How to Use Microfluidics for Nanoscale Particle Manipulation
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics Background and Nanomanipulation Goals
Microfluidics emerged in the early 1990s as a revolutionary field combining fluid mechanics, physics, chemistry, and engineering at the microscale. The technology evolved from initial capillary electrophoresis systems to sophisticated lab-on-a-chip platforms capable of manipulating fluids at volumes as small as picoliters. This progression has been driven by advances in microfabrication techniques, particularly soft lithography using polydimethylsiloxane (PDMS), which enabled rapid prototyping of complex microfluidic architectures.
The fundamental principles of microfluidics leverage unique physical phenomena that dominate at the microscale, including laminar flow, surface tension effects, and enhanced diffusion characteristics. These properties create controlled environments where fluid behavior becomes highly predictable, offering unprecedented precision for particle manipulation. The field has experienced exponential growth, with applications expanding from simple analytical chemistry to complex biological assays, point-of-care diagnostics, and advanced manufacturing processes.
Nanoscale particle manipulation represents the next frontier in microfluidic technology development. As industrial and biomedical applications increasingly require precise control over nanomaterials, microfluidic platforms offer unique advantages for handling particles ranging from 1-1000 nm. The convergence of microfluidics with nanotechnology addresses critical challenges in fields such as drug delivery, biosensing, and advanced materials synthesis.
The primary technical goals for microfluidic nanomanipulation include achieving precise spatial positioning of individual nanoparticles, enabling high-throughput processing while maintaining single-particle resolution, and developing systems capable of sorting heterogeneous nanoparticle populations based on multiple physical parameters simultaneously. Additionally, there is significant interest in creating platforms that can dynamically alter nanoparticle properties or facilitate controlled assembly of complex nanostructures.
Current technological trajectories indicate evolution toward integrated systems combining multiple manipulation mechanisms, including dielectrophoresis, acoustic focusing, optical tweezers, and magnetophoresis. These hybrid approaches aim to overcome the limitations of individual techniques while expanding the range of manipulable particles. The field is also witnessing increased integration with real-time characterization tools, allowing for closed-loop control systems that can adapt to variations in particle properties.
The ultimate vision for microfluidic nanomanipulation technology encompasses fully automated platforms capable of precise, programmable control over diverse nanoparticle types in complex media. Such systems would enable transformative applications in personalized nanomedicine, quantum computing hardware development, and next-generation environmental sensing technologies. Achieving these ambitious goals requires interdisciplinary collaboration spanning microfluidics, nanofabrication, control systems engineering, and application-specific domains.
The fundamental principles of microfluidics leverage unique physical phenomena that dominate at the microscale, including laminar flow, surface tension effects, and enhanced diffusion characteristics. These properties create controlled environments where fluid behavior becomes highly predictable, offering unprecedented precision for particle manipulation. The field has experienced exponential growth, with applications expanding from simple analytical chemistry to complex biological assays, point-of-care diagnostics, and advanced manufacturing processes.
Nanoscale particle manipulation represents the next frontier in microfluidic technology development. As industrial and biomedical applications increasingly require precise control over nanomaterials, microfluidic platforms offer unique advantages for handling particles ranging from 1-1000 nm. The convergence of microfluidics with nanotechnology addresses critical challenges in fields such as drug delivery, biosensing, and advanced materials synthesis.
The primary technical goals for microfluidic nanomanipulation include achieving precise spatial positioning of individual nanoparticles, enabling high-throughput processing while maintaining single-particle resolution, and developing systems capable of sorting heterogeneous nanoparticle populations based on multiple physical parameters simultaneously. Additionally, there is significant interest in creating platforms that can dynamically alter nanoparticle properties or facilitate controlled assembly of complex nanostructures.
Current technological trajectories indicate evolution toward integrated systems combining multiple manipulation mechanisms, including dielectrophoresis, acoustic focusing, optical tweezers, and magnetophoresis. These hybrid approaches aim to overcome the limitations of individual techniques while expanding the range of manipulable particles. The field is also witnessing increased integration with real-time characterization tools, allowing for closed-loop control systems that can adapt to variations in particle properties.
The ultimate vision for microfluidic nanomanipulation technology encompasses fully automated platforms capable of precise, programmable control over diverse nanoparticle types in complex media. Such systems would enable transformative applications in personalized nanomedicine, quantum computing hardware development, and next-generation environmental sensing technologies. Achieving these ambitious goals requires interdisciplinary collaboration spanning microfluidics, nanofabrication, control systems engineering, and application-specific domains.
Market Applications and Demand Analysis for Nanoscale Manipulation
The global market for nanoscale particle manipulation using microfluidics is experiencing robust growth, driven by increasing applications across multiple industries. Current market estimates value this sector at approximately $3.5 billion, with projections indicating a compound annual growth rate of 18.7% through 2028. This growth trajectory reflects the expanding utility of microfluidic technologies in precise manipulation of nanoscale materials.
Healthcare and pharmaceutical sectors represent the largest market segments, accounting for nearly 42% of current applications. The demand is particularly strong for drug delivery systems that utilize microfluidic platforms to create uniform nanoparticles with controlled release properties. Major pharmaceutical companies are increasingly investing in microfluidic technologies to enhance drug efficacy while reducing side effects through targeted delivery mechanisms.
Diagnostic applications constitute another significant market driver, with point-of-care testing devices leveraging microfluidic nanoscale manipulation to achieve higher sensitivity and specificity. The COVID-19 pandemic has accelerated this trend, with numerous microfluidic-based diagnostic platforms receiving emergency approvals and demonstrating commercial viability.
In the biotechnology sector, cell sorting and single-cell analysis applications are creating substantial demand for microfluidic particle manipulation technologies. The ability to precisely control and analyze individual cells at the nanoscale has revolutionized research capabilities in genomics and proteomics, with the market for these applications growing at 22.3% annually.
Materials science and advanced manufacturing industries are emerging as significant new markets, utilizing microfluidic platforms for the synthesis of specialized nanoparticles with unique optical, electrical, or mechanical properties. These engineered nanomaterials find applications in electronics, energy storage, and advanced coatings, expanding the overall market footprint.
Regionally, North America leads with 38% market share, followed closely by Europe at 31% and Asia-Pacific at 26%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 24.1% annually, driven by substantial investments in research infrastructure and manufacturing capabilities in China, Japan, and South Korea.
Customer demand analysis reveals increasing requirements for system integration, automation, and scalability. End-users are seeking complete workflow solutions rather than standalone components, creating opportunities for comprehensive platform development. Additionally, there is growing demand for standardization and validation protocols to facilitate regulatory approval and commercial adoption across different application domains.
Market barriers include high initial investment costs, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding nanomaterial applications. Despite these challenges, the convergence of microfluidics with artificial intelligence and advanced manufacturing techniques is expected to further expand market opportunities and accelerate adoption across diverse industries.
Healthcare and pharmaceutical sectors represent the largest market segments, accounting for nearly 42% of current applications. The demand is particularly strong for drug delivery systems that utilize microfluidic platforms to create uniform nanoparticles with controlled release properties. Major pharmaceutical companies are increasingly investing in microfluidic technologies to enhance drug efficacy while reducing side effects through targeted delivery mechanisms.
Diagnostic applications constitute another significant market driver, with point-of-care testing devices leveraging microfluidic nanoscale manipulation to achieve higher sensitivity and specificity. The COVID-19 pandemic has accelerated this trend, with numerous microfluidic-based diagnostic platforms receiving emergency approvals and demonstrating commercial viability.
In the biotechnology sector, cell sorting and single-cell analysis applications are creating substantial demand for microfluidic particle manipulation technologies. The ability to precisely control and analyze individual cells at the nanoscale has revolutionized research capabilities in genomics and proteomics, with the market for these applications growing at 22.3% annually.
Materials science and advanced manufacturing industries are emerging as significant new markets, utilizing microfluidic platforms for the synthesis of specialized nanoparticles with unique optical, electrical, or mechanical properties. These engineered nanomaterials find applications in electronics, energy storage, and advanced coatings, expanding the overall market footprint.
Regionally, North America leads with 38% market share, followed closely by Europe at 31% and Asia-Pacific at 26%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 24.1% annually, driven by substantial investments in research infrastructure and manufacturing capabilities in China, Japan, and South Korea.
Customer demand analysis reveals increasing requirements for system integration, automation, and scalability. End-users are seeking complete workflow solutions rather than standalone components, creating opportunities for comprehensive platform development. Additionally, there is growing demand for standardization and validation protocols to facilitate regulatory approval and commercial adoption across different application domains.
Market barriers include high initial investment costs, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding nanomaterial applications. Despite these challenges, the convergence of microfluidics with artificial intelligence and advanced manufacturing techniques is expected to further expand market opportunities and accelerate adoption across diverse industries.
Current Microfluidic Technologies and Nanomanipulation Challenges
Microfluidics technology has evolved significantly over the past two decades, enabling precise manipulation of fluids at the microscale. Current microfluidic platforms utilize various physical principles including pressure-driven flow, electrokinetics, acoustics, and optics to control fluid behavior. Despite these advancements, manipulating nanoscale particles (1-100 nm) remains challenging due to the dominance of surface forces over inertial forces at this scale, requiring specialized approaches beyond traditional microfluidic techniques.
The state-of-the-art in microfluidic nanomanipulation includes dielectrophoresis (DEP), which uses non-uniform electric fields to induce particle movement based on their dielectric properties. While effective for particles above 100 nm, DEP faces efficiency limitations with smaller nanoparticles due to scaling laws where dielectrophoretic force decreases with the cube of particle radius. Optical tweezers offer another approach, using focused laser beams to trap particles, but struggle with particles below 100 nm unless extremely high laser powers are employed, risking sample damage.
Acoustic manipulation techniques, including surface acoustic waves (SAW) and bulk acoustic waves (BAW), have shown promise for particle focusing and sorting. However, these methods typically operate optimally for microscale particles, with diminishing effectiveness at the nanoscale due to reduced acoustic radiation forces. Magnetophoresis works well for magnetic or magnetically-labeled nanoparticles but has limited applicability for non-magnetic materials without surface modification.
Nanoscale manipulation faces several fundamental challenges. Brownian motion becomes increasingly dominant as particle size decreases, making precise positioning difficult. The Reynolds number at the nanoscale is extremely low, resulting in laminar flow conditions where mixing occurs primarily through diffusion rather than convection. Additionally, surface interactions including van der Waals forces, electrostatic interactions, and steric effects significantly influence nanoparticle behavior, often causing unwanted aggregation or adhesion to channel walls.
Fabrication limitations present another significant hurdle. While conventional microfluidic channels are typically fabricated at dimensions of tens to hundreds of micrometers, manipulating nanoscale particles often requires channel features approaching the nanoscale. Current fabrication techniques like photolithography face resolution limits, while advanced methods such as electron beam lithography offer higher resolution but at significantly increased cost and reduced throughput.
Detection and characterization of nanoscale particles within microfluidic systems present additional challenges. Traditional optical microscopy reaches its diffraction limit at approximately half the wavelength of light, making direct visualization of nanoparticles difficult. Alternative detection methods such as fluorescence labeling, surface plasmon resonance, or electrical impedance measurements each have their own limitations in sensitivity, specificity, or implementation complexity.
The state-of-the-art in microfluidic nanomanipulation includes dielectrophoresis (DEP), which uses non-uniform electric fields to induce particle movement based on their dielectric properties. While effective for particles above 100 nm, DEP faces efficiency limitations with smaller nanoparticles due to scaling laws where dielectrophoretic force decreases with the cube of particle radius. Optical tweezers offer another approach, using focused laser beams to trap particles, but struggle with particles below 100 nm unless extremely high laser powers are employed, risking sample damage.
Acoustic manipulation techniques, including surface acoustic waves (SAW) and bulk acoustic waves (BAW), have shown promise for particle focusing and sorting. However, these methods typically operate optimally for microscale particles, with diminishing effectiveness at the nanoscale due to reduced acoustic radiation forces. Magnetophoresis works well for magnetic or magnetically-labeled nanoparticles but has limited applicability for non-magnetic materials without surface modification.
Nanoscale manipulation faces several fundamental challenges. Brownian motion becomes increasingly dominant as particle size decreases, making precise positioning difficult. The Reynolds number at the nanoscale is extremely low, resulting in laminar flow conditions where mixing occurs primarily through diffusion rather than convection. Additionally, surface interactions including van der Waals forces, electrostatic interactions, and steric effects significantly influence nanoparticle behavior, often causing unwanted aggregation or adhesion to channel walls.
Fabrication limitations present another significant hurdle. While conventional microfluidic channels are typically fabricated at dimensions of tens to hundreds of micrometers, manipulating nanoscale particles often requires channel features approaching the nanoscale. Current fabrication techniques like photolithography face resolution limits, while advanced methods such as electron beam lithography offer higher resolution but at significantly increased cost and reduced throughput.
Detection and characterization of nanoscale particles within microfluidic systems present additional challenges. Traditional optical microscopy reaches its diffraction limit at approximately half the wavelength of light, making direct visualization of nanoparticles difficult. Alternative detection methods such as fluorescence labeling, surface plasmon resonance, or electrical impedance measurements each have their own limitations in sensitivity, specificity, or implementation complexity.
Established Microfluidic Methods for Nanoparticle Control
01 Dielectrophoresis for nanoscale particle manipulation
Dielectrophoresis (DEP) is a technique used in microfluidic systems to manipulate nanoscale particles by applying non-uniform electric fields. This method enables precise control over particle movement, separation, and positioning based on their dielectric properties. The technique allows for label-free manipulation of various particles including cells, DNA, proteins, and synthetic nanoparticles, making it valuable for biomedical applications, diagnostics, and nanomaterial assembly.- Electrokinetic manipulation of nanoparticles: Electrokinetic techniques are employed in microfluidic systems to manipulate nanoscale particles through the application of electric fields. These methods include dielectrophoresis, electrophoresis, and electroosmosis, which enable precise control over particle movement, separation, and concentration. The electric field gradients created within microchannels allow for non-contact manipulation of particles based on their electrical properties, size, and shape, making these approaches particularly valuable for biological samples and diagnostic applications.
- Acoustic manipulation of nanoparticles: Acoustic waves are utilized in microfluidic devices to manipulate nanoscale particles through acoustic radiation forces. By generating standing acoustic waves within microchannels, particles can be precisely positioned, sorted, and concentrated based on their physical properties such as size, density, and compressibility. This non-contact manipulation technique offers advantages including gentle handling of biological particles, high throughput capabilities, and compatibility with various sample types, making it suitable for applications in biomedical research and diagnostics.
- Magnetic manipulation of nanoparticles: Magnetic fields are employed in microfluidic systems to manipulate magnetic or magnetically-labeled nanoscale particles. By integrating magnetic elements within microfluidic devices, particles can be captured, transported, sorted, and concentrated with high precision. This approach allows for selective manipulation of target particles in complex mixtures and can be dynamically controlled by adjusting the magnetic field strength and configuration. Magnetic manipulation is particularly valuable for biomedical applications including cell separation, immunoassays, and targeted drug delivery.
- Optical manipulation of nanoparticles: Optical forces are utilized in microfluidic platforms to manipulate nanoscale particles through techniques such as optical tweezers and optically-induced dielectrophoresis. By focusing laser beams within microchannels, particles can be trapped, moved, and sorted with nanometer precision based on their optical properties. These non-contact manipulation methods offer advantages including high spatial resolution, dynamic control, and compatibility with transparent particles. Optical manipulation is particularly valuable for single-particle analysis, assembly of nanostructures, and studies of biological interactions at the nanoscale.
- Integrated multi-modal manipulation systems: Advanced microfluidic platforms integrate multiple manipulation techniques to achieve enhanced control over nanoscale particles. These systems combine two or more approaches such as electrokinetic, acoustic, magnetic, and optical methods to leverage their complementary advantages. The integration enables more versatile and robust manipulation capabilities, allowing for complex particle handling operations including sequential processing, multi-parameter sorting, and precise positioning. These integrated platforms are particularly valuable for applications requiring high specificity and efficiency, such as point-of-care diagnostics, advanced material synthesis, and single-cell analysis.
02 Acoustic wave-based particle manipulation
Acoustic waves are employed in microfluidic devices to manipulate nanoscale particles through acoustic radiation forces. By generating standing acoustic waves within microchannels, particles can be focused, separated, or trapped at specific positions. This non-contact manipulation technique is particularly useful for biological samples as it minimizes damage to cells and biomolecules. The method offers advantages in terms of throughput, precision, and compatibility with various sample types for applications in biomedical research and diagnostics.Expand Specific Solutions03 Magnetic field-based manipulation of nanoparticles
Magnetic fields are utilized in microfluidic systems to control the movement and positioning of magnetic or magnetically-labeled nanoparticles. This approach enables selective manipulation of target particles in complex mixtures, allowing for separation, concentration, and directed transport within microchannels. The technique is particularly valuable for biomedical applications such as cell sorting, immunoassays, and targeted drug delivery, offering high specificity and remote control capabilities without direct contact with the sample.Expand Specific Solutions04 Integrated microfluidic platforms for multi-modal particle manipulation
Integrated microfluidic platforms combine multiple manipulation techniques (such as electrical, magnetic, optical, and acoustic methods) within a single system to achieve enhanced control over nanoscale particles. These platforms enable sequential or simultaneous application of different forces for complex manipulation tasks. The integration of multiple modalities overcomes limitations of individual techniques and provides versatile solutions for applications requiring precise particle handling, such as point-of-care diagnostics, single-cell analysis, and nanomaterial synthesis.Expand Specific Solutions05 Microfluidic devices for nanoparticle synthesis and functionalization
Specialized microfluidic devices are designed for controlled synthesis and functionalization of nanoparticles with precise size, shape, and composition. These systems leverage laminar flow characteristics and rapid mixing capabilities to achieve uniform reaction conditions. The devices enable continuous production of nanoparticles with high reproducibility and allow for in-line functionalization processes. This approach offers advantages over conventional batch methods, including improved control over particle properties, reduced reagent consumption, and potential for automated production of functionalized nanoparticles for various applications.Expand Specific Solutions
Leading Research Groups and Companies in Microfluidics
Microfluidics for nanoscale particle manipulation is currently in a growth phase, with the market expected to reach significant expansion due to increasing applications in drug delivery, diagnostics, and material science. The technology is maturing rapidly, with key players demonstrating varying levels of advancement. Companies like Roche Diagnostics and IBM are leveraging their extensive R&D capabilities to develop sophisticated microfluidic platforms, while specialized firms such as Advion and Suzhou Precigenome are focusing on innovative niche applications. Academic institutions including Cornell University and East China Normal University are driving fundamental research, while collaborations between industry leaders like Samsung Electronics and BOE Technology are accelerating commercialization efforts. The competitive landscape reflects a blend of established pharmaceutical companies, technology giants, and emerging specialized players working to overcome technical challenges in precise particle control at the nanoscale.
Suzhou Institute of Biomedical Engineering & Technology
Technical Solution: The Suzhou Institute of Biomedical Engineering & Technology (SIBET) has developed specialized microfluidic platforms for nanoscale particle manipulation with a focus on biomedical applications. Their approach integrates multiple physical principles including hydrodynamic focusing, electrokinetics, and magnetophoresis within precisely fabricated microchannels. SIBET's technology features gradient microstructures that create predictable flow patterns for size-based particle separation with resolution down to 100 nm. Their devices incorporate embedded microelectrode arrays that generate non-uniform electric fields for dielectrophoretic manipulation of nanoparticles based on their electrical properties rather than just size. A notable innovation is their development of magnetically-functionalized microfluidic channels that can capture specific nanoparticles when external magnetic fields are applied, enabling selective isolation from complex biological samples. SIBET researchers have also created microfluidic droplet generators capable of producing monodisperse nanoemulsions with precisely controlled particle sizes for drug delivery applications. Their integrated systems combine sample preparation, particle manipulation, and detection capabilities within single devices, making them particularly suitable for point-of-care diagnostics and personalized medicine applications where handling of exosomes, viruses, and other biological nanoparticles is required.
Strengths: Strong focus on practical biomedical applications; excellent integration of multiple manipulation techniques; good balance between performance and manufacturability. Weaknesses: More limited resolution compared to some academic systems; technologies primarily optimized for biological rather than synthetic nanoparticles; moderate throughput limitations for clinical applications.
National Center for Nanoscience & Technology
Technical Solution: The National Center for Nanoscience & Technology (NCNST) has developed comprehensive microfluidic platforms for nanoscale particle manipulation, leveraging their expertise in nanofabrication and materials science. Their approach combines inertial microfluidics with electrokinetic techniques to achieve precise control over nanoparticles. NCNST researchers have created spiral microfluidic channels with carefully designed cross-sections that generate Dean flow patterns, enabling size-based separation of nanoparticles with diameters as small as 50 nm. Their technology incorporates nanopatterned electrode arrays within microchannels that generate highly localized dielectrophoretic forces for particle trapping and manipulation. A significant innovation is their development of stimuli-responsive polymer coatings for microchannels that can dynamically alter surface properties in response to temperature, pH, or light, enabling switchable particle capture and release. NCNST has also pioneered acoustofluidic techniques that use surface acoustic waves to create pressure gradients capable of manipulating sub-micron particles with minimal heating or damage. Their integrated platforms combine multiple manipulation mechanisms within single devices, allowing sequential operations such as concentration, washing, and sorting of nanoscale particles for applications in diagnostics, drug delivery, and materials science.
Strengths: Comprehensive integration of multiple manipulation techniques; strong materials science foundation enabling novel surface functionalization; excellent fabrication capabilities for complex microstructures. Weaknesses: Some technologies remain at laboratory scale with limited commercial translation; complex operational requirements for multi-modal systems; relatively high expertise needed for system operation.
Key Patents and Innovations in Nanoscale Microfluidics
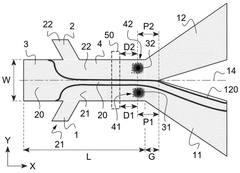
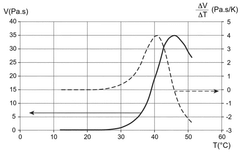
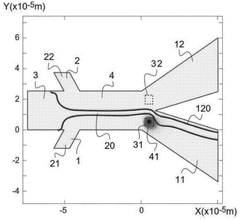
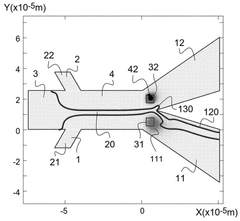
Method and device for microfluidic manipulation using a thermoviscous liquid
PatentWO2025087923A1
Innovation
- A microfluidic handling process that involves injecting a sample liquid and a sheath liquid with a thermo-viscous property into a microfluidic device. The process includes applying a brief, localized energy source to heat the sheath liquid, causing a significant increase in viscosity that allows for the selective diversion of the sample liquid to a specific output channel, enabling high-frequency sorting and concentration of nanometric objects.
Microfluidics Chips Useful in Nanoparticle Preparation and Expandable Pump Network System Useful Therewith
PatentPendingUS20240058817A1
Innovation
- The development of microfluidic chips with high-pressure-resistant materials, 3D channel features, and a network of smart syringe pumps that allow for wireless communication and coordinated control of multiple pumps, enabling precise fluid flow management and integration of electronic elements.
Fabrication Technologies for Microfluidic Devices
The fabrication of microfluidic devices for nanoscale particle manipulation requires sophisticated manufacturing techniques that balance precision, cost-effectiveness, and scalability. Traditional fabrication methods have evolved significantly over the past decade, with photolithography remaining a cornerstone technology adapted from the semiconductor industry. This process involves transferring geometric patterns from photomasks to light-sensitive chemical substrates, enabling the creation of intricate channel networks with feature sizes down to several micrometers.
Soft lithography has emerged as a particularly versatile technique for microfluidic device fabrication, especially using polydimethylsiloxane (PDMS). This elastomeric material offers excellent optical transparency, biocompatibility, and gas permeability, making it ideal for biological applications. The process involves creating a master mold through photolithography, against which PDMS is cast, cured, and bonded to glass or other substrates through plasma treatment or chemical bonding.
For applications requiring higher chemical resistance or more rigid structures, thermoplastic-based fabrication methods have gained prominence. Hot embossing and injection molding enable mass production of devices using materials such as polymethyl methacrylate (PMMA), polycarbonate (PC), and cyclic olefin copolymer (COC). These techniques offer excellent reproducibility and are particularly suitable for commercial-scale manufacturing.
Glass and silicon micromachining represents another important fabrication approach, utilizing wet etching with hydrofluoric acid solutions or dry etching techniques like deep reactive ion etching (DRIE). These materials provide superior chemical resistance and thermal stability compared to polymers, though at higher production costs and with more complex fabrication processes.
Recent advances in 3D printing technologies have revolutionized rapid prototyping for microfluidic devices. Stereolithography (SLA), digital light processing (DLP), and two-photon polymerization enable the direct fabrication of complex three-dimensional microfluidic structures without requiring expensive cleanroom facilities. While resolution limitations currently restrict their application for the smallest nanoscale features, continuous improvements are expanding their utility.
Paper-based microfluidics represents a low-cost alternative, where hydrophobic barriers are patterned on cellulose substrates using wax printing or photolithography. Though limited in resolution compared to other methods, these devices offer advantages in point-of-care diagnostics and resource-limited settings due to their simplicity and affordability.
Hybrid fabrication approaches combining multiple techniques are increasingly common for complex applications in nanoscale particle manipulation, allowing researchers to leverage the strengths of different materials and manufacturing methods to achieve optimal device performance for specific applications.
Soft lithography has emerged as a particularly versatile technique for microfluidic device fabrication, especially using polydimethylsiloxane (PDMS). This elastomeric material offers excellent optical transparency, biocompatibility, and gas permeability, making it ideal for biological applications. The process involves creating a master mold through photolithography, against which PDMS is cast, cured, and bonded to glass or other substrates through plasma treatment or chemical bonding.
For applications requiring higher chemical resistance or more rigid structures, thermoplastic-based fabrication methods have gained prominence. Hot embossing and injection molding enable mass production of devices using materials such as polymethyl methacrylate (PMMA), polycarbonate (PC), and cyclic olefin copolymer (COC). These techniques offer excellent reproducibility and are particularly suitable for commercial-scale manufacturing.
Glass and silicon micromachining represents another important fabrication approach, utilizing wet etching with hydrofluoric acid solutions or dry etching techniques like deep reactive ion etching (DRIE). These materials provide superior chemical resistance and thermal stability compared to polymers, though at higher production costs and with more complex fabrication processes.
Recent advances in 3D printing technologies have revolutionized rapid prototyping for microfluidic devices. Stereolithography (SLA), digital light processing (DLP), and two-photon polymerization enable the direct fabrication of complex three-dimensional microfluidic structures without requiring expensive cleanroom facilities. While resolution limitations currently restrict their application for the smallest nanoscale features, continuous improvements are expanding their utility.
Paper-based microfluidics represents a low-cost alternative, where hydrophobic barriers are patterned on cellulose substrates using wax printing or photolithography. Though limited in resolution compared to other methods, these devices offer advantages in point-of-care diagnostics and resource-limited settings due to their simplicity and affordability.
Hybrid fabrication approaches combining multiple techniques are increasingly common for complex applications in nanoscale particle manipulation, allowing researchers to leverage the strengths of different materials and manufacturing methods to achieve optimal device performance for specific applications.
Integration with Lab-on-a-Chip Systems
The integration of microfluidic technologies with Lab-on-a-Chip (LOC) systems represents a significant advancement in nanoscale particle manipulation capabilities. These integrated systems combine the precise fluid control of microfluidics with the comprehensive analytical functionalities of LOC platforms, creating powerful tools for research, diagnostics, and industrial applications.
Microfluidic-LOC integration enables complete sample-to-answer workflows within a single miniaturized device. This convergence allows for sequential operations including sample preparation, particle separation, concentration, detection, and analysis—all within a unified system. The seamless transition between these processes minimizes sample loss, reduces contamination risks, and enhances overall efficiency in nanoscale particle handling.
Current integration approaches utilize several key architectural strategies. Modular designs incorporate interchangeable microfluidic components that can be assembled according to specific application requirements. Monolithic integration, conversely, fabricates all components simultaneously on a single substrate, offering enhanced reliability but reduced flexibility. Hybrid approaches balance these considerations by combining prefabricated modules with custom-designed elements.
Advanced manufacturing techniques have significantly improved integration capabilities. Multilayer soft lithography enables the creation of complex three-dimensional microfluidic networks with integrated valves and pumps. Digital microfluidics, utilizing electrowetting principles, provides programmable droplet manipulation without physical channels. Additionally, 3D printing technologies are increasingly employed for rapid prototyping of integrated systems with customized geometries.
The integration of sensing technologies represents another crucial aspect of microfluidic-LOC systems. Optical detection methods, including fluorescence and spectroscopy, can be incorporated through integrated waveguides and miniaturized detectors. Electrical sensing approaches utilize embedded electrodes for impedance measurements or electrochemical detection. More recently, surface acoustic wave sensors have been integrated to enable label-free detection of nanoscale particles.
Control systems for integrated platforms have evolved significantly, with automated fluid handling becoming increasingly sophisticated. Programmable pressure controllers, precision syringe pumps, and integrated micropumps enable precise manipulation of nanoliter fluid volumes. These systems can be further enhanced through software interfaces that coordinate multiple operations and provide real-time feedback.
Standardization remains a significant challenge in microfluidic-LOC integration. The field currently lacks universally accepted interconnection standards, complicating the development of plug-and-play components. Industry initiatives are working toward establishing common specifications for fluidic connections, electrical interfaces, and communication protocols to accelerate development and adoption of these integrated technologies.
Microfluidic-LOC integration enables complete sample-to-answer workflows within a single miniaturized device. This convergence allows for sequential operations including sample preparation, particle separation, concentration, detection, and analysis—all within a unified system. The seamless transition between these processes minimizes sample loss, reduces contamination risks, and enhances overall efficiency in nanoscale particle handling.
Current integration approaches utilize several key architectural strategies. Modular designs incorporate interchangeable microfluidic components that can be assembled according to specific application requirements. Monolithic integration, conversely, fabricates all components simultaneously on a single substrate, offering enhanced reliability but reduced flexibility. Hybrid approaches balance these considerations by combining prefabricated modules with custom-designed elements.
Advanced manufacturing techniques have significantly improved integration capabilities. Multilayer soft lithography enables the creation of complex three-dimensional microfluidic networks with integrated valves and pumps. Digital microfluidics, utilizing electrowetting principles, provides programmable droplet manipulation without physical channels. Additionally, 3D printing technologies are increasingly employed for rapid prototyping of integrated systems with customized geometries.
The integration of sensing technologies represents another crucial aspect of microfluidic-LOC systems. Optical detection methods, including fluorescence and spectroscopy, can be incorporated through integrated waveguides and miniaturized detectors. Electrical sensing approaches utilize embedded electrodes for impedance measurements or electrochemical detection. More recently, surface acoustic wave sensors have been integrated to enable label-free detection of nanoscale particles.
Control systems for integrated platforms have evolved significantly, with automated fluid handling becoming increasingly sophisticated. Programmable pressure controllers, precision syringe pumps, and integrated micropumps enable precise manipulation of nanoliter fluid volumes. These systems can be further enhanced through software interfaces that coordinate multiple operations and provide real-time feedback.
Standardization remains a significant challenge in microfluidic-LOC integration. The field currently lacks universally accepted interconnection standards, complicating the development of plug-and-play components. Industry initiatives are working toward establishing common specifications for fluidic connections, electrical interfaces, and communication protocols to accelerate development and adoption of these integrated technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!