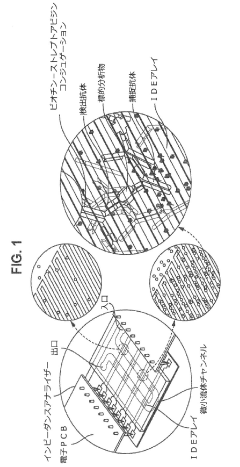
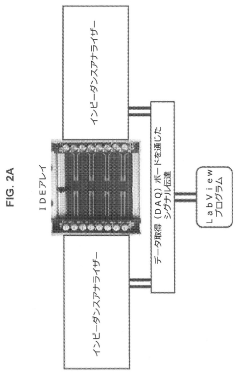
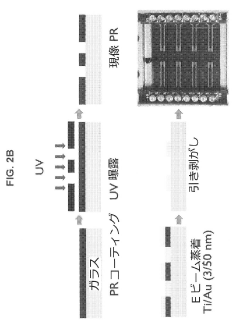
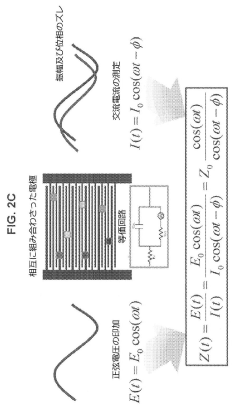
Using Microfluidics to Improve Bioluminescence Assay Sensitivity
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics and Bioluminescence Technology Background
Microfluidics technology has evolved significantly over the past three decades, emerging from the convergence of molecular biology, microfabrication, and fluid mechanics. Initially developed in the 1990s, microfluidic systems manipulate fluids at the microscale level, typically handling volumes in the nanoliter to picoliter range. These systems leverage the unique physical properties of fluids at small scales, where surface tension and laminar flow dominate over turbulence and inertial forces.
Bioluminescence, on the other hand, represents a natural phenomenon where living organisms produce light through biochemical reactions. The most well-characterized bioluminescent system is the luciferase-luciferin reaction found in fireflies (Photinus pyralis), which has been adapted extensively for laboratory applications since the 1980s. This reaction involves the oxidation of luciferin substrate by the luciferase enzyme in the presence of ATP, magnesium, and oxygen, resulting in light emission.
Traditional bioluminescence assays face several limitations, including sensitivity thresholds, signal stability, and reproducibility challenges. These assays typically require relatively large sample volumes (50-200 μL) and suffer from diffusion limitations that affect reaction kinetics and detection efficiency. The sensitivity floor of conventional plate-based assays typically ranges from 10^-15 to 10^-18 moles of analyte, depending on the specific application.
The integration of microfluidics with bioluminescence detection represents a promising technological convergence. Microfluidic platforms offer precise control over reaction conditions, dramatically reduced reagent consumption, enhanced surface-to-volume ratios, and improved mass transfer characteristics. These advantages directly address many limitations of traditional bioluminescence assays, potentially enabling detection of analytes at femtomolar or even attomolar concentrations.
Recent technological advances have further accelerated this field, including the development of droplet microfluidics, digital microfluidics, and paper-based microfluidic systems. These platforms provide diverse approaches for sample handling, reaction compartmentalization, and signal detection. Complementary advances in photon detection technology, including electron-multiplying CCDs and single-photon avalanche diodes, have significantly improved the ability to detect weak bioluminescent signals.
The application landscape for enhanced bioluminescence assays spans multiple sectors, including clinical diagnostics, environmental monitoring, food safety testing, and fundamental research in cell biology. Particularly promising is the potential for point-of-care diagnostic applications, where high sensitivity combined with miniaturization could enable rapid detection of pathogens or biomarkers at clinically relevant concentrations without laboratory infrastructure.
Current research trends focus on optimizing microfluidic geometries for bioluminescent reactions, developing novel surface functionalization strategies to minimize non-specific binding, and creating integrated systems that combine sample preparation, reaction, and detection in single devices. These developments aim to push detection limits while maintaining or improving assay speed, reproducibility, and ease of use.
Bioluminescence, on the other hand, represents a natural phenomenon where living organisms produce light through biochemical reactions. The most well-characterized bioluminescent system is the luciferase-luciferin reaction found in fireflies (Photinus pyralis), which has been adapted extensively for laboratory applications since the 1980s. This reaction involves the oxidation of luciferin substrate by the luciferase enzyme in the presence of ATP, magnesium, and oxygen, resulting in light emission.
Traditional bioluminescence assays face several limitations, including sensitivity thresholds, signal stability, and reproducibility challenges. These assays typically require relatively large sample volumes (50-200 μL) and suffer from diffusion limitations that affect reaction kinetics and detection efficiency. The sensitivity floor of conventional plate-based assays typically ranges from 10^-15 to 10^-18 moles of analyte, depending on the specific application.
The integration of microfluidics with bioluminescence detection represents a promising technological convergence. Microfluidic platforms offer precise control over reaction conditions, dramatically reduced reagent consumption, enhanced surface-to-volume ratios, and improved mass transfer characteristics. These advantages directly address many limitations of traditional bioluminescence assays, potentially enabling detection of analytes at femtomolar or even attomolar concentrations.
Recent technological advances have further accelerated this field, including the development of droplet microfluidics, digital microfluidics, and paper-based microfluidic systems. These platforms provide diverse approaches for sample handling, reaction compartmentalization, and signal detection. Complementary advances in photon detection technology, including electron-multiplying CCDs and single-photon avalanche diodes, have significantly improved the ability to detect weak bioluminescent signals.
The application landscape for enhanced bioluminescence assays spans multiple sectors, including clinical diagnostics, environmental monitoring, food safety testing, and fundamental research in cell biology. Particularly promising is the potential for point-of-care diagnostic applications, where high sensitivity combined with miniaturization could enable rapid detection of pathogens or biomarkers at clinically relevant concentrations without laboratory infrastructure.
Current research trends focus on optimizing microfluidic geometries for bioluminescent reactions, developing novel surface functionalization strategies to minimize non-specific binding, and creating integrated systems that combine sample preparation, reaction, and detection in single devices. These developments aim to push detection limits while maintaining or improving assay speed, reproducibility, and ease of use.
Market Applications for Enhanced Bioluminescence Assays
The enhanced sensitivity of bioluminescence assays through microfluidic technology opens significant market opportunities across multiple sectors. In the pharmaceutical industry, these improved assays enable more efficient drug discovery processes by detecting biomolecular interactions at previously undetectable concentrations. This advancement allows pharmaceutical companies to screen drug candidates more effectively, potentially reducing development timelines and costs while increasing the probability of identifying viable therapeutic compounds.
Clinical diagnostics represents another substantial market, where enhanced bioluminescence assays can revolutionize point-of-care testing. The improved sensitivity enables earlier disease detection, particularly for conditions where biomarkers are present at extremely low concentrations during early stages. This capability is especially valuable for cancer diagnostics, infectious disease monitoring, and autoimmune disorder screening, where early intervention significantly improves patient outcomes.
Environmental monitoring applications benefit tremendously from these advancements. The enhanced sensitivity allows for detection of environmental contaminants, pathogens, and toxins at concentrations well below current regulatory thresholds. Water quality monitoring, food safety testing, and environmental compliance verification can all leverage these improved assays to provide more accurate and timely data for public health protection.
The food and beverage industry represents a growing market for enhanced bioluminescence assays. Microfluidic-improved sensitivity enables rapid detection of foodborne pathogens, allergens, and contaminants throughout the production chain. This capability supports improved quality control processes, reduces product recalls, and enhances consumer safety while potentially extending shelf life through more precise monitoring.
Academic and research institutions constitute a significant market segment, utilizing these enhanced assays for fundamental biological research, including protein-protein interactions, enzyme kinetics, and cellular signaling pathways. The improved sensitivity enables researchers to observe biological processes that were previously undetectable, potentially accelerating scientific discoveries and expanding our understanding of biological systems.
Biodefense and security applications represent a specialized but high-value market. Enhanced bioluminescence assays can detect biological warfare agents and emerging pathogens at extremely low concentrations, enabling faster response to potential threats. Government agencies and security organizations invest substantially in these technologies to protect public health and national security.
Agricultural applications are emerging as farmers and agricultural companies seek more efficient methods to monitor crop health, detect plant pathogens, and optimize growing conditions. The portability and sensitivity of microfluidic-enhanced bioluminescence assays make them ideal for field-based testing, potentially transforming agricultural management practices through data-driven decision making.
Clinical diagnostics represents another substantial market, where enhanced bioluminescence assays can revolutionize point-of-care testing. The improved sensitivity enables earlier disease detection, particularly for conditions where biomarkers are present at extremely low concentrations during early stages. This capability is especially valuable for cancer diagnostics, infectious disease monitoring, and autoimmune disorder screening, where early intervention significantly improves patient outcomes.
Environmental monitoring applications benefit tremendously from these advancements. The enhanced sensitivity allows for detection of environmental contaminants, pathogens, and toxins at concentrations well below current regulatory thresholds. Water quality monitoring, food safety testing, and environmental compliance verification can all leverage these improved assays to provide more accurate and timely data for public health protection.
The food and beverage industry represents a growing market for enhanced bioluminescence assays. Microfluidic-improved sensitivity enables rapid detection of foodborne pathogens, allergens, and contaminants throughout the production chain. This capability supports improved quality control processes, reduces product recalls, and enhances consumer safety while potentially extending shelf life through more precise monitoring.
Academic and research institutions constitute a significant market segment, utilizing these enhanced assays for fundamental biological research, including protein-protein interactions, enzyme kinetics, and cellular signaling pathways. The improved sensitivity enables researchers to observe biological processes that were previously undetectable, potentially accelerating scientific discoveries and expanding our understanding of biological systems.
Biodefense and security applications represent a specialized but high-value market. Enhanced bioluminescence assays can detect biological warfare agents and emerging pathogens at extremely low concentrations, enabling faster response to potential threats. Government agencies and security organizations invest substantially in these technologies to protect public health and national security.
Agricultural applications are emerging as farmers and agricultural companies seek more efficient methods to monitor crop health, detect plant pathogens, and optimize growing conditions. The portability and sensitivity of microfluidic-enhanced bioluminescence assays make them ideal for field-based testing, potentially transforming agricultural management practices through data-driven decision making.
Current Limitations in Bioluminescence Detection Sensitivity
Despite significant advancements in bioluminescence assay technology, several critical limitations continue to hinder the achievement of optimal detection sensitivity. The primary challenge remains the inherently low quantum yield of most bioluminescent reactions, typically ranging from 0.1-0.5, which results in relatively weak signal output compared to fluorescence-based methods. This fundamental limitation becomes particularly problematic when detecting low-abundance analytes in complex biological matrices.
Signal-to-noise ratio (SNR) represents another significant barrier, as background luminescence from sample components and instrument dark current can mask weak bioluminescent signals. Current detection systems struggle to differentiate between true positive signals and background noise when working with samples containing low target concentrations, often below 10^-15 M.
Conventional bioluminescence assay formats suffer from diffusion limitations that impact reaction kinetics and signal generation. In traditional microplate formats, the relatively large reaction volumes (50-200 μL) create significant diffusion distances between reactants, resulting in slower reaction rates and diminished signal intensity. This volume-dependent limitation becomes particularly evident when working with limited sample quantities or rare analytes.
Enzyme stability presents another critical challenge, as many luciferases exhibit reduced activity over time due to thermal denaturation, oxidative damage, or inhibition by assay components. Current stabilization methods often involve chemical additives that may interfere with assay performance or introduce additional variables affecting sensitivity.
The optical properties of conventional assay vessels contribute to sensitivity limitations through light scattering, reflection, and absorption effects. Standard microplates made from polystyrene or glass can absorb or scatter a significant portion of the emitted light, reducing the detectable signal by up to 40% before it reaches the detector.
Detector technology limitations further constrain sensitivity, with most photomultiplier tubes (PMTs) and charge-coupled devices (CCDs) operating at quantum efficiencies below 40% in the spectral range of common bioluminescent emissions (440-620 nm). These detection systems also introduce electronic noise that can mask weak signals.
Sample preparation inconsistencies represent a significant source of variability in bioluminescence assays. Current methods often involve multiple manual handling steps that introduce errors and reduce reproducibility, particularly at low signal levels where precision becomes critical for reliable detection.
Signal-to-noise ratio (SNR) represents another significant barrier, as background luminescence from sample components and instrument dark current can mask weak bioluminescent signals. Current detection systems struggle to differentiate between true positive signals and background noise when working with samples containing low target concentrations, often below 10^-15 M.
Conventional bioluminescence assay formats suffer from diffusion limitations that impact reaction kinetics and signal generation. In traditional microplate formats, the relatively large reaction volumes (50-200 μL) create significant diffusion distances between reactants, resulting in slower reaction rates and diminished signal intensity. This volume-dependent limitation becomes particularly evident when working with limited sample quantities or rare analytes.
Enzyme stability presents another critical challenge, as many luciferases exhibit reduced activity over time due to thermal denaturation, oxidative damage, or inhibition by assay components. Current stabilization methods often involve chemical additives that may interfere with assay performance or introduce additional variables affecting sensitivity.
The optical properties of conventional assay vessels contribute to sensitivity limitations through light scattering, reflection, and absorption effects. Standard microplates made from polystyrene or glass can absorb or scatter a significant portion of the emitted light, reducing the detectable signal by up to 40% before it reaches the detector.
Detector technology limitations further constrain sensitivity, with most photomultiplier tubes (PMTs) and charge-coupled devices (CCDs) operating at quantum efficiencies below 40% in the spectral range of common bioluminescent emissions (440-620 nm). These detection systems also introduce electronic noise that can mask weak signals.
Sample preparation inconsistencies represent a significant source of variability in bioluminescence assays. Current methods often involve multiple manual handling steps that introduce errors and reduce reproducibility, particularly at low signal levels where precision becomes critical for reliable detection.
Microfluidic Solutions for Bioluminescence Signal Enhancement
01 Microfluidic device design for enhanced bioluminescence sensitivity
Specialized microfluidic device designs can significantly enhance the sensitivity of bioluminescence assays. These designs incorporate features such as optimized channel geometries, integrated optical components, and precise flow control mechanisms that maximize signal detection. By minimizing sample volumes while maintaining efficient mixing of reagents and analytes, these devices achieve lower detection limits and improved signal-to-noise ratios for bioluminescent reactions.- Microfluidic device design for enhanced bioluminescence sensitivity: Specialized microfluidic device designs can significantly enhance the sensitivity of bioluminescence assays. These designs incorporate features such as optimized channel geometries, integrated optical components, and precise flow control mechanisms that maximize signal detection. The miniaturized reaction chambers reduce sample and reagent volumes while concentrating the bioluminescent signals, resulting in improved detection limits. Some designs also incorporate specialized surfaces or materials that minimize background noise and enhance signal-to-noise ratios.
- Integration of detection systems with microfluidic platforms: Advanced detection systems integrated directly with microfluidic platforms can significantly improve bioluminescence assay sensitivity. These integrated systems may include photomultiplier tubes, CCD cameras, photodiodes, or specialized optical fibers positioned strategically to capture maximum light emission. The close proximity of detectors to the reaction sites minimizes signal loss and increases detection efficiency. Some systems also incorporate real-time monitoring capabilities that allow for dynamic measurement of bioluminescent reactions as they occur within the microchannels.
- Reagent optimization and mixing strategies: Innovative reagent formulations and mixing strategies within microfluidic systems can enhance bioluminescence assay sensitivity. Controlled laminar flow patterns, gradient generators, and specialized mixing structures enable precise reagent interactions and optimal reaction conditions. Some approaches utilize droplet-based microfluidics to create isolated reaction compartments that concentrate reactants and prevent dilution of signals. Advanced enzyme stabilization techniques and substrate delivery methods also contribute to increased luminescence intensity and prolonged signal duration.
- Surface modifications and immobilization techniques: Surface modifications and biomolecule immobilization techniques within microfluidic channels can significantly improve bioluminescence assay sensitivity. These approaches include specialized coatings that reduce non-specific binding, increase signal retention, and optimize the orientation of bioluminescent enzymes or substrates. Some techniques utilize nanostructured surfaces or hydrogels to increase the effective surface area for reactions. Strategic immobilization of luciferase enzymes or their substrates can create localized reaction zones that concentrate signals and enhance detection limits.
- Signal amplification and processing methods: Advanced signal amplification and processing methods can dramatically improve the sensitivity of microfluidic bioluminescence assays. These include chemical amplification strategies, such as enzymatic cycling reactions, that multiply the number of photons produced per target molecule. Digital processing techniques can filter noise, enhance signal quality, and extract meaningful data from weak bioluminescent emissions. Some approaches utilize machine learning algorithms to identify patterns in low-level signals that would otherwise be undetectable. Integration with nanomaterials or quantum dots can also enhance light emission or facilitate energy transfer for improved sensitivity.
02 Integration of optical detection systems in microfluidic platforms
Advanced optical detection systems integrated directly into microfluidic platforms enable highly sensitive measurement of bioluminescent signals. These systems may include miniaturized photomultiplier tubes, photodiodes, or CCD sensors positioned strategically to capture emitted light with minimal loss. Some designs incorporate waveguides, lenses, or mirrors to direct and concentrate light signals to detectors, further enhancing sensitivity by improving photon collection efficiency.Expand Specific Solutions03 Novel substrate delivery methods for bioluminescent reactions
Innovative approaches to substrate delivery within microfluidic systems can significantly improve the sensitivity of bioluminescence assays. These methods include controlled release mechanisms, gradient generators, and pulsed delivery systems that optimize the interaction between enzymes and substrates. By ensuring precise timing and concentration of reagents at the reaction site, these techniques maximize light output and extend the duration of the bioluminescent signal, resulting in enhanced detection sensitivity.Expand Specific Solutions04 Microfluidic sample preparation techniques for bioluminescence assays
Advanced sample preparation techniques integrated into microfluidic platforms can significantly improve the sensitivity of bioluminescence assays. These techniques include on-chip cell lysis, filtration, concentration, and purification steps that remove inhibitors and concentrate target analytes. By processing samples directly within the microfluidic device, these methods minimize sample loss, reduce contamination risks, and preserve the activity of bioluminescent reagents, ultimately leading to enhanced assay sensitivity.Expand Specific Solutions05 Multiplexed bioluminescence detection in microfluidic systems
Multiplexed detection strategies in microfluidic platforms allow for simultaneous measurement of multiple bioluminescent reactions with high sensitivity. These approaches utilize spatial separation of reactions in different channels, temporal separation through controlled reaction timing, or spectral separation using different bioluminescent reporters with distinct emission wavelengths. Advanced signal processing algorithms help distinguish between multiple signals, reducing background noise and improving overall assay sensitivity in complex sample analysis.Expand Specific Solutions
Leading Companies and Research Institutions in Bioluminescence
The microfluidics-enhanced bioluminescence assay market is currently in a growth phase, with increasing adoption across biomedical research and diagnostics. The global market size is expanding rapidly, driven by demands for higher sensitivity detection methods in pharmaceutical development and clinical applications. Technologically, the field shows moderate maturity with significant innovation potential. Leading academic institutions (MIT, Harvard, Johns Hopkins, Cornell) are advancing fundamental research, while commercial players demonstrate varying levels of expertise. Companies like Illumina and Caliper Life Sciences have established strong positions in microfluidics integration, while BOE Technology and Siemens Healthcare Diagnostics are leveraging their manufacturing capabilities to improve assay platforms. Specialized firms such as Gyros AB and NanoEntek are developing proprietary microfluidic structures specifically optimized for bioluminescence applications, creating a competitive landscape balanced between established corporations and innovative startups.
Caliper Life Sciences, Inc.
Technical Solution: Caliper Life Sciences has developed advanced microfluidic platforms specifically designed to enhance bioluminescence assay sensitivity. Their LabChip technology integrates microfluidic channels with precise flow control mechanisms to create optimized reaction environments for bioluminescent reactions. The system employs specialized microchambers that concentrate the sample and reagents into smaller volumes (nanoliter to picoliter range), significantly increasing the local concentration of luminescent molecules and improving signal detection. Their proprietary surface treatments minimize biomolecule adsorption to channel walls, reducing sample loss and background noise. Caliper's platforms incorporate integrated optical detection systems with high-sensitivity photomultiplier tubes positioned in close proximity to the reaction chambers, maximizing photon capture efficiency. The company has also developed automated sample handling and precise reagent mixing capabilities that ensure optimal timing of substrate addition and signal measurement, critical factors in maximizing bioluminescence output.
Strengths: Highly integrated systems combining microfluidics with sensitive detection; established commercial presence with validated platforms; comprehensive automation capabilities reducing user variability. Weaknesses: Higher cost compared to traditional plate-based assays; requires specialized equipment and expertise; limited flexibility for customization of certain assay parameters.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have pioneered innovative microfluidic approaches to enhance bioluminescence assay sensitivity through their "Digital Bioluminescence" platform. This technology utilizes droplet microfluidics to partition samples into thousands of nanoliter-sized droplets, effectively creating isolated reaction chambers that concentrate the bioluminescent reactions. By implementing a digital counting approach rather than analog intensity measurements, MIT's system can detect single-molecule events, dramatically lowering detection limits. Their platform incorporates specialized PDMS (polydimethylsiloxane) microchannels with optimized geometries that maximize light collection efficiency while minimizing optical losses. MIT has also developed novel surface chemistry modifications that prevent protein adsorption and enzyme denaturation within microchannels, preserving enzymatic activity crucial for bioluminescent reactions. Additionally, they've integrated CMOS-based imaging systems directly with their microfluidic chips, creating compact, highly sensitive detection systems capable of real-time monitoring of bioluminescent signals with spatial resolution.
Strengths: Cutting-edge technology enabling single-molecule detection; digital approach provides superior quantification compared to traditional methods; highly miniaturized systems suitable for point-of-care applications. Weaknesses: Complex fabrication processes may limit mass production; relatively early-stage technology with limited commercial validation; requires specialized expertise in both microfluidics and optical detection systems.
Key Patents and Innovations in Microfluidic Bioluminescence
Microfluidic particulate-labeled impedance sensor arrays for enhancing bioassay sensitivity
PatentPendingJP2024513691A
Innovation
- A microfluidic biosensing platform is designed, using multi-layer microfluidic channels, buffer inlets, sample inlets and waste outlets, combined with a multi-layer fluid network, using multi-layer microfluidic channels and the base layer of the first antibody attached to the surface, detecting electrical impedance changes to detect and quantify biomolecules, and using micro-particle labeling technology to improve sensitivity.
A microfluidic microparticle-labeled impedance sensor array for enhancing bioassay sensitivity
PatentWO2022192571A1
Innovation
- A microfluidic device with a multi-layer network and microparticle-labeled impedance sensor array that uses capillary-driven flow and microparticles to enhance sensitivity, integrating a microfluidic chip with interdigitated electrodes and a detector for real-time impedance measurement.
Scalability and Manufacturing Considerations
The scalability of microfluidic systems for bioluminescence assays represents a critical consideration for their widespread adoption in research and commercial applications. Current manufacturing approaches for microfluidic devices include soft lithography using polydimethylsiloxane (PDMS), injection molding, hot embossing, and 3D printing. Each method offers distinct advantages in terms of resolution, throughput, and material compatibility. PDMS-based fabrication, while excellent for prototyping due to its optical clarity and gas permeability, faces challenges in mass production scenarios due to labor-intensive processes and batch-to-batch variability.
For industrial-scale implementation, thermoplastic-based manufacturing methods such as injection molding offer superior cost-effectiveness when production volumes exceed approximately 10,000 units. Recent advances in high-precision injection molding have enabled the creation of microchannels with dimensions approaching 10 micrometers, suitable for many bioluminescence applications. However, the initial tooling costs remain substantial, typically ranging from $10,000 to $100,000 depending on complexity.
Material selection significantly impacts both manufacturing feasibility and assay performance. While PDMS remains dominant in research settings, its hydrophobicity and potential for non-specific protein adsorption can interfere with sensitive bioluminescence measurements. Alternative materials such as cyclic olefin copolymer (COC) and polymethyl methacrylate (PMMA) offer improved optical properties and reduced autofluorescence, critical for detecting low-level bioluminescent signals.
Integration of detection components presents another manufacturing challenge. Current approaches include post-fabrication assembly of photomultiplier tubes or photodiodes, which increases production complexity and cost. Emerging solutions involve direct integration of thin-film photodetectors during the manufacturing process, potentially reducing assembly steps by 40-60% while improving signal detection uniformity.
Quality control represents a significant consideration in scaled production. Microfluidic channels must maintain consistent dimensions to ensure reproducible fluid dynamics and reaction kinetics. Industry standards typically require dimensional tolerances below ±2% for critical features. Advanced inspection techniques such as automated optical verification and micro-CT scanning are increasingly employed to maintain these tolerances in production environments.
Economic viability ultimately depends on achieving sufficient production volumes to amortize initial tooling and setup costs. Market analysis indicates that for diagnostic applications, production volumes exceeding 50,000 units annually typically justify dedicated manufacturing lines. For research applications, where customization requirements are higher but volumes lower, modular manufacturing approaches that balance flexibility with efficiency are gaining traction, potentially reducing setup costs by 30-50% compared to traditional methods.
For industrial-scale implementation, thermoplastic-based manufacturing methods such as injection molding offer superior cost-effectiveness when production volumes exceed approximately 10,000 units. Recent advances in high-precision injection molding have enabled the creation of microchannels with dimensions approaching 10 micrometers, suitable for many bioluminescence applications. However, the initial tooling costs remain substantial, typically ranging from $10,000 to $100,000 depending on complexity.
Material selection significantly impacts both manufacturing feasibility and assay performance. While PDMS remains dominant in research settings, its hydrophobicity and potential for non-specific protein adsorption can interfere with sensitive bioluminescence measurements. Alternative materials such as cyclic olefin copolymer (COC) and polymethyl methacrylate (PMMA) offer improved optical properties and reduced autofluorescence, critical for detecting low-level bioluminescent signals.
Integration of detection components presents another manufacturing challenge. Current approaches include post-fabrication assembly of photomultiplier tubes or photodiodes, which increases production complexity and cost. Emerging solutions involve direct integration of thin-film photodetectors during the manufacturing process, potentially reducing assembly steps by 40-60% while improving signal detection uniformity.
Quality control represents a significant consideration in scaled production. Microfluidic channels must maintain consistent dimensions to ensure reproducible fluid dynamics and reaction kinetics. Industry standards typically require dimensional tolerances below ±2% for critical features. Advanced inspection techniques such as automated optical verification and micro-CT scanning are increasingly employed to maintain these tolerances in production environments.
Economic viability ultimately depends on achieving sufficient production volumes to amortize initial tooling and setup costs. Market analysis indicates that for diagnostic applications, production volumes exceeding 50,000 units annually typically justify dedicated manufacturing lines. For research applications, where customization requirements are higher but volumes lower, modular manufacturing approaches that balance flexibility with efficiency are gaining traction, potentially reducing setup costs by 30-50% compared to traditional methods.
Regulatory Pathway for Microfluidic Diagnostic Platforms
The regulatory landscape for microfluidic platforms used in bioluminescence assay applications presents a complex pathway that developers must navigate carefully. In the United States, the FDA categorizes these platforms primarily under in vitro diagnostic (IVD) devices, with classification depending on intended use and risk profile. Class II designation is most common for microfluidic diagnostic tools, requiring 510(k) clearance demonstrating substantial equivalence to predefined predicate devices.
For novel microfluidic technologies specifically enhancing bioluminescence assay sensitivity, developers may face additional regulatory scrutiny due to the innovative nature of these platforms. The FDA's De Novo classification process offers an alternative pathway when no suitable predicate exists, allowing breakthrough technologies to enter the market with appropriate controls.
European market access requires CE marking under the In Vitro Diagnostic Regulation (IVDR), which replaced the previous IVDD in 2022. The IVDR introduces more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Microfluidic platforms enhancing bioluminescence sensitivity would likely fall under Class C devices, necessitating conformity assessment involving a notified body.
Quality management systems compliant with ISO 13485 standards are essential for both FDA and European regulatory pathways. For microfluidic bioluminescence platforms, specific considerations include validation of fluid dynamics, material biocompatibility, and demonstration of enhanced analytical sensitivity compared to conventional methods.
Clinical validation requirements vary by intended use, with diagnostic applications demanding robust clinical performance studies. Developers must establish clear performance characteristics including limits of detection, analytical specificity, and reproducibility—particularly critical for microfluidic platforms claiming improved bioluminescence sensitivity.
Regulatory bodies increasingly recognize the value of real-world evidence and adaptive licensing approaches for innovative technologies. The FDA's Breakthrough Devices Program and Europe's IVDR provisions for innovative devices offer accelerated pathways for technologies demonstrating substantial clinical advantages, which may benefit advanced microfluidic platforms for bioluminescence assays.
Global harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually standardizing requirements across major markets, potentially streamlining the regulatory process for developers targeting multiple regions. However, country-specific requirements remain in emerging markets, requiring tailored regulatory strategies for global commercialization of microfluidic diagnostic platforms.
For novel microfluidic technologies specifically enhancing bioluminescence assay sensitivity, developers may face additional regulatory scrutiny due to the innovative nature of these platforms. The FDA's De Novo classification process offers an alternative pathway when no suitable predicate exists, allowing breakthrough technologies to enter the market with appropriate controls.
European market access requires CE marking under the In Vitro Diagnostic Regulation (IVDR), which replaced the previous IVDD in 2022. The IVDR introduces more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Microfluidic platforms enhancing bioluminescence sensitivity would likely fall under Class C devices, necessitating conformity assessment involving a notified body.
Quality management systems compliant with ISO 13485 standards are essential for both FDA and European regulatory pathways. For microfluidic bioluminescence platforms, specific considerations include validation of fluid dynamics, material biocompatibility, and demonstration of enhanced analytical sensitivity compared to conventional methods.
Clinical validation requirements vary by intended use, with diagnostic applications demanding robust clinical performance studies. Developers must establish clear performance characteristics including limits of detection, analytical specificity, and reproducibility—particularly critical for microfluidic platforms claiming improved bioluminescence sensitivity.
Regulatory bodies increasingly recognize the value of real-world evidence and adaptive licensing approaches for innovative technologies. The FDA's Breakthrough Devices Program and Europe's IVDR provisions for innovative devices offer accelerated pathways for technologies demonstrating substantial clinical advantages, which may benefit advanced microfluidic platforms for bioluminescence assays.
Global harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually standardizing requirements across major markets, potentially streamlining the regulatory process for developers targeting multiple regions. However, country-specific requirements remain in emerging markets, requiring tailored regulatory strategies for global commercialization of microfluidic diagnostic platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!