfluidic platforms frequently demands specialized knowledge in fluid dynamics and microfabrication, whereas conventional laboratory techniques benefit from decades of established training protocols and widespread familiarity among laboratory personnel.
Economic considerations further complicate the comparison. Despite the theoretical cost advantages of reduced reagent consumption in microfluidic systems, the initial investment for specialized equipment and design expertise often outweighs these savings in the short term. Conventional laboratories, while reagent-intensive, operate with established economic models and predictable operational costs.
Regulatory and validation challenges represent significant hurdles for microfluidic implementation in regulated environments. Conventional laboratory methods typically have well-established validation protocols and regulatory acceptance, while microfluidic alternatives must navigate complex approval pathways with limited precedent, particularly in clinical and pharmaceutical applications.
Existing Modular Design Approaches and Implementations
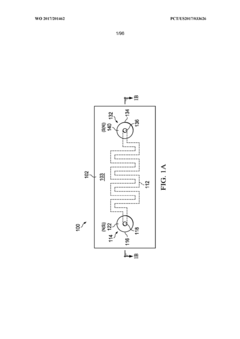
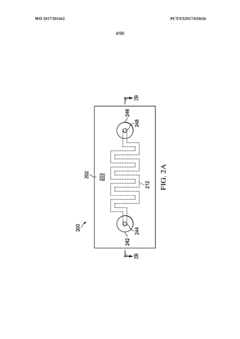
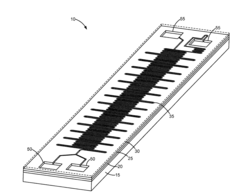
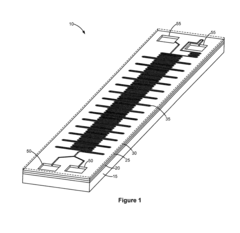
01 Modular microfluidic device architecture
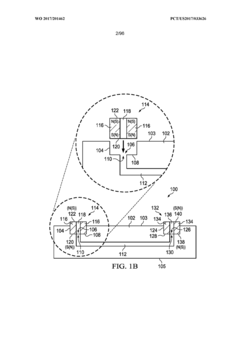
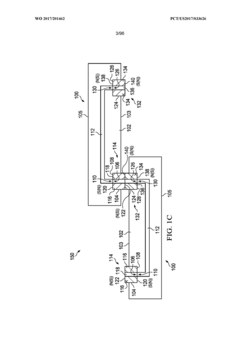
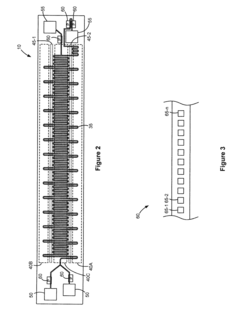
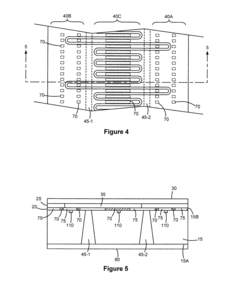
Modular microfluidic systems allow for flexible configuration of different functional components that can be assembled and reconfigured as needed. These systems typically feature standardized interfaces between modules, enabling researchers to create customized microfluidic setups without designing entirely new devices. The modular approach facilitates rapid prototyping, reduces development time, and allows for scalable and adaptable microfluidic platforms that can be tailored to specific applications.- Modular microfluidic device architecture: Modular microfluidic systems allow for flexible configuration of different functional components. These architectures enable users to assemble customized microfluidic platforms by connecting standardized modules with specific functions. The modular approach facilitates rapid prototyping, easy replacement of components, and adaptation to various applications without redesigning the entire system. This design philosophy enhances scalability and reusability while reducing development time and costs.
- Interconnection systems for microfluidic modules: Specialized connection mechanisms enable reliable fluid transfer between microfluidic modules while maintaining system integrity. These interconnection systems include standardized ports, quick-connect fittings, and alignment features that ensure proper module assembly. Advanced designs incorporate leak-proof seals, contamination prevention measures, and mechanisms to minimize dead volume at connection points. These interconnection technologies are crucial for building reconfigurable microfluidic systems with reliable fluid handling capabilities.
- Control systems for modular microfluidics: Integrated control systems coordinate the operation of multiple microfluidic modules within a unified platform. These systems manage fluid flow rates, pressure gradients, temperature conditions, and timing sequences across interconnected modules. Advanced control architectures incorporate feedback mechanisms, automated calibration, and user-friendly interfaces for programming complex protocols. Some implementations feature distributed control capabilities that allow individual modules to operate semi-autonomously while maintaining coordination with the overall system.
- Microfluidic module fabrication techniques: Specialized manufacturing methods enable production of standardized microfluidic modules with consistent performance characteristics. These techniques include precision molding, 3D printing, micromachining, and advanced bonding processes that create reliable fluid channels and functional elements. Some approaches incorporate multi-material fabrication to integrate different properties within a single module. Manufacturing innovations focus on cost-effective production of modules with standardized interfaces while maintaining tight tolerances for critical dimensions and surface properties.
- Application-specific microfluidic module designs: Specialized microfluidic modules are designed to perform specific functions within modular systems, such as mixing, separation, detection, or cell culture. These purpose-built modules incorporate optimized internal geometries and surface treatments tailored to their intended applications. The modular approach allows researchers to select and combine appropriate functional units based on experimental requirements. This enables the creation of customized microfluidic systems for diverse applications in diagnostics, drug discovery, chemical synthesis, and biological research.
02 Interconnection systems for microfluidic modules
Specialized connection systems enable reliable fluid transfer between microfluidic modules while maintaining flow integrity. These interconnection technologies include standardized ports, quick-connect fittings, gasket-based seals, and alignment mechanisms that ensure precise module positioning. Advanced interconnection systems minimize dead volume, prevent leakage, and allow for pressure-resistant connections, enabling the creation of complex microfluidic networks from individual modules without compromising system performance.Expand Specific Solutions03 Reconfigurable microfluidic platforms
Reconfigurable microfluidic platforms allow users to dynamically change the arrangement and functionality of microfluidic components during experiments. These systems often incorporate plug-and-play modules that can be rearranged to create different flow paths and reaction conditions. The reconfigurable nature enables iterative optimization of experimental parameters, facilitates multiple assay types on a single platform, and supports adaptive protocols that can be modified based on real-time results.Expand Specific Solutions04 Automated control systems for modular microfluidics
Automated control systems integrate with modular microfluidic platforms to provide precise regulation of fluid flow, temperature, pressure, and other parameters across multiple modules. These systems typically include programmable controllers, sensor arrays, and actuators that coordinate the operation of different microfluidic components. Advanced control architectures enable complex experimental sequences, feedback-based adjustments, and remote operation capabilities, enhancing the reproducibility and throughput of microfluidic experiments.Expand Specific Solutions05 Application-specific microfluidic module designs
Specialized microfluidic modules are designed for specific applications such as cell culture, particle separation, mixing, detection, or sample preparation. These purpose-built modules incorporate optimized geometries and surface properties for their intended functions while maintaining compatibility with standardized connection interfaces. Libraries of application-specific modules enable researchers to assemble customized microfluidic systems by selecting and combining modules that perform required functions for particular experimental workflows.Expand Specific Solutions
Leading Companies in Modular Microfluidic Solutions
Microfluidics technology is currently in a growth phase, transitioning from early adoption to mainstream implementation across laboratory sciences. The global microfluidics market is expanding rapidly, projected to reach approximately $50 billion by 2026, driven by advantages in modular design that conventional labs cannot match. While established players like Agilent Technologies and Corning have developed commercial platforms, universities (Harvard, Tsinghua, Michigan) continue to drive fundamental innovation. Companies like IntegenX and Atonomics are advancing specialized applications, while Abbott Laboratories and Samsung Electro-Mechanics are integrating microfluidic technologies into broader product ecosystems. The technology has reached moderate maturity in diagnostics and analytical applications, but emerging areas like organ-on-chip systems remain in early development stages, presenting significant growth opportunities for both established corporations and specialized startups.
Corning, Inc.
Technical Solution: Corning has pioneered advanced microfluidic solutions through their Corning® Microfluidic Discovery Platform, which utilizes proprietary glass fabrication techniques to create highly precise microchannels with exceptional optical clarity and chemical resistance. Their modular approach incorporates standardized connection interfaces that allow researchers to combine different functional modules—such as mixing chambers, reaction zones, and detection areas—into customized analytical systems. Corning's technology features microchannels with dimensions ranging from 5-500 μm, precisely controlled using their advanced etching processes. The platform employs a "building block" architecture where pre-validated functional modules can be connected through standardized ports, enabling rapid reconfiguration for different applications. Their glass-based microfluidic chips offer superior thermal stability and optical properties compared to polymer alternatives, making them particularly suitable for applications requiring precise temperature control or sensitive optical detection.
Strengths: Exceptional material properties including chemical resistance, optical clarity, and thermal stability; highly precise manufacturing capabilities ensure consistent channel dimensions. Weaknesses: Glass-based systems typically have higher production costs than polymer alternatives; less flexibility in rapid prototyping compared to soft lithography techniques.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed an integrated microfluidic lab-on-a-chip platform that combines multiple laboratory functions on a single chip measuring just a few square centimeters. Their technology utilizes precision-manufactured microchannels (typically 20-100 μm wide) etched into glass or polymer substrates, with standardized fluidic interfaces that enable modular connectivity between different functional units. The system incorporates automated sample preparation, reagent mixing, and detection capabilities within a unified architecture. Agilent's approach emphasizes standardized microfluidic building blocks that can be reconfigured for different analytical applications, from genomics to proteomics. Their chips feature integrated electrodes for electrokinetic sample manipulation and built-in optical detection windows compatible with their analytical instruments. The modular design allows researchers to swap out functional components while maintaining system integrity, significantly reducing development time compared to conventional laboratory setups.
Strengths: Seamless integration with existing Agilent analytical instruments provides end-to-end workflow solutions; high manufacturing precision ensures reproducible results across different chips. Weaknesses: Proprietary connection standards may limit interoperability with third-party components; higher initial investment compared to traditional laboratory equipment.
Key Technical Innovations in Microfluidic Modularity
Microfluidic module, system and kit having magnetic interconnects on same side of inlet and outlet openings
PatentWO2017201462A1
Innovation
- The integration of magnetic interconnects, specifically ring magnets with sealing gaskets, into microfluidic modules and systems, allowing for magnetic coupling and easy reconfiguration of modules while maintaining fluidic communication.
Micro-fluidic modules on a chip for diagnostic applications
PatentActiveUS20160199839A1
Innovation
- A micro-fluidic system is developed where pump modules and fluidic structures are monolithically fabricated on a substrate using heater chip fabrication methods, creating a continuous flow system with distinct temperature regions and integrated pumps to facilitate thermal cycling of fluids.
Standardization Efforts in Microfluidic Interfaces
Standardization efforts in microfluidic interfaces have become increasingly critical as the field matures from academic research to commercial applications. The microfluidics industry currently faces significant challenges due to the lack of universal standards for device connections, component interfaces, and system integration protocols. Unlike conventional laboratory equipment with well-established standards, microfluidic systems often feature proprietary designs that limit interoperability between different manufacturers' products.
Several international organizations have initiated standardization projects to address these challenges. The International Organization for Standardization (ISO) has established technical committees focused on developing standards for microfluidic connections, while the ASTM International has published guidelines for testing microfluidic device performance. These efforts aim to create a common language for microfluidic interfaces that would enable plug-and-play functionality similar to USB standards in computing.
Industry consortia have also emerged to drive standardization forward. The Microfluidics Consortium, comprising leading manufacturers and research institutions, has proposed specifications for standardized chip-to-world interfaces, including fluidic, electrical, and optical connections. Their recommendations include standardized port dimensions, connection mechanisms, and communication protocols that would allow devices from different vendors to work seamlessly together.
Academic research centers have contributed significantly by developing open-source interface designs and protocols. The Microfluidic Innovation Hub at MIT has created reference designs for modular microfluidic connections that have been adopted by several commercial entities. Similarly, the European Microfluidics Standardization Initiative has published white papers outlining recommended practices for interface design and validation.
The economic benefits of standardization are substantial. Market analysis indicates that standardized interfaces could reduce integration costs by up to 40% and accelerate product development cycles by 30%. Companies adopting open standards have reported increased customer adoption rates due to the flexibility offered by modular systems that can be reconfigured for different applications without proprietary constraints.
Despite progress, challenges remain in achieving widespread adoption of standards. Technical hurdles include accommodating the diverse requirements of different application domains, from clinical diagnostics to environmental monitoring. Additionally, some established manufacturers have been reluctant to abandon proprietary interfaces that lock customers into their ecosystem. However, the growing demand for interoperable systems is gradually shifting the industry toward embracing standardization as a competitive advantage rather than a threat to proprietary technologies.
Several international organizations have initiated standardization projects to address these challenges. The International Organization for Standardization (ISO) has established technical committees focused on developing standards for microfluidic connections, while the ASTM International has published guidelines for testing microfluidic device performance. These efforts aim to create a common language for microfluidic interfaces that would enable plug-and-play functionality similar to USB standards in computing.
Industry consortia have also emerged to drive standardization forward. The Microfluidics Consortium, comprising leading manufacturers and research institutions, has proposed specifications for standardized chip-to-world interfaces, including fluidic, electrical, and optical connections. Their recommendations include standardized port dimensions, connection mechanisms, and communication protocols that would allow devices from different vendors to work seamlessly together.
Academic research centers have contributed significantly by developing open-source interface designs and protocols. The Microfluidic Innovation Hub at MIT has created reference designs for modular microfluidic connections that have been adopted by several commercial entities. Similarly, the European Microfluidics Standardization Initiative has published white papers outlining recommended practices for interface design and validation.
The economic benefits of standardization are substantial. Market analysis indicates that standardized interfaces could reduce integration costs by up to 40% and accelerate product development cycles by 30%. Companies adopting open standards have reported increased customer adoption rates due to the flexibility offered by modular systems that can be reconfigured for different applications without proprietary constraints.
Despite progress, challenges remain in achieving widespread adoption of standards. Technical hurdles include accommodating the diverse requirements of different application domains, from clinical diagnostics to environmental monitoring. Additionally, some established manufacturers have been reluctant to abandon proprietary interfaces that lock customers into their ecosystem. However, the growing demand for interoperable systems is gradually shifting the industry toward embracing standardization as a competitive advantage rather than a threat to proprietary technologies.
Cost-Benefit Analysis of Modular vs Traditional Systems
The economic analysis of modular microfluidic systems versus traditional laboratory setups reveals significant cost differentials across initial investment, operational expenses, and long-term financial implications. Initial capital expenditure for conventional laboratory infrastructure typically ranges between $500,000 to several million dollars, encompassing specialized equipment, dedicated facilities, and extensive utility installations. Conversely, modular microfluidic platforms generally require $50,000-200,000 for comparable analytical capabilities, representing a 60-80% reduction in upfront investment.
Operational cost structures demonstrate even more pronounced disparities. Traditional laboratories incur substantial ongoing expenses including reagent consumption (approximately $15-25 per sample), specialized personnel ($70,000-120,000 annual salaries), and facility maintenance costs averaging 15-20% of initial investment annually. Microfluidic systems dramatically reduce these figures, with reagent usage decreased by 90-95% through nanoliter-scale processing, simplified operation reducing personnel requirements by 30-50%, and minimal maintenance needs typically under 5% of system cost annually.
Return on investment calculations indicate modular microfluidic systems achieve breakeven points within 12-18 months for most applications, compared to 3-5 years for conventional laboratory setups. This accelerated ROI stems from both reduced capital requirements and significantly lower operational costs, creating compelling financial incentives for adoption.
Scalability economics further favor modular approaches. Traditional laboratories face near-linear cost increases when expanding capacity, requiring proportional increases in space, equipment, and personnel. Microfluidic platforms demonstrate more favorable scaling economics, with incremental capacity additions typically costing 40-60% less per unit of analytical capability compared to initial implementation.
Risk assessment from a financial perspective also advantages modular systems. The compartmentalized nature of microfluidic platforms allows for targeted upgrades and replacements, limiting financial exposure during technology transitions. Traditional laboratories often require wholesale system replacements, creating significant financial vulnerabilities during technological evolution cycles.
Recent case studies from pharmaceutical research operations demonstrate these economic differentials in practice. A mid-sized drug discovery operation reported 68% reduction in per-sample analysis costs after transitioning to modular microfluidic platforms, with initial investment recovered within 14 months despite transition costs. Similar economic advantages have been documented across clinical diagnostics, environmental monitoring, and food safety applications.
Operational cost structures demonstrate even more pronounced disparities. Traditional laboratories incur substantial ongoing expenses including reagent consumption (approximately $15-25 per sample), specialized personnel ($70,000-120,000 annual salaries), and facility maintenance costs averaging 15-20% of initial investment annually. Microfluidic systems dramatically reduce these figures, with reagent usage decreased by 90-95% through nanoliter-scale processing, simplified operation reducing personnel requirements by 30-50%, and minimal maintenance needs typically under 5% of system cost annually.
Return on investment calculations indicate modular microfluidic systems achieve breakeven points within 12-18 months for most applications, compared to 3-5 years for conventional laboratory setups. This accelerated ROI stems from both reduced capital requirements and significantly lower operational costs, creating compelling financial incentives for adoption.
Scalability economics further favor modular approaches. Traditional laboratories face near-linear cost increases when expanding capacity, requiring proportional increases in space, equipment, and personnel. Microfluidic platforms demonstrate more favorable scaling economics, with incremental capacity additions typically costing 40-60% less per unit of analytical capability compared to initial implementation.
Risk assessment from a financial perspective also advantages modular systems. The compartmentalized nature of microfluidic platforms allows for targeted upgrades and replacements, limiting financial exposure during technology transitions. Traditional laboratories often require wholesale system replacements, creating significant financial vulnerabilities during technological evolution cycles.
Recent case studies from pharmaceutical research operations demonstrate these economic differentials in practice. A mid-sized drug discovery operation reported 68% reduction in per-sample analysis costs after transitioning to modular microfluidic platforms, with initial investment recovered within 14 months despite transition costs. Similar economic advantages have been documented across clinical diagnostics, environmental monitoring, and food safety applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!