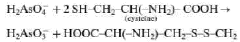How to Correctly Calibrate Microfluidics for Trace Analysis
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Calibration Background and Objectives
Microfluidic technology has evolved significantly over the past three decades, transforming from a conceptual framework in the 1990s to a sophisticated analytical platform today. The miniaturization of fluid handling systems has enabled unprecedented precision in chemical and biological analyses, particularly in scenarios requiring detection of trace elements at parts-per-billion or even parts-per-trillion levels. This evolution has been driven by advancements in materials science, microfabrication techniques, and detection methodologies, creating increasingly integrated and automated systems.
The calibration of microfluidic systems represents a critical challenge in ensuring accurate trace analysis. Historically, calibration approaches have been adapted from conventional analytical chemistry, but these methods often fail to address the unique physical phenomena occurring at the microscale, including surface tension effects, laminar flow dynamics, and material-fluid interactions that can significantly impact analytical results.
Recent technological trends indicate a shift toward digital microfluidics, organ-on-a-chip platforms, and paper-based microfluidic systems, each presenting distinct calibration challenges. The integration of artificial intelligence and machine learning algorithms for real-time calibration adjustment represents an emerging frontier, potentially enabling adaptive calibration protocols that respond to changing experimental conditions.
The primary objective of microfluidic calibration for trace analysis is to establish standardized, reproducible methodologies that ensure measurement accuracy across different devices, operators, and environmental conditions. This includes developing robust calibration protocols that account for system-specific variables such as channel geometry, surface properties, and flow dynamics, while maintaining sensitivity to analytes at extremely low concentrations.
Secondary objectives include reducing calibration complexity to facilitate broader adoption in field settings, minimizing calibration drift over time, and developing internal calibration standards that can be integrated directly into microfluidic chips. These objectives align with the broader goal of democratizing advanced analytical capabilities through portable, user-friendly microfluidic platforms.
The technical goals extend to creating calibration approaches that remain effective across diverse sample matrices, from environmental water samples to complex biological fluids. This requires addressing matrix effects that can interfere with trace analysis, such as protein binding, ionic strength variations, and the presence of competing analytes.
Ultimately, advances in microfluidic calibration aim to bridge the gap between laboratory precision and real-world applicability, enabling reliable trace analysis in resource-limited settings, point-of-care diagnostics, environmental monitoring, and industrial quality control. The achievement of these objectives would significantly expand the practical utility of microfluidic technologies across scientific disciplines and commercial applications.
The calibration of microfluidic systems represents a critical challenge in ensuring accurate trace analysis. Historically, calibration approaches have been adapted from conventional analytical chemistry, but these methods often fail to address the unique physical phenomena occurring at the microscale, including surface tension effects, laminar flow dynamics, and material-fluid interactions that can significantly impact analytical results.
Recent technological trends indicate a shift toward digital microfluidics, organ-on-a-chip platforms, and paper-based microfluidic systems, each presenting distinct calibration challenges. The integration of artificial intelligence and machine learning algorithms for real-time calibration adjustment represents an emerging frontier, potentially enabling adaptive calibration protocols that respond to changing experimental conditions.
The primary objective of microfluidic calibration for trace analysis is to establish standardized, reproducible methodologies that ensure measurement accuracy across different devices, operators, and environmental conditions. This includes developing robust calibration protocols that account for system-specific variables such as channel geometry, surface properties, and flow dynamics, while maintaining sensitivity to analytes at extremely low concentrations.
Secondary objectives include reducing calibration complexity to facilitate broader adoption in field settings, minimizing calibration drift over time, and developing internal calibration standards that can be integrated directly into microfluidic chips. These objectives align with the broader goal of democratizing advanced analytical capabilities through portable, user-friendly microfluidic platforms.
The technical goals extend to creating calibration approaches that remain effective across diverse sample matrices, from environmental water samples to complex biological fluids. This requires addressing matrix effects that can interfere with trace analysis, such as protein binding, ionic strength variations, and the presence of competing analytes.
Ultimately, advances in microfluidic calibration aim to bridge the gap between laboratory precision and real-world applicability, enabling reliable trace analysis in resource-limited settings, point-of-care diagnostics, environmental monitoring, and industrial quality control. The achievement of these objectives would significantly expand the practical utility of microfluidic technologies across scientific disciplines and commercial applications.
Market Demand for Precise Trace Analysis Systems
The global market for precise trace analysis systems has been experiencing robust growth, driven primarily by increasing demands in pharmaceutical research, environmental monitoring, clinical diagnostics, and food safety sectors. The microfluidics trace analysis market was valued at approximately $3.2 billion in 2022 and is projected to reach $7.5 billion by 2028, representing a compound annual growth rate of 15.3% during the forecast period.
Healthcare and pharmaceutical industries constitute the largest market segment, accounting for nearly 40% of the total demand. This is attributed to the growing need for accurate detection of biomarkers, pathogens, and drug metabolites at extremely low concentrations. The ability to detect analytes at parts-per-billion or parts-per-trillion levels has become crucial for early disease diagnosis and personalized medicine approaches.
Environmental monitoring represents another significant market driver, with regulatory bodies worldwide imposing stricter standards for contaminant detection in water, soil, and air samples. The EPA, EU Environmental Standards, and similar organizations in Asia have continuously lowered acceptable limits for various pollutants, necessitating more sensitive and precise analytical techniques. This regulatory pressure has created a substantial market for advanced microfluidic calibration technologies.
Food and beverage safety testing has emerged as a rapidly growing segment, expanding at approximately 18% annually. Consumer awareness regarding food contaminants and the implementation of stringent safety regulations have compelled manufacturers to adopt sophisticated trace analysis systems. The detection of pesticides, antibiotics, mycotoxins, and allergens at trace levels has become standard practice in quality control laboratories worldwide.
Industrial applications, particularly in semiconductor manufacturing and materials science, have also contributed significantly to market growth. As device dimensions continue to shrink in the electronics industry, the detection and control of impurities at ever-decreasing concentrations have become critical for maintaining product quality and performance.
The geographical distribution of market demand shows North America leading with approximately 35% market share, followed closely by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is experiencing the fastest growth rate at 17.2% annually, driven by expanding healthcare infrastructure, increasing environmental concerns, and growing industrial base.
End-users consistently emphasize three key requirements: improved sensitivity to detect lower concentrations, enhanced reproducibility for reliable results, and increased automation to reduce human error. Market surveys indicate that 78% of users rank calibration accuracy as the most critical factor when selecting microfluidic systems for trace analysis, highlighting the significant market opportunity for advanced calibration technologies.
Healthcare and pharmaceutical industries constitute the largest market segment, accounting for nearly 40% of the total demand. This is attributed to the growing need for accurate detection of biomarkers, pathogens, and drug metabolites at extremely low concentrations. The ability to detect analytes at parts-per-billion or parts-per-trillion levels has become crucial for early disease diagnosis and personalized medicine approaches.
Environmental monitoring represents another significant market driver, with regulatory bodies worldwide imposing stricter standards for contaminant detection in water, soil, and air samples. The EPA, EU Environmental Standards, and similar organizations in Asia have continuously lowered acceptable limits for various pollutants, necessitating more sensitive and precise analytical techniques. This regulatory pressure has created a substantial market for advanced microfluidic calibration technologies.
Food and beverage safety testing has emerged as a rapidly growing segment, expanding at approximately 18% annually. Consumer awareness regarding food contaminants and the implementation of stringent safety regulations have compelled manufacturers to adopt sophisticated trace analysis systems. The detection of pesticides, antibiotics, mycotoxins, and allergens at trace levels has become standard practice in quality control laboratories worldwide.
Industrial applications, particularly in semiconductor manufacturing and materials science, have also contributed significantly to market growth. As device dimensions continue to shrink in the electronics industry, the detection and control of impurities at ever-decreasing concentrations have become critical for maintaining product quality and performance.
The geographical distribution of market demand shows North America leading with approximately 35% market share, followed closely by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is experiencing the fastest growth rate at 17.2% annually, driven by expanding healthcare infrastructure, increasing environmental concerns, and growing industrial base.
End-users consistently emphasize three key requirements: improved sensitivity to detect lower concentrations, enhanced reproducibility for reliable results, and increased automation to reduce human error. Market surveys indicate that 78% of users rank calibration accuracy as the most critical factor when selecting microfluidic systems for trace analysis, highlighting the significant market opportunity for advanced calibration technologies.
Current Challenges in Microfluidic Calibration
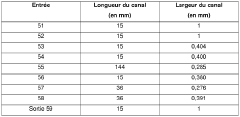
Despite significant advancements in microfluidic technology, calibration for trace analysis remains a formidable challenge in the field. The primary difficulty stems from the microscale dimensions of microfluidic channels, typically ranging from 1-1000 micrometers, which necessitate extremely precise calibration methods. When dealing with trace analysis, where analyte concentrations can be in the parts-per-billion or even parts-per-trillion range, even minor calibration errors can lead to significant analytical inaccuracies.
One persistent challenge is achieving uniform flow rates across microfluidic channels. Variations in channel geometry, surface roughness, and material properties can create unpredictable flow patterns, affecting the reliability of calibration standards. These variations become particularly problematic when scaling up from single-channel prototypes to multi-channel systems for high-throughput applications.
Surface adsorption presents another significant obstacle. Trace analytes often adsorb onto channel surfaces, leading to sample loss and cross-contamination between analyses. This phenomenon is especially pronounced with biomolecules and certain organic compounds, making quantitative calibration exceedingly difficult as the effective concentration reaching the detection zone may differ substantially from the input concentration.
Temperature fluctuations, even minor ones, can dramatically impact fluid properties in microchannels. Viscosity changes affect flow rates, while thermal expansion can alter channel dimensions. For trace analysis, these temperature-dependent variations can introduce systematic errors that are difficult to account for in calibration protocols.
Integration of detection systems with microfluidic platforms introduces additional calibration complexities. The coupling efficiency between microfluidic channels and detection systems often varies between devices, necessitating individual calibration for each system. This lack of standardization hampers reproducibility across different laboratories and platforms.
Matrix effects represent a particularly challenging aspect of microfluidic calibration. Real-world samples contain numerous components that can interfere with analyte detection or alter fluid dynamics within microchannels. Creating calibration standards that accurately mimic these complex matrices while maintaining known trace analyte concentrations remains technically demanding.
Temporal stability of calibration is another critical issue. Many microfluidic systems experience drift over time due to surface fouling, material degradation, or mechanical stress. This necessitates frequent recalibration, which is time-consuming and can introduce additional variability into analytical results.
The lack of universally accepted calibration protocols and reference materials specifically designed for microfluidic trace analysis further complicates the field. While standards exist for conventional analytical techniques, their adaptation to microfluidic platforms is often suboptimal due to the unique physical phenomena occurring at the microscale.
One persistent challenge is achieving uniform flow rates across microfluidic channels. Variations in channel geometry, surface roughness, and material properties can create unpredictable flow patterns, affecting the reliability of calibration standards. These variations become particularly problematic when scaling up from single-channel prototypes to multi-channel systems for high-throughput applications.
Surface adsorption presents another significant obstacle. Trace analytes often adsorb onto channel surfaces, leading to sample loss and cross-contamination between analyses. This phenomenon is especially pronounced with biomolecules and certain organic compounds, making quantitative calibration exceedingly difficult as the effective concentration reaching the detection zone may differ substantially from the input concentration.
Temperature fluctuations, even minor ones, can dramatically impact fluid properties in microchannels. Viscosity changes affect flow rates, while thermal expansion can alter channel dimensions. For trace analysis, these temperature-dependent variations can introduce systematic errors that are difficult to account for in calibration protocols.
Integration of detection systems with microfluidic platforms introduces additional calibration complexities. The coupling efficiency between microfluidic channels and detection systems often varies between devices, necessitating individual calibration for each system. This lack of standardization hampers reproducibility across different laboratories and platforms.
Matrix effects represent a particularly challenging aspect of microfluidic calibration. Real-world samples contain numerous components that can interfere with analyte detection or alter fluid dynamics within microchannels. Creating calibration standards that accurately mimic these complex matrices while maintaining known trace analyte concentrations remains technically demanding.
Temporal stability of calibration is another critical issue. Many microfluidic systems experience drift over time due to surface fouling, material degradation, or mechanical stress. This necessitates frequent recalibration, which is time-consuming and can introduce additional variability into analytical results.
The lack of universally accepted calibration protocols and reference materials specifically designed for microfluidic trace analysis further complicates the field. While standards exist for conventional analytical techniques, their adaptation to microfluidic platforms is often suboptimal due to the unique physical phenomena occurring at the microscale.
Established Calibration Protocols for Trace Analysis
01 Calibration methods for microfluidic devices
Various calibration methods are employed to ensure accuracy and precision in microfluidic systems. These methods include reference standards, automated calibration procedures, and mathematical models that compensate for environmental factors. Calibration techniques often involve comparing measured values with known standards to establish correction factors, which are then applied to subsequent measurements to improve accuracy.- Calibration methods for microfluidic devices: Various calibration methods are employed to ensure accuracy and precision in microfluidic systems. These methods include reference standards, automated calibration procedures, and real-time calibration techniques that compensate for environmental variations. Proper calibration of microfluidic devices is essential for maintaining measurement accuracy across different operating conditions and ensuring reliable experimental results.
- Precision measurement techniques in microfluidics: Advanced measurement techniques are implemented to achieve high precision in microfluidic applications. These include optical detection methods, electrical impedance measurements, and flow rate monitoring systems. These techniques enable precise control and monitoring of fluid behavior at microscale levels, which is critical for applications requiring high accuracy such as medical diagnostics and analytical chemistry.
- Error compensation and correction algorithms: Sophisticated algorithms are developed to compensate for systematic errors and improve measurement accuracy in microfluidic systems. These algorithms account for temperature variations, pressure fluctuations, and other environmental factors that can affect measurement precision. By implementing these error correction mechanisms, microfluidic devices can maintain consistent performance even under varying operating conditions.
- Integrated quality control systems: Microfluidic platforms incorporate integrated quality control systems to continuously monitor and verify measurement accuracy. These systems include internal reference standards, self-diagnostic capabilities, and automated validation protocols. The integration of quality control mechanisms ensures reliable operation and helps identify potential calibration issues before they affect experimental results.
- Standardization and validation protocols: Standardized protocols are established for validating the accuracy and precision of microfluidic measurements. These protocols define procedures for initial calibration, periodic verification, and performance qualification. Adherence to these standardized approaches ensures consistency across different laboratories and enables reliable comparison of results obtained from different microfluidic platforms.
02 Precision measurement techniques in microfluidics
Advanced measurement techniques are crucial for achieving high precision in microfluidic applications. These techniques include optical detection methods, electrical impedance measurements, and laser-based sensing systems. By implementing precise measurement techniques, microfluidic systems can detect and quantify minute changes in fluid properties, ensuring reliable and reproducible results in applications such as medical diagnostics and analytical chemistry.Expand Specific Solutions03 Error compensation and correction algorithms
Sophisticated algorithms are developed to compensate for systematic errors and improve the accuracy of microfluidic measurements. These algorithms analyze data patterns, identify sources of error, and apply appropriate corrections. Machine learning approaches can be used to adapt calibration parameters based on historical data, leading to continuous improvement in measurement accuracy and precision over time.Expand Specific Solutions04 Temperature and environmental control for accuracy
Environmental factors, particularly temperature fluctuations, significantly impact the accuracy of microfluidic measurements. Systems incorporating temperature sensors, feedback control mechanisms, and thermal stabilization components can maintain optimal operating conditions. Some advanced systems include pressure compensation and humidity control to further enhance measurement stability and reliability across varying environmental conditions.Expand Specific Solutions05 Automated validation and verification systems
Automated systems for continuous validation and verification ensure that microfluidic devices maintain calibration over time. These systems periodically check device performance against established standards, flagging when recalibration is needed. Real-time monitoring capabilities allow for immediate detection of drift or malfunction, while digital twin technologies can predict calibration needs before accuracy is compromised, reducing downtime and ensuring consistent precision.Expand Specific Solutions
Leading Organizations in Microfluidic Technology
The microfluidics calibration for trace analysis market is currently in a growth phase, with increasing adoption across pharmaceutical, clinical diagnostics, and environmental monitoring sectors. The global microfluidics market is projected to reach approximately $30 billion by 2025, with trace analysis applications representing a significant segment. Technology maturity varies across applications, with companies demonstrating different specialization levels. Leading players include Agilent Technologies and Siemens Healthineers, who offer comprehensive calibration solutions, while PARC and miDIAGNOSTICS are advancing innovative microfluidic platforms. Academic institutions like MIT and University of Washington collaborate with industry partners to bridge fundamental research with practical applications. Emerging companies like Skyphos Industries are disrupting traditional approaches with specialized 3D printing technologies for microfluidic chip production, enabling rapid prototyping and customization capabilities.
Cyvek, Inc.
Technical Solution: Cyvek has developed an innovative microfluidic calibration platform specifically designed for multiplexed protein trace analysis. Their system employs proprietary microfluidic cartridges with integrated calibration chambers containing lyophilized multi-analyte calibration standards that are automatically reconstituted during the analysis process. The technology utilizes a sequential dilution network that generates on-chip calibration curves spanning five orders of magnitude, enabling accurate quantification from pg/mL to μg/mL concentrations. Cyvek's approach incorporates magnetic microbeads with precisely controlled surface properties as mobile calibration standards, allowing dynamic recalibration throughout the analytical process. Their system features integrated optical detection with ratiometric measurement capabilities, normalizing analyte signals against reference dyes to compensate for variations in illumination intensity and detector sensitivity. The platform also employs automated fluidic control with real-time flow verification, ensuring precise delivery of samples and calibrants with coefficient of variation below 2% across multiple microchannels.
Strengths: Excellent multiplexing capabilities for simultaneous analysis of multiple analytes; automated calibration process minimizing manual intervention; high sensitivity suitable for protein biomarker detection. Weaknesses: Limited application scope primarily focused on protein analysis; proprietary consumables increasing per-test costs; requires specialized instrumentation limiting compatibility with existing laboratory equipment.
miDIAGNOSTICS NV
Technical Solution: miDIAGNOSTICS has pioneered a silicon-based microfluidic calibration approach specifically optimized for ultra-sensitive trace analysis in clinical diagnostics. Their technology employs nanofabricated silicon microfluidic channels with precisely controlled surface properties to minimize analyte adsorption, achieving detection limits in the femtomolar range. The calibration system utilizes a multi-point dynamic calibration protocol that automatically generates calibration curves across five orders of magnitude, enabling accurate quantification from picogram to nanogram levels. Their proprietary "Calibration-on-Chip" technology incorporates lyophilized calibration standards directly into microfluidic reservoirs that are automatically reconstituted during the analysis process, eliminating manual calibration steps and reducing human error. The system employs real-time flow monitoring with integrated impedance sensors that continuously verify fluid movement through critical junctions, ensuring sample and calibrant delivery with volumetric precision better than 2%.
Strengths: Exceptional sensitivity for trace analysis applications; automated calibration process minimizing human error; integrated quality control features ensuring reliable results. Weaknesses: Specialized chip design limits flexibility for different applications; higher cost per test compared to conventional methods; requires proprietary instrumentation for optimal performance.
Critical Technologies for Microfluidic Precision
Microfluidic method for analysing metals
PatentWO2020064987A1
Innovation
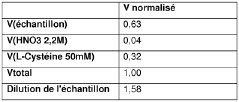
- A microfluidic method using a circuit with electrochemical detection involving gold and platinum electrodes, combined with nitric acid and L-cysteine, allows for rapid, reliable, and reproducible analysis of arsenic without sample denaturation, achieving detection limits compatible with regulatory standards.
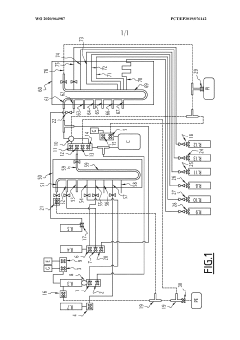
Calibration method for a liquid system
PatentPendingUS20250085151A1
Innovation
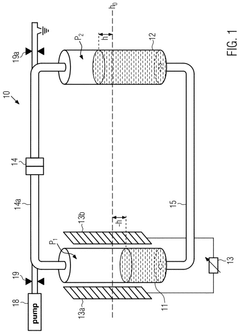
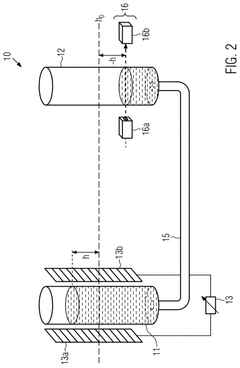
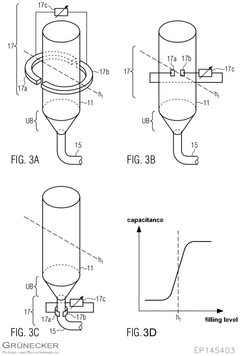
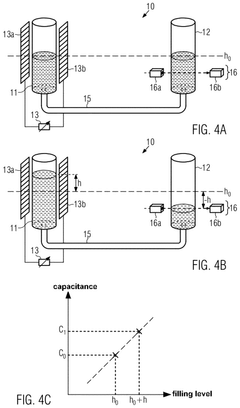
- A calibration method that uses a capacitance sensor to measure electrical capacitance at a reservoir, allowing for the determination of a relationship between filling levels and capacitance, thereby enabling precise measurement of flow.
Quality Control Standards for Microfluidic Systems
Quality control standards for microfluidic systems represent a critical framework for ensuring reliable and reproducible results in trace analysis applications. These standards must address the unique challenges posed by the microscale nature of fluid handling and the sensitivity required for detecting trace analytes. Established quality control protocols typically encompass calibration verification, system performance monitoring, and validation of analytical methods specific to microfluidic platforms.
The development of standardized quality control measures requires consideration of both international standards (ISO, ASTM) and industry-specific guidelines that govern analytical instrumentation. For microfluidic trace analysis, these standards must be adapted to account for the reduced sample volumes, potential surface interactions, and flow dynamics unique to microscale channels. Organizations such as NIST (National Institute of Standards and Technology) have begun developing certified reference materials specifically designed for microfluidic applications, enabling more accurate calibration procedures.
Key quality control parameters for microfluidic systems include flow rate accuracy, pressure stability, temperature uniformity, and channel dimension consistency. These parameters must be regularly verified using standardized procedures to ensure system performance remains within acceptable tolerances. Documentation requirements typically mandate detailed records of calibration schedules, verification results, and corrective actions taken when deviations occur.
Statistical process control methods play an essential role in microfluidic quality assurance, with control charts and trend analysis helping to identify system drift before it impacts analytical results. Acceptance criteria must be established based on the specific requirements of the trace analysis application, with tighter tolerances generally necessary for lower detection limits.
Proficiency testing represents another crucial component of quality control standards, allowing laboratories to verify their microfluidic trace analysis capabilities against peer organizations. These interlaboratory comparisons help identify systematic errors and establish confidence in analytical results across different microfluidic platforms and operator skill levels.
Automated quality control systems are increasingly being integrated into microfluidic platforms, enabling real-time monitoring of critical parameters and automatic flagging of out-of-specification conditions. These systems often incorporate internal standards and calibration verification samples that can be analyzed periodically to ensure consistent performance throughout analytical runs.
The evolution of quality control standards for microfluidics continues to advance alongside technological developments, with emerging areas such as digital microfluidics and organ-on-chip systems requiring specialized approaches to quality assurance. As the field matures, consensus standards developed through collaborative efforts between academic researchers, industry stakeholders, and regulatory bodies will be essential for establishing widely accepted quality control frameworks.
The development of standardized quality control measures requires consideration of both international standards (ISO, ASTM) and industry-specific guidelines that govern analytical instrumentation. For microfluidic trace analysis, these standards must be adapted to account for the reduced sample volumes, potential surface interactions, and flow dynamics unique to microscale channels. Organizations such as NIST (National Institute of Standards and Technology) have begun developing certified reference materials specifically designed for microfluidic applications, enabling more accurate calibration procedures.
Key quality control parameters for microfluidic systems include flow rate accuracy, pressure stability, temperature uniformity, and channel dimension consistency. These parameters must be regularly verified using standardized procedures to ensure system performance remains within acceptable tolerances. Documentation requirements typically mandate detailed records of calibration schedules, verification results, and corrective actions taken when deviations occur.
Statistical process control methods play an essential role in microfluidic quality assurance, with control charts and trend analysis helping to identify system drift before it impacts analytical results. Acceptance criteria must be established based on the specific requirements of the trace analysis application, with tighter tolerances generally necessary for lower detection limits.
Proficiency testing represents another crucial component of quality control standards, allowing laboratories to verify their microfluidic trace analysis capabilities against peer organizations. These interlaboratory comparisons help identify systematic errors and establish confidence in analytical results across different microfluidic platforms and operator skill levels.
Automated quality control systems are increasingly being integrated into microfluidic platforms, enabling real-time monitoring of critical parameters and automatic flagging of out-of-specification conditions. These systems often incorporate internal standards and calibration verification samples that can be analyzed periodically to ensure consistent performance throughout analytical runs.
The evolution of quality control standards for microfluidics continues to advance alongside technological developments, with emerging areas such as digital microfluidics and organ-on-chip systems requiring specialized approaches to quality assurance. As the field matures, consensus standards developed through collaborative efforts between academic researchers, industry stakeholders, and regulatory bodies will be essential for establishing widely accepted quality control frameworks.
Cross-Contamination Prevention Strategies
Cross-contamination represents a critical challenge in microfluidic trace analysis, where even minute contaminants can significantly impact analytical results. Effective prevention strategies must be implemented throughout the entire analytical workflow, from sample preparation to final detection. The implementation of physical barriers between different processing zones within microfluidic devices serves as a fundamental approach, utilizing hydrophobic patches, air gaps, or specialized valve systems to prevent fluid mixing between adjacent channels.
Surface treatment protocols play a vital role in contamination prevention. Techniques such as silanization, plasma treatment, or polymer coating can modify channel surfaces to reduce analyte adsorption and subsequent release. These treatments create surfaces with controlled hydrophobicity or hydrophilicity, minimizing carryover effects between sequential analyses. Regular renewal of these surface treatments maintains their effectiveness over extended operational periods.
Rigorous cleaning procedures constitute another essential component of contamination control. Standardized protocols involving sequential rinses with appropriate solvents, enzymatic cleaners, and ultrapure water effectively remove residual analytes and reagents. For particularly sensitive applications, dedicated cleaning solutions tailored to specific contaminants may be necessary. Automated cleaning cycles integrated into microfluidic platforms ensure consistent decontamination between analytical runs.
Single-use components represent an increasingly popular strategy for eliminating cross-contamination risks. Disposable microfluidic cartridges, sample introduction interfaces, and detection chambers can be employed for critical applications. While this approach increases consumable costs, it significantly reduces contamination risks in high-sensitivity trace analysis applications where result integrity is paramount.
Advanced fluidic control systems provide dynamic contamination prevention through precise flow management. Unidirectional flow designs, coupled with optimized channel geometries, minimize backflow and cross-channel diffusion. Sophisticated pumping systems maintaining consistent pressure gradients prevent unwanted fluid migration between analytical zones. Additionally, temporal separation strategies, where different samples or reagents are processed with intervening cleaning cycles, further reduce contamination risks.
Validation protocols must be established to verify the effectiveness of contamination prevention measures. These typically involve analyzing blank samples between test samples to detect potential carryover, conducting recovery tests with known standards, and implementing statistical process control to monitor system cleanliness over time. Regular system audits using fluorescent tracers or other visualization techniques can identify potential contamination pathways before they impact analytical results.
Surface treatment protocols play a vital role in contamination prevention. Techniques such as silanization, plasma treatment, or polymer coating can modify channel surfaces to reduce analyte adsorption and subsequent release. These treatments create surfaces with controlled hydrophobicity or hydrophilicity, minimizing carryover effects between sequential analyses. Regular renewal of these surface treatments maintains their effectiveness over extended operational periods.
Rigorous cleaning procedures constitute another essential component of contamination control. Standardized protocols involving sequential rinses with appropriate solvents, enzymatic cleaners, and ultrapure water effectively remove residual analytes and reagents. For particularly sensitive applications, dedicated cleaning solutions tailored to specific contaminants may be necessary. Automated cleaning cycles integrated into microfluidic platforms ensure consistent decontamination between analytical runs.
Single-use components represent an increasingly popular strategy for eliminating cross-contamination risks. Disposable microfluidic cartridges, sample introduction interfaces, and detection chambers can be employed for critical applications. While this approach increases consumable costs, it significantly reduces contamination risks in high-sensitivity trace analysis applications where result integrity is paramount.
Advanced fluidic control systems provide dynamic contamination prevention through precise flow management. Unidirectional flow designs, coupled with optimized channel geometries, minimize backflow and cross-channel diffusion. Sophisticated pumping systems maintaining consistent pressure gradients prevent unwanted fluid migration between analytical zones. Additionally, temporal separation strategies, where different samples or reagents are processed with intervening cleaning cycles, further reduce contamination risks.
Validation protocols must be established to verify the effectiveness of contamination prevention measures. These typically involve analyzing blank samples between test samples to detect potential carryover, conducting recovery tests with known standards, and implementing statistical process control to monitor system cleanliness over time. Regular system audits using fluorescent tracers or other visualization techniques can identify potential contamination pathways before they impact analytical results.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!