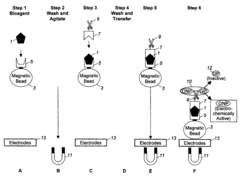
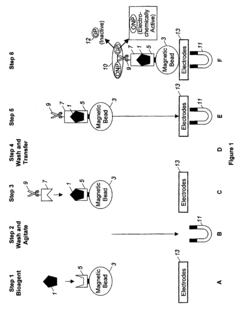
Automated Sample Handling in Microfluidic ELISA Systems
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Automation Background and Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has evolved significantly since its inception in the 1970s, transforming from a manual laboratory technique to an increasingly automated process. The integration of microfluidic technologies with ELISA represents a pivotal advancement in diagnostic capabilities, offering enhanced sensitivity, reduced sample volumes, and accelerated analysis times. This technological convergence has been driven by growing demands for point-of-care testing, personalized medicine, and high-throughput screening applications across healthcare, pharmaceutical research, and environmental monitoring sectors.
The evolution of microfluidic ELISA systems has progressed through several distinct phases: from initial proof-of-concept demonstrations to the development of integrated platforms incorporating multiple analytical steps. Early systems focused primarily on miniaturization of reaction chambers, while contemporary approaches emphasize comprehensive sample-to-answer workflows. Despite these advances, sample handling remains a critical bottleneck in achieving fully automated microfluidic ELISA systems.
Current technological trends indicate a shift toward multifunctional microfluidic platforms that combine sample preparation, reagent delivery, incubation, washing, and detection within unified systems. This integration is increasingly supported by advances in materials science, microfabrication techniques, and control systems that enable precise manipulation of micro-scale fluid volumes. Parallel developments in biosensor technologies and signal amplification methods are further enhancing the analytical capabilities of these systems.
The primary objective of automated sample handling in microfluidic ELISA systems is to eliminate manual intervention throughout the analytical workflow, thereby reducing operator-dependent variability, minimizing contamination risks, and enabling standardized testing protocols. Additional goals include reducing reagent consumption, decreasing analysis time, and facilitating multiplexed assays for simultaneous detection of multiple analytes.
Technical objectives specifically focus on developing robust mechanisms for sample introduction, precise aliquoting, controlled mixing with reagents, and effective washing procedures—all within the constraints of microfluidic architectures. These objectives necessitate innovations in flow control, surface chemistry, and system integration to overcome challenges related to bubble formation, protein adsorption, and cross-contamination.
The advancement of automated sample handling in microfluidic ELISA systems aligns with broader industry trends toward decentralized testing, increased accessibility of diagnostic technologies, and data-driven healthcare approaches. Success in this domain has the potential to transform diagnostic practices across various settings, from sophisticated clinical laboratories to resource-limited environments, by providing reliable, user-friendly analytical platforms with minimal training requirements.
The evolution of microfluidic ELISA systems has progressed through several distinct phases: from initial proof-of-concept demonstrations to the development of integrated platforms incorporating multiple analytical steps. Early systems focused primarily on miniaturization of reaction chambers, while contemporary approaches emphasize comprehensive sample-to-answer workflows. Despite these advances, sample handling remains a critical bottleneck in achieving fully automated microfluidic ELISA systems.
Current technological trends indicate a shift toward multifunctional microfluidic platforms that combine sample preparation, reagent delivery, incubation, washing, and detection within unified systems. This integration is increasingly supported by advances in materials science, microfabrication techniques, and control systems that enable precise manipulation of micro-scale fluid volumes. Parallel developments in biosensor technologies and signal amplification methods are further enhancing the analytical capabilities of these systems.
The primary objective of automated sample handling in microfluidic ELISA systems is to eliminate manual intervention throughout the analytical workflow, thereby reducing operator-dependent variability, minimizing contamination risks, and enabling standardized testing protocols. Additional goals include reducing reagent consumption, decreasing analysis time, and facilitating multiplexed assays for simultaneous detection of multiple analytes.
Technical objectives specifically focus on developing robust mechanisms for sample introduction, precise aliquoting, controlled mixing with reagents, and effective washing procedures—all within the constraints of microfluidic architectures. These objectives necessitate innovations in flow control, surface chemistry, and system integration to overcome challenges related to bubble formation, protein adsorption, and cross-contamination.
The advancement of automated sample handling in microfluidic ELISA systems aligns with broader industry trends toward decentralized testing, increased accessibility of diagnostic technologies, and data-driven healthcare approaches. Success in this domain has the potential to transform diagnostic practices across various settings, from sophisticated clinical laboratories to resource-limited environments, by providing reliable, user-friendly analytical platforms with minimal training requirements.
Market Analysis for Automated Microfluidic Immunoassays
The global market for automated microfluidic immunoassays is experiencing robust growth, driven by increasing demand for rapid, accurate, and cost-effective diagnostic solutions. The market size was valued at approximately $2.3 billion in 2022 and is projected to reach $5.7 billion by 2028, representing a compound annual growth rate (CAGR) of 16.4% during the forecast period.
Healthcare facilities constitute the largest end-user segment, accounting for nearly 45% of the market share. This dominance stems from the rising prevalence of chronic diseases and the growing emphasis on early diagnosis and preventive healthcare. Research institutions represent the second-largest segment, driven by extensive R&D activities in life sciences and pharmaceutical development.
Geographically, North America leads the market with a 38% share, attributed to advanced healthcare infrastructure, substantial R&D investments, and favorable reimbursement policies. Europe follows closely at 30%, while the Asia-Pacific region is emerging as the fastest-growing market with a projected CAGR of 19.2%, fueled by improving healthcare access, increasing healthcare expenditure, and growing awareness about advanced diagnostic technologies.
The COVID-19 pandemic significantly accelerated market growth, highlighting the critical importance of rapid diagnostic capabilities. This catalyzed increased adoption of automated microfluidic immunoassay systems across various healthcare settings, from centralized laboratories to point-of-care facilities.
Key market drivers include technological advancements in microfluidics, increasing incidence of infectious and chronic diseases, growing demand for point-of-care testing, and the shift toward personalized medicine. The integration of artificial intelligence and machine learning with microfluidic platforms is creating new opportunities for enhanced diagnostic accuracy and data analysis capabilities.
However, the market faces challenges such as high initial investment costs, technical complexities in system integration, and regulatory hurdles. The average cost of implementing a fully automated microfluidic ELISA system ranges from $50,000 to $200,000, depending on throughput capacity and level of automation, which may limit adoption in resource-constrained settings.
Consumer trends indicate growing preference for multiplex testing capabilities, reduced sample volume requirements, and faster time-to-result. Systems offering sample-to-answer solutions in under 30 minutes are gaining significant market traction, particularly in emergency care and infectious disease management settings.
Healthcare facilities constitute the largest end-user segment, accounting for nearly 45% of the market share. This dominance stems from the rising prevalence of chronic diseases and the growing emphasis on early diagnosis and preventive healthcare. Research institutions represent the second-largest segment, driven by extensive R&D activities in life sciences and pharmaceutical development.
Geographically, North America leads the market with a 38% share, attributed to advanced healthcare infrastructure, substantial R&D investments, and favorable reimbursement policies. Europe follows closely at 30%, while the Asia-Pacific region is emerging as the fastest-growing market with a projected CAGR of 19.2%, fueled by improving healthcare access, increasing healthcare expenditure, and growing awareness about advanced diagnostic technologies.
The COVID-19 pandemic significantly accelerated market growth, highlighting the critical importance of rapid diagnostic capabilities. This catalyzed increased adoption of automated microfluidic immunoassay systems across various healthcare settings, from centralized laboratories to point-of-care facilities.
Key market drivers include technological advancements in microfluidics, increasing incidence of infectious and chronic diseases, growing demand for point-of-care testing, and the shift toward personalized medicine. The integration of artificial intelligence and machine learning with microfluidic platforms is creating new opportunities for enhanced diagnostic accuracy and data analysis capabilities.
However, the market faces challenges such as high initial investment costs, technical complexities in system integration, and regulatory hurdles. The average cost of implementing a fully automated microfluidic ELISA system ranges from $50,000 to $200,000, depending on throughput capacity and level of automation, which may limit adoption in resource-constrained settings.
Consumer trends indicate growing preference for multiplex testing capabilities, reduced sample volume requirements, and faster time-to-result. Systems offering sample-to-answer solutions in under 30 minutes are gaining significant market traction, particularly in emergency care and infectious disease management settings.
Technical Challenges in Microfluidic Sample Handling
Microfluidic ELISA systems face several significant technical challenges in sample handling that impede their widespread adoption in clinical and research settings. The miniaturization of conventional ELISA processes introduces complex fluid dynamics considerations that do not exist at macro scales. Surface tension, capillary forces, and laminar flow dominate at the microscale, requiring precise control mechanisms to ensure accurate sample movement and processing.
One primary challenge is achieving consistent and precise sample introduction into microfluidic channels. Traditional manual pipetting introduces variability and potential contamination, while automated systems must overcome issues related to bubble formation, which can disrupt flow patterns and affect assay reliability. Even minor air bubbles can block channels or chambers, causing test failures or erroneous results.
Sample volume management presents another significant hurdle. Microfluidic ELISA typically operates with nanoliter to microliter volumes, requiring extremely precise metering systems. The evaporation of these minute volumes becomes problematic during extended incubation periods, potentially altering sample concentration and affecting assay sensitivity and specificity.
Cross-contamination between samples represents a critical concern in automated systems. Without proper channel cleaning or the implementation of disposable components, residual material from previous samples may contaminate subsequent tests. This is particularly challenging when designing systems for sequential or high-throughput testing.
The integration of multiple sample preparation steps poses considerable engineering challenges. Conventional ELISA requires numerous washing steps, reagent additions, and incubation periods. Translating these processes to an automated microfluidic format demands sophisticated valve systems, pumping mechanisms, and timing controls that must function reliably at microscale dimensions.
Material compatibility issues further complicate sample handling. Biomolecules may adsorb to channel surfaces, reducing assay sensitivity. Additionally, certain polymers used in microfluidic fabrication can leach compounds that interfere with enzymatic reactions or fluorescence detection. Finding materials that are both manufacturable and biologically inert remains challenging.
Temperature control represents another technical obstacle. Enzymatic reactions in ELISA are temperature-sensitive, yet achieving uniform heating across microfluidic channels is difficult due to thermal gradients. Integrating effective heating elements while maintaining the compact nature of microfluidic devices requires innovative engineering approaches.
Finally, interfacing microfluidic systems with external equipment for sample loading and result detection presents significant design challenges. Creating user-friendly systems that maintain the benefits of microfluidics while allowing seamless integration with existing laboratory workflows requires careful consideration of both technical and human factors engineering principles.
One primary challenge is achieving consistent and precise sample introduction into microfluidic channels. Traditional manual pipetting introduces variability and potential contamination, while automated systems must overcome issues related to bubble formation, which can disrupt flow patterns and affect assay reliability. Even minor air bubbles can block channels or chambers, causing test failures or erroneous results.
Sample volume management presents another significant hurdle. Microfluidic ELISA typically operates with nanoliter to microliter volumes, requiring extremely precise metering systems. The evaporation of these minute volumes becomes problematic during extended incubation periods, potentially altering sample concentration and affecting assay sensitivity and specificity.
Cross-contamination between samples represents a critical concern in automated systems. Without proper channel cleaning or the implementation of disposable components, residual material from previous samples may contaminate subsequent tests. This is particularly challenging when designing systems for sequential or high-throughput testing.
The integration of multiple sample preparation steps poses considerable engineering challenges. Conventional ELISA requires numerous washing steps, reagent additions, and incubation periods. Translating these processes to an automated microfluidic format demands sophisticated valve systems, pumping mechanisms, and timing controls that must function reliably at microscale dimensions.
Material compatibility issues further complicate sample handling. Biomolecules may adsorb to channel surfaces, reducing assay sensitivity. Additionally, certain polymers used in microfluidic fabrication can leach compounds that interfere with enzymatic reactions or fluorescence detection. Finding materials that are both manufacturable and biologically inert remains challenging.
Temperature control represents another technical obstacle. Enzymatic reactions in ELISA are temperature-sensitive, yet achieving uniform heating across microfluidic channels is difficult due to thermal gradients. Integrating effective heating elements while maintaining the compact nature of microfluidic devices requires innovative engineering approaches.
Finally, interfacing microfluidic systems with external equipment for sample loading and result detection presents significant design challenges. Creating user-friendly systems that maintain the benefits of microfluidics while allowing seamless integration with existing laboratory workflows requires careful consideration of both technical and human factors engineering principles.
Current Automated Sample Handling Solutions
01 Microfluidic chip designs for ELISA sample handling
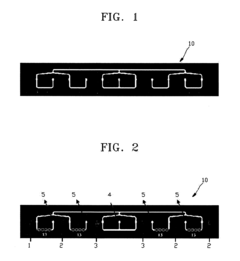
Various microfluidic chip designs have been developed specifically for ELISA applications, featuring channels, chambers, and reservoirs optimized for sample handling. These designs incorporate structures that facilitate efficient mixing, incubation, and washing steps required in ELISA protocols. The chips are often fabricated using materials like PDMS, glass, or polymers that provide optical transparency for detection and biocompatibility for biological samples.- Microfluidic channel designs for ELISA sample handling: Specialized microfluidic channel designs can enhance ELISA sample handling efficiency. These designs include serpentine channels, parallel processing lanes, and gradient generators that control sample flow rates and residence times. The optimized geometries enable precise control over sample volumes, reduce reagent consumption, and improve mixing efficiency, leading to more sensitive and reproducible ELISA results.
- Automated sample loading and processing systems: Automated systems for microfluidic ELISA incorporate robotic sample loading, programmable fluid handling, and sequential processing steps. These systems can manage multiple samples simultaneously while maintaining precise control over incubation times and washing procedures. The automation reduces human error, increases throughput, and enables standardized protocols for consistent results across large sample sets.
- Integration of sample preparation with detection: Integrated microfluidic ELISA platforms combine sample preparation steps with detection mechanisms in a single device. These systems incorporate on-chip sample filtration, cell lysis, protein extraction, and antibody binding within connected microchannels. The integration minimizes sample transfer steps, reduces contamination risks, and enables rapid analysis from raw samples to quantitative results.
- Novel surface treatments for improved sample interaction: Surface modifications within microfluidic ELISA systems enhance protein binding and reduce non-specific interactions. Techniques include plasma treatment, chemical functionalization, and biomolecule immobilization strategies that optimize antibody orientation and density. These treatments improve signal-to-noise ratios, increase detection sensitivity, and enable more efficient sample handling with smaller volumes.
- Portable and point-of-care microfluidic ELISA systems: Portable microfluidic ELISA platforms enable point-of-care testing with simplified sample handling requirements. These systems incorporate miniaturized pumps, valves, and detection components that can operate with minimal external equipment. The designs focus on user-friendly interfaces, stable reagent storage, and robust sample processing that can function in resource-limited settings while maintaining analytical performance comparable to laboratory-based systems.
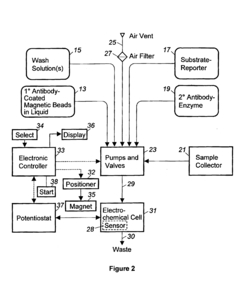
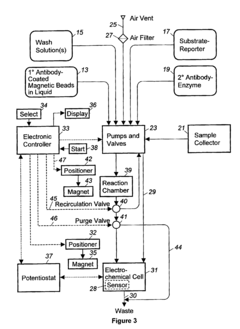
02 Automated sample handling and processing systems
Automated systems for microfluidic ELISA integrate sample preparation, reagent dispensing, incubation, washing, and detection into a single platform. These systems utilize precise fluid control mechanisms such as micropumps, microvalves, and pressure-driven flow to manipulate small sample volumes with high accuracy. Automation reduces human error, increases throughput, and improves reproducibility in ELISA assays while minimizing sample and reagent consumption.Expand Specific Solutions03 Sample preparation and pre-treatment techniques
Specialized techniques for sample preparation and pre-treatment in microfluidic ELISA systems include on-chip filtration, cell lysis, protein extraction, and sample concentration. These integrated preparation steps help to isolate target analytes from complex biological matrices such as blood, saliva, or tissue samples. The pre-treatment processes are designed to be compatible with downstream ELISA steps while maintaining the integrity of target biomarkers.Expand Specific Solutions04 Multiplexed sample analysis capabilities
Microfluidic ELISA platforms with multiplexed capabilities allow for simultaneous analysis of multiple samples or detection of multiple analytes in a single sample. These systems incorporate parallel processing channels, array-based detection zones, or droplet-based compartmentalization to achieve high-throughput analysis. Multiplexed designs maximize analytical efficiency while maintaining sensitivity and specificity comparable to traditional ELISA methods.Expand Specific Solutions05 Integration of detection systems with sample handling
Advanced microfluidic ELISA platforms integrate optical, electrochemical, or other detection systems directly with sample handling components. These integrated systems feature detection zones positioned strategically within the microfluidic path to enable real-time monitoring or endpoint measurements. The integration minimizes sample transfer steps, reduces signal loss, and enables more sensitive detection of analytes even at low concentrations in small sample volumes.Expand Specific Solutions
Leading Companies in Microfluidic Automation
The automated sample handling in microfluidic ELISA systems market is currently in a growth phase, with increasing adoption across clinical diagnostics and research applications. The global market size is expanding rapidly, driven by demand for point-of-care testing and lab automation. Leading players include established healthcare giants like Roche Diagnostics and Hologic, alongside specialized microfluidic technology companies such as Biomeme and Novel Microdevices. Samsung Electronics and Robert Bosch bring significant engineering capabilities, while academic institutions like Beijing University of Chemical Technology contribute research innovations. The technology is approaching maturity in standardized applications but remains in development for more complex automated workflows, with companies like 908 Devices and Fialab Instruments advancing miniaturization and integration capabilities for next-generation systems.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed an integrated microfluidic ELISA platform called cobas® that incorporates automated sample handling through a combination of centrifugal forces and capillary action. Their system utilizes a disc-based microfluidic cartridge where samples are automatically distributed to multiple reaction chambers through rotation-induced fluid movement. The platform incorporates pre-loaded reagents in sealed compartments that are automatically released in sequence during the assay process. Roche's technology employs precision microvalves and hydrophobic/hydrophilic surface treatments to control fluid movement without external pumps. The system also features integrated optical detection components that allow for real-time monitoring of the ELISA reactions. Their automated sample preparation includes cell lysis, protein extraction, and washing steps all contained within the microfluidic circuit, minimizing human intervention and contamination risks[1][3].
Strengths: High throughput capability with multiplexed testing, excellent reproducibility due to minimal human handling, and seamless integration with laboratory information systems. Weaknesses: Relatively high cost of instrumentation, proprietary consumables that limit customization, and larger footprint compared to some competing portable systems.
Fialab Instruments, Inc.
Technical Solution: Fialab has developed the Flow Injection Analysis (FIA) approach for automated microfluidic ELISA systems. Their technology utilizes sequential injection analysis principles where precise volumes of samples and reagents are aspirated into a holding coil and then propelled through a microfluidic reaction chamber. The system employs a multi-position valve that allows for automated selection between different reagents and washing solutions without cross-contamination. Fialab's microfluidic cartridges incorporate functionalized surfaces where capture antibodies are immobilized in defined regions, enabling spatial multiplexing of different assays. Their automated sample handling includes in-line dilution capabilities, where samples can be automatically prepared at various concentrations before analysis. The platform features integrated optical detection with LED light sources and photodiode arrays for quantitative measurement of ELISA endpoints. Fialab's systems also incorporate automated calibration procedures where standard curves are generated within each analytical run to ensure accurate quantification[4][6].
Strengths: Low reagent consumption (typically 50-200 μL per test), rapid analysis times (2-5 minutes per sample), and excellent reproducibility with minimal carryover between samples. Weaknesses: Limited throughput compared to plate-based systems, more complex fluidic control requirements, and challenges with highly viscous samples.
Key Patents in Microfluidic Sample Manipulation
Automated enzyme-linked immunosorbent assay device with ONP-GP
PatentInactiveUS6933143B2
Innovation
- The use of o-nitrophenyl beta-D-galactopyranoside (ONP-GP) as a substrate, which is cleaved by the enzyme at a faster rate than p-nitrophenyl beta-D-galactopyranoside, reducing the duration of the ELISA test and enabling quicker production of test results through an automated system controlled by a microcontroller that sequences fluid pumping and positions the reporter carrier adjacent to the sensor.
Method and automated fluidic system for detecting protein in biological sample
PatentInactiveUS20040152200A1
Innovation
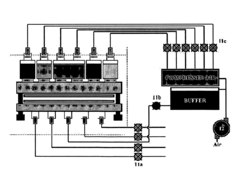
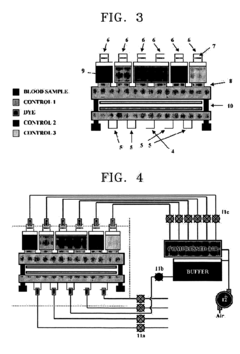
- An automated microfluidic system that includes a cartridge with reservoirs for samples and control solutions, a microfluidic channel with immobilized antibodies, and a reader for measuring antigen-antibody reactions, which automates sample injection, washing, and detection using compressed air and buffer solutions, eliminating the need for manual manipulation.
Integration with Lab-on-a-Chip Technologies
The integration of automated sample handling systems with Lab-on-a-Chip (LOC) technologies represents a significant advancement in microfluidic ELISA platforms. This convergence creates comprehensive analytical systems capable of performing complex immunoassays with minimal human intervention, enhancing both efficiency and reliability.
Lab-on-a-Chip technologies fundamentally transform traditional laboratory processes by miniaturizing and integrating multiple analytical functions onto a single chip platform. When combined with automated sample handling in microfluidic ELISA systems, these technologies enable seamless sample preparation, reagent mixing, incubation, washing, and detection—all within a compact footprint.
Key integration approaches include modular designs where automated sample handling components interface with standardized microfluidic cartridges. These systems typically incorporate programmable pumping mechanisms, precision valve controls, and sophisticated flow management to ensure accurate sample delivery to reaction chambers. Advanced implementations feature integrated temperature control zones, allowing for optimized reaction kinetics during critical ELISA steps.
The integration challenges primarily revolve around interface standardization between automated handlers and microfluidic chips. Researchers have developed various solutions including standardized fluidic connectors, alignment mechanisms, and universal docking stations that facilitate reliable connections while maintaining system integrity. Material compatibility between handling components and chip substrates presents another critical consideration, particularly when biological samples and specialized reagents are involved.
Recent technological breakthroughs have introduced digital microfluidics into these integrated systems, employing electrowetting principles for droplet manipulation without mechanical pumps. This approach offers unprecedented flexibility in sample routing and reaction sequencing, further enhancing automation capabilities within LOC platforms.
Commercial systems demonstrating successful integration include platforms from companies like Fluidigm, Abbott Point-of-Care, and Illumina, which have developed integrated solutions for specific diagnostic applications. These systems showcase the practical implementation of automated sample handling within microfluidic ELISA frameworks, providing valuable benchmarks for future development efforts.
The integration pathway typically progresses from proof-of-concept demonstrations to fully validated systems through iterative optimization. Successful integration strategies emphasize scalable manufacturing approaches, user-friendly interfaces, and robust operation under varied environmental conditions—factors essential for widespread adoption in clinical and research settings.
Lab-on-a-Chip technologies fundamentally transform traditional laboratory processes by miniaturizing and integrating multiple analytical functions onto a single chip platform. When combined with automated sample handling in microfluidic ELISA systems, these technologies enable seamless sample preparation, reagent mixing, incubation, washing, and detection—all within a compact footprint.
Key integration approaches include modular designs where automated sample handling components interface with standardized microfluidic cartridges. These systems typically incorporate programmable pumping mechanisms, precision valve controls, and sophisticated flow management to ensure accurate sample delivery to reaction chambers. Advanced implementations feature integrated temperature control zones, allowing for optimized reaction kinetics during critical ELISA steps.
The integration challenges primarily revolve around interface standardization between automated handlers and microfluidic chips. Researchers have developed various solutions including standardized fluidic connectors, alignment mechanisms, and universal docking stations that facilitate reliable connections while maintaining system integrity. Material compatibility between handling components and chip substrates presents another critical consideration, particularly when biological samples and specialized reagents are involved.
Recent technological breakthroughs have introduced digital microfluidics into these integrated systems, employing electrowetting principles for droplet manipulation without mechanical pumps. This approach offers unprecedented flexibility in sample routing and reaction sequencing, further enhancing automation capabilities within LOC platforms.
Commercial systems demonstrating successful integration include platforms from companies like Fluidigm, Abbott Point-of-Care, and Illumina, which have developed integrated solutions for specific diagnostic applications. These systems showcase the practical implementation of automated sample handling within microfluidic ELISA frameworks, providing valuable benchmarks for future development efforts.
The integration pathway typically progresses from proof-of-concept demonstrations to fully validated systems through iterative optimization. Successful integration strategies emphasize scalable manufacturing approaches, user-friendly interfaces, and robust operation under varied environmental conditions—factors essential for widespread adoption in clinical and research settings.
Standardization and Quality Control Considerations
Standardization and quality control represent critical challenges in automated microfluidic ELISA systems, particularly as these platforms transition from research environments to clinical applications. The miniaturization and integration of multiple analytical steps within microfluidic devices necessitate robust standardization protocols to ensure reproducibility and reliability across different laboratories and manufacturing batches.
Current standardization efforts focus on establishing reference materials and calibration standards specifically designed for microfluidic platforms. These standards must account for the unique fluid dynamics and surface interactions that occur at the microscale level. Organizations such as NIST (National Institute of Standards and Technology) and ISO (International Organization for Standardization) have begun developing guidelines for microfluidic device characterization, though comprehensive standards for automated ELISA systems remain under development.
Quality control in automated sample handling requires multi-level monitoring approaches. At the component level, manufacturers must implement stringent quality assurance for microfluidic chips, pumps, valves, and detection systems. Statistical process control methods adapted for microfluidic manufacturing help maintain consistent device performance across production batches. Dimensional tolerances for microchannels typically must be maintained within ±2-5% to ensure reproducible fluid behavior.
Real-time quality monitoring during operation presents unique challenges in microfluidic systems. Advanced systems incorporate internal control channels that run parallel to sample processing pathways, allowing continuous verification of fluid movement, mixing efficiency, and reaction kinetics. Optical monitoring of flow patterns and droplet formation provides valuable data for quality assessment during operation, with machine learning algorithms increasingly employed to detect anomalies in system performance.
Validation protocols for automated microfluidic ELISA systems must address both analytical performance and operational reliability. This includes assessment of precision (CV typically <5% for intra-assay variation), accuracy (recovery rates of 90-110% compared to reference methods), and robustness under varying environmental conditions. Stability testing must verify consistent performance across the shelf-life of pre-loaded reagents, particularly important for point-of-care applications.
Regulatory considerations further complicate standardization efforts. The FDA's approach to microfluidic diagnostic devices continues to evolve, with recent guidance documents addressing aspects of validation for novel microfluidic technologies. The European Union's IVDR (In Vitro Diagnostic Regulation) implementation has created additional requirements for technical documentation and performance evaluation of automated diagnostic systems, including those utilizing microfluidic technologies.
Current standardization efforts focus on establishing reference materials and calibration standards specifically designed for microfluidic platforms. These standards must account for the unique fluid dynamics and surface interactions that occur at the microscale level. Organizations such as NIST (National Institute of Standards and Technology) and ISO (International Organization for Standardization) have begun developing guidelines for microfluidic device characterization, though comprehensive standards for automated ELISA systems remain under development.
Quality control in automated sample handling requires multi-level monitoring approaches. At the component level, manufacturers must implement stringent quality assurance for microfluidic chips, pumps, valves, and detection systems. Statistical process control methods adapted for microfluidic manufacturing help maintain consistent device performance across production batches. Dimensional tolerances for microchannels typically must be maintained within ±2-5% to ensure reproducible fluid behavior.
Real-time quality monitoring during operation presents unique challenges in microfluidic systems. Advanced systems incorporate internal control channels that run parallel to sample processing pathways, allowing continuous verification of fluid movement, mixing efficiency, and reaction kinetics. Optical monitoring of flow patterns and droplet formation provides valuable data for quality assessment during operation, with machine learning algorithms increasingly employed to detect anomalies in system performance.
Validation protocols for automated microfluidic ELISA systems must address both analytical performance and operational reliability. This includes assessment of precision (CV typically <5% for intra-assay variation), accuracy (recovery rates of 90-110% compared to reference methods), and robustness under varying environmental conditions. Stability testing must verify consistent performance across the shelf-life of pre-loaded reagents, particularly important for point-of-care applications.
Regulatory considerations further complicate standardization efforts. The FDA's approach to microfluidic diagnostic devices continues to evolve, with recent guidance documents addressing aspects of validation for novel microfluidic technologies. The European Union's IVDR (In Vitro Diagnostic Regulation) implementation has created additional requirements for technical documentation and performance evaluation of automated diagnostic systems, including those utilizing microfluidic technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!