How to Validate Microfluidics for Reliable Organ-on-Chip Modelling
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics Validation Background and Objectives
Microfluidics technology has evolved significantly over the past three decades, transforming from simple flow channels to sophisticated platforms capable of mimicking complex biological systems. The journey began in the 1990s with basic proof-of-concept devices and has now reached a critical juncture where organ-on-chip (OOC) models represent one of the most promising applications. These biomimetic systems aim to recapitulate the structural and functional aspects of human organs within microfluidic environments, offering unprecedented opportunities for drug development, toxicology testing, and personalized medicine.
Despite remarkable progress, the validation of microfluidic systems for reliable organ-on-chip modeling remains a significant challenge. The field currently lacks standardized validation protocols, making it difficult to compare results across different platforms and laboratories. This inconsistency hampers the broader adoption of OOC technology in pharmaceutical research and clinical applications, where reproducibility and reliability are paramount.
The primary objective of microfluidics validation is to establish robust methodologies that ensure these systems accurately represent human physiology at both cellular and tissue levels. This includes validating fluid dynamics, cellular responses, tissue-tissue interactions, and pharmacokinetic/pharmacodynamic parameters. Effective validation must bridge the gap between engineering precision and biological relevance, addressing the inherent variability of biological systems while maintaining the controllability of microfluidic platforms.
Current validation approaches typically focus on individual aspects such as flow characteristics, cell viability, or specific biological responses. However, comprehensive validation frameworks that integrate multiple parameters and account for system-level behaviors are still emerging. The field is moving toward multi-parametric validation strategies that combine computational modeling, real-time monitoring, and correlation with in vivo data to establish the predictive power of OOC models.
Recent technological advances in sensors, imaging, and data analytics have created new opportunities for more sophisticated validation methods. These include integrated sensing capabilities for continuous monitoring of cellular responses, advanced imaging techniques for structural and functional assessment, and machine learning approaches for pattern recognition and predictive modeling. These tools are enabling more comprehensive validation protocols that can address the complexity of organ-on-chip systems.
The trajectory of microfluidics validation is increasingly aligned with regulatory considerations, as agencies like the FDA and EMA begin to explore frameworks for qualifying these novel models as drug development tools. This regulatory perspective is driving the development of more rigorous validation standards that can demonstrate the reliability and relevance of OOC models for specific applications, ultimately aiming to establish these technologies as accepted alternatives to traditional testing methods.
Despite remarkable progress, the validation of microfluidic systems for reliable organ-on-chip modeling remains a significant challenge. The field currently lacks standardized validation protocols, making it difficult to compare results across different platforms and laboratories. This inconsistency hampers the broader adoption of OOC technology in pharmaceutical research and clinical applications, where reproducibility and reliability are paramount.
The primary objective of microfluidics validation is to establish robust methodologies that ensure these systems accurately represent human physiology at both cellular and tissue levels. This includes validating fluid dynamics, cellular responses, tissue-tissue interactions, and pharmacokinetic/pharmacodynamic parameters. Effective validation must bridge the gap between engineering precision and biological relevance, addressing the inherent variability of biological systems while maintaining the controllability of microfluidic platforms.
Current validation approaches typically focus on individual aspects such as flow characteristics, cell viability, or specific biological responses. However, comprehensive validation frameworks that integrate multiple parameters and account for system-level behaviors are still emerging. The field is moving toward multi-parametric validation strategies that combine computational modeling, real-time monitoring, and correlation with in vivo data to establish the predictive power of OOC models.
Recent technological advances in sensors, imaging, and data analytics have created new opportunities for more sophisticated validation methods. These include integrated sensing capabilities for continuous monitoring of cellular responses, advanced imaging techniques for structural and functional assessment, and machine learning approaches for pattern recognition and predictive modeling. These tools are enabling more comprehensive validation protocols that can address the complexity of organ-on-chip systems.
The trajectory of microfluidics validation is increasingly aligned with regulatory considerations, as agencies like the FDA and EMA begin to explore frameworks for qualifying these novel models as drug development tools. This regulatory perspective is driving the development of more rigorous validation standards that can demonstrate the reliability and relevance of OOC models for specific applications, ultimately aiming to establish these technologies as accepted alternatives to traditional testing methods.
Market Analysis for Organ-on-Chip Technologies
The global Organ-on-Chip (OoC) market is experiencing significant growth, valued at approximately $30 million in 2021 and projected to reach $220 million by 2028, representing a CAGR of 39.2%. This remarkable expansion is driven by increasing concerns over animal testing ethics, rising R&D costs in pharmaceutical development, and growing regulatory pressure for more predictive preclinical models.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 60% of the total market share. These organizations are increasingly adopting OoC technologies to reduce drug development costs, which currently average $2.6 billion per approved drug, and to address the high failure rates of 90% in clinical trials. The implementation of reliable microfluidic validation protocols could potentially reduce these failure rates by 15-20%.
Academic research institutions represent the second-largest market segment at 25%, focusing primarily on advancing fundamental OoC technology and exploring novel applications. Contract research organizations (CROs) are rapidly adopting these technologies, showing the fastest growth rate at 42% annually, as they seek to offer differentiated services to pharmaceutical clients.
Geographically, North America dominates the market with 45% share, followed by Europe (30%) and Asia-Pacific (20%). China and South Korea are emerging as significant players, with government initiatives specifically targeting OoC development. Japan has established a $100 million national program focused on integrating OoC technologies into their pharmaceutical evaluation processes.
The market is currently fragmented, with over 30 companies offering various OoC solutions. Key players include Emulate, TissUse, Mimetas, and CN Bio Innovations, collectively holding approximately 65% market share. Strategic partnerships between technology developers and pharmaceutical companies have increased by 200% in the past three years, indicating growing industry confidence in the technology.
Investors have recognized the potential of OoC technologies, with venture capital funding exceeding $500 million since 2018. Recent funding rounds have specifically emphasized the importance of validation protocols, with investors increasingly requiring robust validation data before committing capital.
Customer demand is shifting from single-organ models to more complex multi-organ systems, with 78% of end-users expressing interest in validated interconnected organ models. This trend underscores the critical importance of developing standardized validation protocols for microfluidic systems that can ensure reliable performance across different organ models and their interactions.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 60% of the total market share. These organizations are increasingly adopting OoC technologies to reduce drug development costs, which currently average $2.6 billion per approved drug, and to address the high failure rates of 90% in clinical trials. The implementation of reliable microfluidic validation protocols could potentially reduce these failure rates by 15-20%.
Academic research institutions represent the second-largest market segment at 25%, focusing primarily on advancing fundamental OoC technology and exploring novel applications. Contract research organizations (CROs) are rapidly adopting these technologies, showing the fastest growth rate at 42% annually, as they seek to offer differentiated services to pharmaceutical clients.
Geographically, North America dominates the market with 45% share, followed by Europe (30%) and Asia-Pacific (20%). China and South Korea are emerging as significant players, with government initiatives specifically targeting OoC development. Japan has established a $100 million national program focused on integrating OoC technologies into their pharmaceutical evaluation processes.
The market is currently fragmented, with over 30 companies offering various OoC solutions. Key players include Emulate, TissUse, Mimetas, and CN Bio Innovations, collectively holding approximately 65% market share. Strategic partnerships between technology developers and pharmaceutical companies have increased by 200% in the past three years, indicating growing industry confidence in the technology.
Investors have recognized the potential of OoC technologies, with venture capital funding exceeding $500 million since 2018. Recent funding rounds have specifically emphasized the importance of validation protocols, with investors increasingly requiring robust validation data before committing capital.
Customer demand is shifting from single-organ models to more complex multi-organ systems, with 78% of end-users expressing interest in validated interconnected organ models. This trend underscores the critical importance of developing standardized validation protocols for microfluidic systems that can ensure reliable performance across different organ models and their interactions.
Current Challenges in Microfluidic Validation
Despite significant advancements in microfluidic technology for organ-on-chip (OOC) applications, validation remains one of the most critical challenges facing researchers and industry professionals. The fundamental issue lies in establishing standardized validation protocols that can ensure reproducibility and reliability across different platforms and research settings. Currently, there is a notable absence of consensus regarding which parameters should be measured and what thresholds constitute acceptable performance for microfluidic devices in biological modeling.
The complexity of biological systems presents a significant validation hurdle. Unlike traditional microfluidic applications in chemistry or physics, biological systems exhibit inherent variability and unpredictability. This biological variability makes it difficult to distinguish between normal biological responses and artifacts introduced by the microfluidic system itself, complicating validation efforts substantially.
Scale-related challenges further compound validation difficulties. Microscale phenomena in microfluidic channels often behave differently than their macroscale counterparts, particularly regarding fluid dynamics, diffusion rates, and surface tension effects. These scale-dependent behaviors can significantly impact cellular responses and tissue function, yet current validation methods often fail to adequately account for these differences.
Technical limitations in measurement capabilities present another major obstacle. Many critical parameters in microfluidic systems operate at scales that challenge current sensing technologies. For instance, accurately measuring localized shear stress, oxygen gradients, or metabolite concentrations at the cellular level remains technically challenging, limiting comprehensive validation of these crucial parameters.
Material-related issues also impede validation efforts. The interaction between biological samples and device materials can lead to protein adsorption, cell adhesion patterns, or even release of compounds that affect cellular behavior. These material-interface effects are often inconsistent across different manufacturing batches and difficult to standardize, introducing variability that complicates validation.
Cross-platform comparability represents another significant challenge. Results obtained from one microfluidic platform are frequently difficult to reproduce on different systems due to variations in design, materials, and operational parameters. This lack of standardization hampers the establishment of universal validation criteria and limits the broader applicability of research findings.
Long-term stability validation remains particularly problematic for OOC applications. Many biological models require extended culture periods to develop physiologically relevant functions, yet validating microfluidic performance over these extended timeframes presents unique challenges related to material degradation, bubble formation, and maintaining sterility without disrupting the system.
The complexity of biological systems presents a significant validation hurdle. Unlike traditional microfluidic applications in chemistry or physics, biological systems exhibit inherent variability and unpredictability. This biological variability makes it difficult to distinguish between normal biological responses and artifacts introduced by the microfluidic system itself, complicating validation efforts substantially.
Scale-related challenges further compound validation difficulties. Microscale phenomena in microfluidic channels often behave differently than their macroscale counterparts, particularly regarding fluid dynamics, diffusion rates, and surface tension effects. These scale-dependent behaviors can significantly impact cellular responses and tissue function, yet current validation methods often fail to adequately account for these differences.
Technical limitations in measurement capabilities present another major obstacle. Many critical parameters in microfluidic systems operate at scales that challenge current sensing technologies. For instance, accurately measuring localized shear stress, oxygen gradients, or metabolite concentrations at the cellular level remains technically challenging, limiting comprehensive validation of these crucial parameters.
Material-related issues also impede validation efforts. The interaction between biological samples and device materials can lead to protein adsorption, cell adhesion patterns, or even release of compounds that affect cellular behavior. These material-interface effects are often inconsistent across different manufacturing batches and difficult to standardize, introducing variability that complicates validation.
Cross-platform comparability represents another significant challenge. Results obtained from one microfluidic platform are frequently difficult to reproduce on different systems due to variations in design, materials, and operational parameters. This lack of standardization hampers the establishment of universal validation criteria and limits the broader applicability of research findings.
Long-term stability validation remains particularly problematic for OOC applications. Many biological models require extended culture periods to develop physiologically relevant functions, yet validating microfluidic performance over these extended timeframes presents unique challenges related to material degradation, bubble formation, and maintaining sterility without disrupting the system.
Established Validation Protocols for Microfluidics
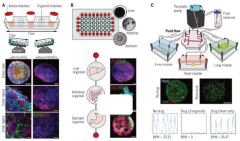
01 Microfluidic systems for organ-on-chip validation
Microfluidic systems provide controlled environments for organ-on-chip platforms, enabling precise validation of their functionality. These systems incorporate channels, chambers, and sensors that mimic physiological conditions and fluid dynamics found in living organs. The integration of microfluidic components allows for accurate measurement of cellular responses, tissue function, and drug interactions, enhancing the reliability of organ-on-chip models for research and pharmaceutical testing.- Microfluidic systems for organ-on-chip validation: Microfluidic systems provide controlled environments for organ-on-chip models, enabling precise validation of their functionality. These systems incorporate channels, chambers, and sensors that mimic physiological conditions and fluid dynamics found in living organs. The integration of microfluidic components allows for continuous perfusion of culture media, controlled delivery of test compounds, and real-time monitoring of cellular responses, enhancing the reliability and reproducibility of organ-on-chip platforms for drug testing and disease modeling.
- Reliability assessment methods for organ-on-chip devices: Various methods have been developed to assess the reliability of organ-on-chip devices, including standardized protocols for performance evaluation. These methods involve measuring parameters such as flow stability, temperature control, oxygen diffusion, and cellular viability over extended periods. Advanced imaging techniques and computational models are employed to verify the structural integrity and functional consistency of the microfluidic platforms. Reliability assessment ensures that organ-on-chip systems can consistently replicate organ functions and provide dependable data for research and clinical applications.
- Integration of sensors for real-time monitoring: The integration of various sensors within microfluidic organ-on-chip platforms enables real-time monitoring of critical parameters, enhancing validation capabilities. These sensors can measure pH, oxygen levels, temperature, pressure, and specific biomarkers released by cells. The continuous data collection allows researchers to verify the physiological relevance of the model and detect any deviations from expected behavior. Sensor integration improves the reliability of organ-on-chip systems by providing immediate feedback on cellular responses to experimental conditions and identifying potential issues before they affect experimental outcomes.
- Standardization approaches for organ-on-chip validation: Standardization approaches are crucial for ensuring consistent validation and reliability of organ-on-chip technologies across different laboratories and applications. These approaches include the development of reference materials, calibration protocols, and performance benchmarks that enable meaningful comparison between different organ-on-chip platforms. Standardized cell sources, culture media formulations, and operating procedures help minimize variability and improve reproducibility. Industry collaborations and regulatory frameworks are being established to define quality control measures and validation criteria for organ-on-chip systems used in drug development and toxicity testing.
- Multi-organ integration and system validation: Advanced microfluidic platforms enable the integration of multiple organ models into interconnected systems, requiring comprehensive validation strategies to ensure reliability. These multi-organ systems aim to replicate complex physiological interactions and systemic responses observed in the human body. Validation of such integrated platforms involves assessing the maintenance of organ-specific functions, inter-organ communication, and metabolic activities across the system. Specialized microfluidic designs facilitate controlled fluid exchange between different organ compartments while maintaining appropriate residence times and concentration gradients. This approach enhances the predictive capability of organ-on-chip technology for studying drug efficacy, toxicity, and disease progression at a systemic level.
02 Reliability assessment methods for organ-on-chip platforms
Various methods have been developed to assess the reliability of organ-on-chip platforms, including standardized protocols for validation and quality control. These methods involve measuring parameters such as cell viability, tissue functionality, reproducibility of results, and long-term stability. Advanced imaging techniques, biochemical assays, and computational models are employed to evaluate the performance and consistency of organ-on-chip systems, ensuring their reliability for applications in drug development and disease modeling.Expand Specific Solutions03 Sensor integration for real-time monitoring and validation
Integration of sensors within microfluidic organ-on-chip devices enables real-time monitoring of physiological parameters, enhancing validation capabilities. These sensors can measure various factors including pH, oxygen levels, temperature, pressure, and specific biomarkers. The continuous data collection provides comprehensive insights into the dynamic behavior of cultured tissues, allowing for immediate detection of anomalies and improved reliability assessment of the organ-on-chip models under different experimental conditions.Expand Specific Solutions04 Multi-organ integration and cross-validation techniques
Advanced microfluidic platforms enable the integration of multiple organ models within a single system, allowing for cross-validation between different tissue types. These multi-organ-on-chip systems simulate complex physiological interactions and systemic responses, providing more comprehensive validation of drug effects and toxicity. The interconnected nature of these platforms improves the reliability of predictions by accounting for organ-organ interactions that influence drug metabolism, distribution, and overall physiological response.Expand Specific Solutions05 Standardization protocols for microfluidic organ-on-chip validation
Standardization protocols have been developed to ensure consistent validation and reliability assessment of microfluidic organ-on-chip platforms. These protocols establish uniform methods for device fabrication, cell culture conditions, fluid flow parameters, and performance metrics. Standardized approaches facilitate comparison between different organ-on-chip models and improve reproducibility across laboratories. The implementation of these protocols enhances the credibility of organ-on-chip technology for regulatory applications and pharmaceutical development.Expand Specific Solutions
Leading Organizations in Organ-on-Chip Development
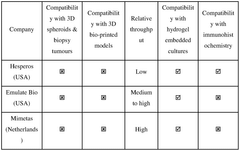
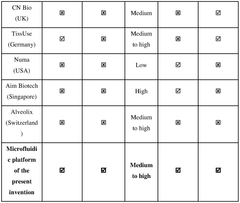
The organ-on-chip microfluidics market is currently in a growth phase, with an estimated global market size of $100-150 million and projected annual growth of 35-40%. The technology is transitioning from early adoption to broader implementation, with varying degrees of technical maturity. Leading academic institutions (MIT, Tsinghua University, University of Tokyo) are advancing fundamental research, while commercial entities like Emulate, Inc. have developed more mature platforms. Research centers at National University of Singapore and Cedars-Sinai are bridging the gap between academic innovation and clinical application. The competitive landscape features collaboration between academic institutions and industry partners, with increasing focus on standardization and validation protocols to ensure reliable organ modeling for pharmaceutical testing and personalized medicine applications.
Emulate, Inc.
Technical Solution: Emulate has developed a comprehensive validation framework for their Organ-Chip technology that integrates microfluidic systems with living human cells. Their approach includes multi-parameter quality control protocols that validate both the physical microfluidic components and the biological responses. The company's proprietary Organ-Chip platform features precisely engineered microchannels that recreate tissue-tissue interfaces and dynamic mechanical forces. Their validation methodology encompasses mechanical testing of chip materials, flow rate verification across physiologically relevant ranges (0.1-1 mL/hour), pressure differential measurements, and assessment of oxygen/nutrient diffusion characteristics. Emulate implements automated imaging systems to verify channel dimensions with micron-level precision and employs computational fluid dynamics modeling to predict and validate flow patterns within their chips. Additionally, they've established standardized biological validation protocols using reference cell lines to ensure consistent cellular responses across different chip lots.
Strengths: Industry-leading standardization with comprehensive validation protocols that address both physical and biological parameters; automated quality control systems enable high reproducibility across manufacturing lots. Weaknesses: Proprietary closed system limits customization options for researchers; relatively high cost compared to academic solutions; validation methods are optimized for their specific platform and may not translate to other microfluidic designs.
Shanghai Jiao Tong University
Technical Solution: Shanghai Jiao Tong University has developed a systematic validation framework for microfluidic organ-on-chip platforms that emphasizes reproducibility and physiological relevance. Their approach integrates physical characterization of microfluidic parameters with biological validation metrics. The university's research teams have pioneered methods for precise control and measurement of fluid dynamics within microchannels, including advanced particle tracking velocimetry techniques that can map flow profiles with submicron resolution. Their validation protocol includes comprehensive material testing for biocompatibility, focusing particularly on surface chemistry characterization and protein adsorption profiles. The university has developed specialized imaging platforms that enable real-time monitoring of cellular responses within microfluidic environments, allowing correlation between fluid mechanical parameters and biological outcomes. Their validation methodology incorporates computational fluid dynamics modeling to predict shear stress distributions and oxygen/nutrient gradients, with subsequent experimental verification using oxygen-sensitive fluorescent probes and microsensors. Additionally, they've established standardized biological validation assays using primary human cells to verify physiological responses across different microfluidic designs and manufacturing batches.
Strengths: Strong integration of computational modeling with experimental validation; comprehensive characterization of both physical and biological parameters; emphasis on standardization and reproducibility. Weaknesses: Validation protocols primarily developed in research settings may require adaptation for industrial applications; complex multi-parameter validation increases time and resource requirements; limited focus on long-term stability validation.
Critical Technologies for Reliable Microfluidic Validation
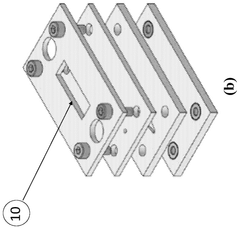
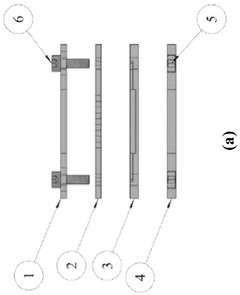
A versatile microfluidic apparatus and an attachment thereof
PatentWO2024246945A9
Innovation
- A versatile microfluidic apparatus with a multi-compartment design and attachment-based concept that allows for the culture of various 3D cell models, such as spheroids, biopsy tissues, and organoids, without the need for continuous membranes, enabling multi-cellular interactions and mimicking organ-specific micro-physiological environments.
A method for the development of organoids and organ on a chip system
PatentPendingIN202211068827A
Innovation
- The development of an organ-on-a-chip system that combines microfabrication with tissue engineering to create microfluidic devices capable of simulating human organs, using stem cell-derived organoids to replicate the structural and functional traits of in vivo organs, enabling precise control and monitoring of the microenvironment for drug testing and toxicity assessment.
Regulatory Framework for Organ-on-Chip Technologies
The regulatory landscape for Organ-on-Chip (OoC) technologies is evolving rapidly as these platforms transition from research tools to clinical applications. Currently, there is no unified global regulatory framework specifically designed for OoC technologies, creating significant challenges for validation and commercialization efforts. The FDA has initiated programs like the Tissue Chip for Drug Screening to explore how these technologies might be incorporated into regulatory decision-making processes.
In the United States, OoC technologies primarily fall under the FDA's jurisdiction, potentially classified as medical devices or in vitro diagnostic tools depending on their intended use. The FDA's Center for Devices and Radiological Health (CDRH) has shown interest in developing specialized guidance for microphysiological systems, recognizing their unique position between traditional cell culture and animal models.
The European Medicines Agency (EMA) has established working groups focused on advanced therapy medicinal products that include considerations for OoC technologies. The EU's emphasis on reducing animal testing through the 3Rs principle (Replacement, Reduction, Refinement) has created a favorable regulatory environment for OoC adoption, though specific validation frameworks remain under development.
International harmonization efforts are being led by organizations such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the Organization for Economic Co-operation and Development (OECD). These bodies are working to establish standardized validation protocols that can be recognized across jurisdictions, facilitating global development and implementation.
Key regulatory considerations for microfluidic validation include demonstrating reproducibility across devices, establishing physiological relevance compared to human tissues, and developing appropriate reference standards. Regulatory bodies increasingly require evidence of robust quality control systems, including documentation of materials biocompatibility, manufacturing consistency, and performance characterization.
Industry consortia like the European Organ-on-Chip Society (EUROoCS) and the IQ Consortium's Microphysiological Systems Working Group are collaborating with regulatory agencies to develop consensus standards and validation frameworks. These public-private partnerships are essential for creating practical regulatory pathways that balance innovation with safety considerations.
For companies developing microfluidic OoC platforms, early engagement with regulatory authorities through programs like the FDA's Pre-Submission Program is highly recommended to navigate the complex and evolving regulatory landscape. This proactive approach can help identify potential regulatory hurdles and develop appropriate validation strategies aligned with emerging regulatory expectations.
In the United States, OoC technologies primarily fall under the FDA's jurisdiction, potentially classified as medical devices or in vitro diagnostic tools depending on their intended use. The FDA's Center for Devices and Radiological Health (CDRH) has shown interest in developing specialized guidance for microphysiological systems, recognizing their unique position between traditional cell culture and animal models.
The European Medicines Agency (EMA) has established working groups focused on advanced therapy medicinal products that include considerations for OoC technologies. The EU's emphasis on reducing animal testing through the 3Rs principle (Replacement, Reduction, Refinement) has created a favorable regulatory environment for OoC adoption, though specific validation frameworks remain under development.
International harmonization efforts are being led by organizations such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the Organization for Economic Co-operation and Development (OECD). These bodies are working to establish standardized validation protocols that can be recognized across jurisdictions, facilitating global development and implementation.
Key regulatory considerations for microfluidic validation include demonstrating reproducibility across devices, establishing physiological relevance compared to human tissues, and developing appropriate reference standards. Regulatory bodies increasingly require evidence of robust quality control systems, including documentation of materials biocompatibility, manufacturing consistency, and performance characterization.
Industry consortia like the European Organ-on-Chip Society (EUROoCS) and the IQ Consortium's Microphysiological Systems Working Group are collaborating with regulatory agencies to develop consensus standards and validation frameworks. These public-private partnerships are essential for creating practical regulatory pathways that balance innovation with safety considerations.
For companies developing microfluidic OoC platforms, early engagement with regulatory authorities through programs like the FDA's Pre-Submission Program is highly recommended to navigate the complex and evolving regulatory landscape. This proactive approach can help identify potential regulatory hurdles and develop appropriate validation strategies aligned with emerging regulatory expectations.
Standardization Efforts in Microfluidic Validation
The standardization of microfluidic validation protocols represents a critical frontier in advancing organ-on-chip (OOC) technology toward widespread adoption in pharmaceutical research, toxicology testing, and personalized medicine. Currently, several international organizations are spearheading efforts to establish consensus guidelines that would enable reproducible and reliable microfluidic validation.
The International Organization for Standardization (ISO) has established the Technical Committee 276 on Biotechnology, which includes a working group specifically addressing microfluidic technologies. Their ongoing work aims to standardize terminology, measurement methods, and validation protocols specific to organ-on-chip systems, with draft standards expected to reach publication within the next two years.
Similarly, the European Union's Organ-on-Chip Society (EUROoCS) has launched a dedicated initiative to develop validation frameworks through their "Good In Vitro Method Practices" (GIVIMP) adaptation for microfluidic devices. This initiative focuses on establishing minimum reporting requirements for experimental parameters, quality control metrics, and validation benchmarks specific to different organ models.
In the United States, the National Institute of Standards and Technology (NIST) collaborates with the FDA to develop reference materials and standard operating procedures for microfluidic device validation. Their recent publication of the "Microfluidic Device Qualification Framework" provides a tiered approach to validation, encompassing physical characterization, biological functionality, and physiological relevance assessment.
Industry consortia are also making significant contributions to standardization efforts. The IQ Consortium's Microphysiological Systems Working Group, comprising over 30 pharmaceutical companies, has published best practice recommendations for implementing microfluidic organ models in drug development pipelines, including validation criteria for different application contexts.
Academic-industry partnerships have established round-robin testing initiatives, where identical microfluidic platforms are validated across multiple laboratories to assess reproducibility and identify critical parameters affecting system performance. The NIH-funded Tissue Chip Testing Centers represent a formalized version of this approach, providing independent validation services for emerging organ-on-chip technologies.
Despite these advances, significant challenges remain in harmonizing validation approaches across different microfluidic platforms and organ models. The inherent biological variability, coupled with the diversity of engineering designs, complicates the establishment of universal standards. Moving forward, a balance between standardization and flexibility will be essential to accommodate innovation while ensuring reliability in this rapidly evolving field.
The International Organization for Standardization (ISO) has established the Technical Committee 276 on Biotechnology, which includes a working group specifically addressing microfluidic technologies. Their ongoing work aims to standardize terminology, measurement methods, and validation protocols specific to organ-on-chip systems, with draft standards expected to reach publication within the next two years.
Similarly, the European Union's Organ-on-Chip Society (EUROoCS) has launched a dedicated initiative to develop validation frameworks through their "Good In Vitro Method Practices" (GIVIMP) adaptation for microfluidic devices. This initiative focuses on establishing minimum reporting requirements for experimental parameters, quality control metrics, and validation benchmarks specific to different organ models.
In the United States, the National Institute of Standards and Technology (NIST) collaborates with the FDA to develop reference materials and standard operating procedures for microfluidic device validation. Their recent publication of the "Microfluidic Device Qualification Framework" provides a tiered approach to validation, encompassing physical characterization, biological functionality, and physiological relevance assessment.
Industry consortia are also making significant contributions to standardization efforts. The IQ Consortium's Microphysiological Systems Working Group, comprising over 30 pharmaceutical companies, has published best practice recommendations for implementing microfluidic organ models in drug development pipelines, including validation criteria for different application contexts.
Academic-industry partnerships have established round-robin testing initiatives, where identical microfluidic platforms are validated across multiple laboratories to assess reproducibility and identify critical parameters affecting system performance. The NIH-funded Tissue Chip Testing Centers represent a formalized version of this approach, providing independent validation services for emerging organ-on-chip technologies.
Despite these advances, significant challenges remain in harmonizing validation approaches across different microfluidic platforms and organ models. The inherent biological variability, coupled with the diversity of engineering designs, complicates the establishment of universal standards. Moving forward, a balance between standardization and flexibility will be essential to accommodate innovation while ensuring reliability in this rapidly evolving field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!