Microfluidic ELISA for Early Sepsis Biomarker Detection
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sepsis Biomarker Detection Background and Objectives
Sepsis represents a life-threatening condition characterized by a dysregulated host response to infection, leading to organ dysfunction and high mortality rates. Early detection remains crucial for effective intervention, with mortality increasing by approximately 8% for each hour of delayed treatment. Traditional diagnostic methods often lack the sensitivity and specificity required for early intervention, creating an urgent need for innovative detection technologies.
The evolution of sepsis biomarker research has progressed significantly over the past decades. Initially focused on cytokines like TNF-α and IL-6, the field has expanded to include procalcitonin (PCT), C-reactive protein (CRP), and more recently, promising markers such as presepsin, soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), and various microRNAs. Each generation of biomarkers has offered incremental improvements in diagnostic accuracy, yet challenges in sensitivity and specificity persist.
Microfluidic technologies have emerged as a transformative approach in biomedical diagnostics, offering miniaturization, reduced sample volumes, faster reaction kinetics, and potential for point-of-care applications. The integration of microfluidics with Enzyme-Linked Immunosorbent Assay (ELISA) represents a significant advancement, combining the sensitivity of traditional ELISA with the efficiency and portability of microfluidic platforms.
The global burden of sepsis remains substantial, with an estimated 48.9 million cases and 11 million sepsis-related deaths worldwide annually. The economic impact is equally significant, with sepsis care in the United States alone costing approximately $24 billion annually. These statistics underscore the critical need for improved diagnostic tools.
The primary objective of microfluidic ELISA development for sepsis biomarker detection is to create a rapid, sensitive, and specific diagnostic platform capable of detecting sepsis biomarkers at clinically relevant concentrations during the disease's earliest stages. This technology aims to reduce the time-to-result from hours to minutes while maintaining or exceeding the analytical performance of conventional laboratory methods.
Secondary objectives include developing multiplexed detection capabilities for simultaneous analysis of multiple biomarkers, enhancing the system's predictive value through biomarker panels, and creating a user-friendly platform suitable for point-of-care implementation in various healthcare settings, including resource-limited environments.
The successful development of microfluidic ELISA for sepsis biomarker detection could revolutionize sepsis management by enabling earlier intervention, facilitating more precise therapeutic decisions, and ultimately improving patient outcomes while reducing healthcare costs associated with prolonged intensive care and complications of advanced sepsis.
The evolution of sepsis biomarker research has progressed significantly over the past decades. Initially focused on cytokines like TNF-α and IL-6, the field has expanded to include procalcitonin (PCT), C-reactive protein (CRP), and more recently, promising markers such as presepsin, soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), and various microRNAs. Each generation of biomarkers has offered incremental improvements in diagnostic accuracy, yet challenges in sensitivity and specificity persist.
Microfluidic technologies have emerged as a transformative approach in biomedical diagnostics, offering miniaturization, reduced sample volumes, faster reaction kinetics, and potential for point-of-care applications. The integration of microfluidics with Enzyme-Linked Immunosorbent Assay (ELISA) represents a significant advancement, combining the sensitivity of traditional ELISA with the efficiency and portability of microfluidic platforms.
The global burden of sepsis remains substantial, with an estimated 48.9 million cases and 11 million sepsis-related deaths worldwide annually. The economic impact is equally significant, with sepsis care in the United States alone costing approximately $24 billion annually. These statistics underscore the critical need for improved diagnostic tools.
The primary objective of microfluidic ELISA development for sepsis biomarker detection is to create a rapid, sensitive, and specific diagnostic platform capable of detecting sepsis biomarkers at clinically relevant concentrations during the disease's earliest stages. This technology aims to reduce the time-to-result from hours to minutes while maintaining or exceeding the analytical performance of conventional laboratory methods.
Secondary objectives include developing multiplexed detection capabilities for simultaneous analysis of multiple biomarkers, enhancing the system's predictive value through biomarker panels, and creating a user-friendly platform suitable for point-of-care implementation in various healthcare settings, including resource-limited environments.
The successful development of microfluidic ELISA for sepsis biomarker detection could revolutionize sepsis management by enabling earlier intervention, facilitating more precise therapeutic decisions, and ultimately improving patient outcomes while reducing healthcare costs associated with prolonged intensive care and complications of advanced sepsis.
Market Analysis for Point-of-Care Sepsis Diagnostics
The global market for point-of-care (POC) sepsis diagnostics is experiencing significant growth, driven by the critical need for early detection and treatment of sepsis. Currently valued at approximately $650 million, this market segment is projected to reach $1.2 billion by 2027, representing a compound annual growth rate (CAGR) of 8.5%. This growth trajectory is supported by increasing awareness of sepsis as a medical emergency and the substantial economic burden it places on healthcare systems worldwide.
North America dominates the market with a 42% share, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 8% is distributed across other regions. This regional distribution reflects differences in healthcare infrastructure, reimbursement policies, and clinical practice guidelines. The United States specifically represents the largest single-country market, accounting for approximately 35% of global demand.
Hospital emergency departments constitute the primary end-user segment (45%), followed by intensive care units (30%), and other clinical settings (25%). This distribution highlights the critical importance of rapid diagnosis in emergency and critical care environments where time-to-treatment directly impacts patient outcomes.
Key market drivers include the rising incidence of sepsis globally, growing geriatric population with increased susceptibility to infections, and heightened awareness among healthcare providers about the importance of early sepsis detection. Additionally, favorable reimbursement policies in developed markets and increasing healthcare expenditure in emerging economies are contributing to market expansion.
The demand for microfluidic ELISA-based sepsis biomarker detection systems is particularly strong due to their potential to significantly reduce diagnosis time from hours to minutes. Healthcare providers are increasingly recognizing the value proposition of these systems, which can potentially reduce sepsis-related mortality by 25-30% through earlier intervention.
Market challenges include high initial implementation costs, regulatory hurdles for novel diagnostic technologies, and the need for clinical validation across diverse patient populations. The average cost per test remains a significant barrier to widespread adoption, especially in resource-limited settings.
Customer preferences are evolving toward integrated systems that offer multiplexed biomarker detection, electronic health record integration, and decision support algorithms. There is also growing demand for systems that can be operated by personnel with minimal specialized training, particularly in emergency settings where laboratory technicians may not be immediately available.
The competitive landscape features both established diagnostic companies and innovative startups. Market concentration is moderate, with the top five players controlling approximately 65% of the market share. Recent strategic partnerships between technology developers and healthcare providers indicate a trend toward collaborative approaches to market penetration and clinical validation.
North America dominates the market with a 42% share, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 8% is distributed across other regions. This regional distribution reflects differences in healthcare infrastructure, reimbursement policies, and clinical practice guidelines. The United States specifically represents the largest single-country market, accounting for approximately 35% of global demand.
Hospital emergency departments constitute the primary end-user segment (45%), followed by intensive care units (30%), and other clinical settings (25%). This distribution highlights the critical importance of rapid diagnosis in emergency and critical care environments where time-to-treatment directly impacts patient outcomes.
Key market drivers include the rising incidence of sepsis globally, growing geriatric population with increased susceptibility to infections, and heightened awareness among healthcare providers about the importance of early sepsis detection. Additionally, favorable reimbursement policies in developed markets and increasing healthcare expenditure in emerging economies are contributing to market expansion.
The demand for microfluidic ELISA-based sepsis biomarker detection systems is particularly strong due to their potential to significantly reduce diagnosis time from hours to minutes. Healthcare providers are increasingly recognizing the value proposition of these systems, which can potentially reduce sepsis-related mortality by 25-30% through earlier intervention.
Market challenges include high initial implementation costs, regulatory hurdles for novel diagnostic technologies, and the need for clinical validation across diverse patient populations. The average cost per test remains a significant barrier to widespread adoption, especially in resource-limited settings.
Customer preferences are evolving toward integrated systems that offer multiplexed biomarker detection, electronic health record integration, and decision support algorithms. There is also growing demand for systems that can be operated by personnel with minimal specialized training, particularly in emergency settings where laboratory technicians may not be immediately available.
The competitive landscape features both established diagnostic companies and innovative startups. Market concentration is moderate, with the top five players controlling approximately 65% of the market share. Recent strategic partnerships between technology developers and healthcare providers indicate a trend toward collaborative approaches to market penetration and clinical validation.
Microfluidic ELISA Technology Landscape and Barriers
The global landscape of microfluidic ELISA technology for sepsis biomarker detection has evolved significantly over the past decade, with research centers across North America, Europe, and Asia contributing to advancements. The United States leads in innovation with major research clusters at MIT, Stanford, and Harvard developing novel microfluidic platforms. European contributions are centered in Germany, Switzerland, and the UK, focusing on clinical validation and standardization protocols. Asian research, particularly from China, Japan, and Singapore, has emphasized cost-effective manufacturing and point-of-care applications.
Despite these advancements, significant technical barriers persist in microfluidic ELISA implementation for early sepsis detection. Surface chemistry optimization remains challenging, as protein adsorption and biofouling can compromise assay sensitivity and reproducibility. Current surface modification techniques often fail to maintain stability under clinical sample conditions, leading to inconsistent results when detecting low-abundance sepsis biomarkers like procalcitonin and interleukins.
Fluid handling presents another major obstacle, particularly in achieving precise control over nanoliter volumes required for high-sensitivity detection. Capillary forces, evaporation effects, and bubble formation frequently disrupt flow patterns in microchannels, affecting reaction kinetics and quantification accuracy. These issues become more pronounced when dealing with complex biological fluids like whole blood or serum from sepsis patients.
Integration challenges between microfluidic components and detection systems constitute a significant barrier. While individual microfluidic elements may function well in isolation, creating fully integrated systems that maintain sensitivity while enabling automation remains difficult. The interface between sample preparation modules and detection chambers often introduces contamination risks and signal loss, particularly problematic for early-stage sepsis biomarkers present at picogram/mL concentrations.
Manufacturing scalability represents a persistent limitation, with most current microfluidic ELISA platforms remaining in laboratory prototype stages. The transition from PDMS-based research devices to mass-producible materials like thermoplastics introduces performance variations that affect assay reliability. Additionally, quality control processes for microfluidic channels at commercial scale have not been fully standardized, leading to device-to-device variability.
Regulatory hurdles further complicate technology adoption, with FDA and equivalent international bodies requiring extensive validation data for diagnostic applications. The lack of standardized performance metrics specifically for microfluidic immunoassays creates uncertainty in approval pathways. This regulatory landscape has slowed clinical translation, with most technologies remaining in research or pre-clinical validation phases despite their potential for revolutionizing early sepsis detection.
Despite these advancements, significant technical barriers persist in microfluidic ELISA implementation for early sepsis detection. Surface chemistry optimization remains challenging, as protein adsorption and biofouling can compromise assay sensitivity and reproducibility. Current surface modification techniques often fail to maintain stability under clinical sample conditions, leading to inconsistent results when detecting low-abundance sepsis biomarkers like procalcitonin and interleukins.
Fluid handling presents another major obstacle, particularly in achieving precise control over nanoliter volumes required for high-sensitivity detection. Capillary forces, evaporation effects, and bubble formation frequently disrupt flow patterns in microchannels, affecting reaction kinetics and quantification accuracy. These issues become more pronounced when dealing with complex biological fluids like whole blood or serum from sepsis patients.
Integration challenges between microfluidic components and detection systems constitute a significant barrier. While individual microfluidic elements may function well in isolation, creating fully integrated systems that maintain sensitivity while enabling automation remains difficult. The interface between sample preparation modules and detection chambers often introduces contamination risks and signal loss, particularly problematic for early-stage sepsis biomarkers present at picogram/mL concentrations.
Manufacturing scalability represents a persistent limitation, with most current microfluidic ELISA platforms remaining in laboratory prototype stages. The transition from PDMS-based research devices to mass-producible materials like thermoplastics introduces performance variations that affect assay reliability. Additionally, quality control processes for microfluidic channels at commercial scale have not been fully standardized, leading to device-to-device variability.
Regulatory hurdles further complicate technology adoption, with FDA and equivalent international bodies requiring extensive validation data for diagnostic applications. The lack of standardized performance metrics specifically for microfluidic immunoassays creates uncertainty in approval pathways. This regulatory landscape has slowed clinical translation, with most technologies remaining in research or pre-clinical validation phases despite their potential for revolutionizing early sepsis detection.
Current Microfluidic ELISA Implementation Approaches
01 Microfluidic chip designs for ELISA-based early detection
Various microfluidic chip designs have been developed specifically for ELISA-based early detection of diseases. These designs incorporate channels, chambers, and reaction zones optimized for sample handling, reagent mixing, and signal detection. The miniaturized platforms enable rapid analysis with minimal sample volumes while maintaining or improving sensitivity compared to conventional ELISA methods. These chip designs often include integrated components for sample preparation, incubation, washing, and detection steps.- Microfluidic chip designs for ELISA-based early detection: Various microfluidic chip designs have been developed specifically for ELISA-based early detection of diseases. These designs incorporate channels, chambers, and reaction zones optimized for sample processing, reagent mixing, and signal detection. The miniaturized platforms enable faster analysis times, reduced sample volumes, and improved sensitivity compared to conventional ELISA methods, making them suitable for point-of-care diagnostics and early disease screening.
- Integration of detection technologies with microfluidic ELISA: Advanced detection technologies have been integrated with microfluidic ELISA platforms to enhance sensitivity and enable early detection of biomarkers. These include optical detection methods, electrochemical sensors, fluorescence-based detection, and colorimetric analysis. The integration of these technologies with microfluidic systems allows for real-time monitoring of ELISA reactions and detection of biomarkers at very low concentrations, facilitating early disease diagnosis.
- Automated sample processing for high-throughput screening: Automated sample processing systems have been developed for microfluidic ELISA platforms to enable high-throughput screening for early disease detection. These systems incorporate automated sample loading, reagent dispensing, incubation, washing, and detection steps. The automation reduces human error, increases reproducibility, and allows for the processing of multiple samples simultaneously, making these platforms suitable for large-scale screening programs and early detection initiatives.
- Novel biomarker detection methods for early disease diagnosis: Innovative approaches for detecting disease-specific biomarkers have been implemented in microfluidic ELISA systems for early diagnosis. These methods include multiplexed detection of multiple biomarkers, amplification techniques to enhance signal strength, and specialized antibody configurations to improve specificity. The ability to detect disease-specific biomarkers at very early stages of disease progression enables timely intervention and improved patient outcomes.
- Portable and point-of-care microfluidic ELISA devices: Portable and point-of-care microfluidic ELISA devices have been developed for early detection in resource-limited settings. These devices are designed to be compact, user-friendly, and operate with minimal external equipment. Some incorporate smartphone-based detection systems, battery-powered components, and simplified user interfaces. The portability and ease of use enable early detection of diseases in field settings, remote locations, and primary healthcare facilities where traditional laboratory infrastructure may be unavailable.
02 Integration of detection technologies with microfluidic ELISA
Advanced detection technologies have been integrated with microfluidic ELISA platforms to enhance sensitivity and enable early detection of biomarkers. These include optical detection methods (fluorescence, chemiluminescence), electrochemical sensors, and colorimetric detection systems. The integration allows for real-time monitoring of ELISA reactions and quantification of target analytes at lower concentrations than traditional methods, facilitating earlier disease detection when biomarker levels are still low.Expand Specific Solutions03 Automated microfluidic ELISA systems for point-of-care applications
Automated microfluidic ELISA systems have been developed for point-of-care applications, enabling early detection outside of laboratory settings. These systems incorporate pumps, valves, and control mechanisms to automate sample processing, reagent delivery, and waste handling. The automation reduces user intervention, minimizes human error, and standardizes testing procedures, making early detection more accessible in resource-limited settings or for rapid screening programs.Expand Specific Solutions04 Novel biomarker detection strategies using microfluidic ELISA
Innovative approaches for detecting disease-specific biomarkers using microfluidic ELISA have been developed for early diagnosis. These strategies include multiplexed detection of multiple biomarkers simultaneously, signal amplification techniques to detect ultra-low concentrations of biomarkers, and specialized antibody configurations to improve specificity. The ability to detect panels of biomarkers or identify novel biomarkers at early disease stages significantly improves diagnostic capabilities and enables earlier intervention.Expand Specific Solutions05 Sample preparation and processing techniques for microfluidic ELISA
Advanced sample preparation and processing techniques have been developed specifically for microfluidic ELISA platforms to enhance early detection capabilities. These include on-chip sample filtration, cell separation, plasma extraction from whole blood, and pre-concentration of target analytes. These techniques improve the quality of samples for analysis, remove potential interferents, and concentrate biomarkers to detectable levels, thereby increasing the sensitivity and reliability of early detection assays.Expand Specific Solutions
Leading Companies in Microfluidic Diagnostic Platforms
The microfluidic ELISA for early sepsis biomarker detection market is in its growth phase, characterized by increasing adoption of point-of-care diagnostics and rising sepsis awareness. The global market is projected to reach approximately $1.5 billion by 2027, driven by the critical need for rapid sepsis diagnosis. Major players like Becton, Dickinson & Co., F. Hoffmann-La Roche, and Hologic are advancing the technology's maturity through significant R&D investments. Academic institutions including Institut Pasteur, McMaster University, and Chongqing Medical University are collaborating with industry leaders like Magnolia Medical Technologies and Biocartis to develop more sensitive, rapid, and cost-effective microfluidic platforms. The competitive landscape features established diagnostic companies expanding their portfolios alongside innovative startups focusing on novel microfluidic approaches for early sepsis detection.
Becton, Dickinson & Co.
Technical Solution: BD has developed an advanced microfluidic ELISA platform for early sepsis detection that integrates their proprietary microfluidic technology with high-sensitivity immunoassays. Their system utilizes a lab-on-a-chip approach with miniaturized reaction chambers that require minimal sample volumes (5-10 μL) while maintaining clinical sensitivity. The platform incorporates automated sample preparation, precise fluid handling, and multiplexed detection of key sepsis biomarkers including procalcitonin (PCT), interleukin-6 (IL-6), and C-reactive protein (CRP) simultaneously. BD's technology employs enhanced chemiluminescence detection methods that improve signal-to-noise ratios, enabling detection of biomarkers at concentrations as low as 0.1 pg/mL, which is critical for early sepsis identification before clinical symptoms fully manifest. The system provides results in under 30 minutes, significantly faster than traditional laboratory-based ELISA methods that typically require 2-4 hours.
Strengths: Established global distribution network and manufacturing capabilities; extensive experience in clinical diagnostics; strong regulatory expertise for medical device approvals. Weaknesses: Higher cost structure compared to emerging competitors; potentially slower innovation cycle due to corporate size and regulatory compliance requirements.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has pioneered a microfluidic ELISA platform for sepsis biomarker detection that integrates their proprietary electrochemiluminescence (ECL) technology with advanced microfluidics. Their system, based on the IMPACT (Immuno-Magnetic Particle Capture and Transfer) platform, utilizes paramagnetic microbeads coated with specific antibodies to capture sepsis biomarkers from whole blood samples. The microfluidic channels incorporate multiple detection zones for simultaneous quantification of procalcitonin, IL-6, presepsin, and emerging biomarkers like serum amyloid A. Roche's technology employs a novel sample preparation module that automatically separates plasma from whole blood within the device, eliminating manual preprocessing steps. The system achieves detection limits in the pg/mL range with a dynamic range spanning five orders of magnitude, allowing for accurate quantification across the clinical decision spectrum. Results are available within 15-20 minutes, enabling rapid clinical decision-making in emergency and intensive care settings.
Strengths: Extensive experience in immunoassay development; strong intellectual property portfolio; established presence in clinical laboratory markets with integrated diagnostic solutions. Weaknesses: Premium pricing model may limit adoption in resource-constrained settings; heavy reliance on proprietary consumables creates ongoing cost considerations for healthcare systems.
Key Patents in Microfluidic Sepsis Biomarker Detection
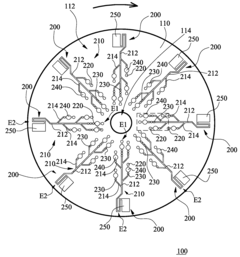
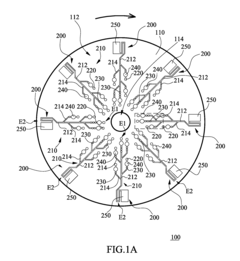
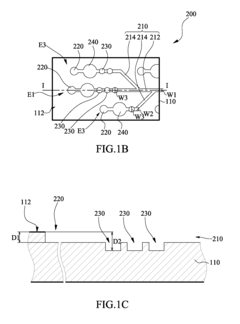
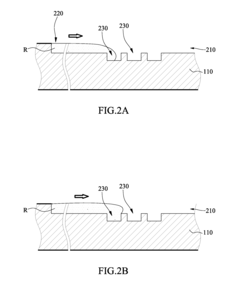
Microfluidic chip
PatentInactiveUS20100304470A1
Innovation
- A microfluidic chip with a substrate and channel sets, including filler and well fillisters that function as valves to control fluid flow, allowing for automated and controlled fluid handling, adaptable for ELISA and other biological or chemical applications.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Clinical Validation Requirements and Protocols
Clinical validation represents a critical phase in the development pathway for microfluidic ELISA platforms targeting early sepsis biomarker detection. The validation process must adhere to stringent regulatory frameworks, including FDA guidelines for in vitro diagnostic devices and ISO 13485 standards for medical device quality management systems. These frameworks ensure that the developed technology meets necessary safety and efficacy requirements before clinical implementation.
The validation protocol should begin with analytical validation to establish performance characteristics such as sensitivity, specificity, precision, and accuracy. For sepsis biomarkers like procalcitonin, IL-6, and C-reactive protein, the microfluidic platform must demonstrate detection limits in clinically relevant ranges (e.g., <0.5 ng/mL for procalcitonin) with coefficient of variation below 10% across the measurement range.
Clinical validation requires a multi-phase approach, starting with retrospective studies using banked samples from confirmed sepsis patients and appropriate controls. These studies should include samples representing various sepsis stages and etiologies to assess the platform's performance across diverse clinical presentations. Sample size calculations should be performed to ensure statistical power, typically requiring 200-300 patient samples for preliminary validation.
Prospective clinical trials represent the gold standard for validation and should be designed as multi-center studies to account for population and practice variations. A recommended approach involves a two-arm study comparing the microfluidic ELISA platform against current standard laboratory methods, with outcomes including time-to-result, diagnostic accuracy, and correlation with clinical outcomes. Special attention must be paid to patient stratification based on comorbidities, infection types, and severity scores.
Validation protocols must address pre-analytical variables that could affect test performance, including sample collection procedures, storage conditions, and processing methods. Standardized protocols for blood collection, handling, and processing are essential to minimize variability and ensure reproducible results across different clinical settings.
Quality control measures must be integrated into validation protocols, including the use of reference standards, calibrators, and control samples. Regular system performance verification should be conducted throughout the validation period to ensure consistent and reliable results. Additionally, operator training requirements and competency assessment protocols should be established to minimize user-dependent variations.
The final validation report must include comprehensive data analysis, including receiver operating characteristic (ROC) curves, positive and negative predictive values, and likelihood ratios across different clinical contexts and patient populations. This information will be crucial for establishing appropriate clinical decision thresholds and interpreting test results in various clinical scenarios.
The validation protocol should begin with analytical validation to establish performance characteristics such as sensitivity, specificity, precision, and accuracy. For sepsis biomarkers like procalcitonin, IL-6, and C-reactive protein, the microfluidic platform must demonstrate detection limits in clinically relevant ranges (e.g., <0.5 ng/mL for procalcitonin) with coefficient of variation below 10% across the measurement range.
Clinical validation requires a multi-phase approach, starting with retrospective studies using banked samples from confirmed sepsis patients and appropriate controls. These studies should include samples representing various sepsis stages and etiologies to assess the platform's performance across diverse clinical presentations. Sample size calculations should be performed to ensure statistical power, typically requiring 200-300 patient samples for preliminary validation.
Prospective clinical trials represent the gold standard for validation and should be designed as multi-center studies to account for population and practice variations. A recommended approach involves a two-arm study comparing the microfluidic ELISA platform against current standard laboratory methods, with outcomes including time-to-result, diagnostic accuracy, and correlation with clinical outcomes. Special attention must be paid to patient stratification based on comorbidities, infection types, and severity scores.
Validation protocols must address pre-analytical variables that could affect test performance, including sample collection procedures, storage conditions, and processing methods. Standardized protocols for blood collection, handling, and processing are essential to minimize variability and ensure reproducible results across different clinical settings.
Quality control measures must be integrated into validation protocols, including the use of reference standards, calibrators, and control samples. Regular system performance verification should be conducted throughout the validation period to ensure consistent and reliable results. Additionally, operator training requirements and competency assessment protocols should be established to minimize user-dependent variations.
The final validation report must include comprehensive data analysis, including receiver operating characteristic (ROC) curves, positive and negative predictive values, and likelihood ratios across different clinical contexts and patient populations. This information will be crucial for establishing appropriate clinical decision thresholds and interpreting test results in various clinical scenarios.
Regulatory Pathway for Sepsis Diagnostic Devices
The regulatory landscape for sepsis diagnostic devices is complex and multifaceted, requiring careful navigation to achieve market approval. In the United States, the Food and Drug Administration (FDA) classifies most sepsis diagnostic devices as Class II medical devices, necessitating a 510(k) premarket notification pathway. However, novel microfluidic ELISA technologies for early sepsis biomarker detection may require a De Novo classification request if substantial equivalence to predicate devices cannot be established.
For these innovative diagnostic platforms, the FDA's Breakthrough Devices Program offers potential advantages, including prioritized review and enhanced communication channels with regulatory officials. This pathway is particularly relevant for microfluidic ELISA technologies that demonstrate significant advantages over conventional methods in terms of detection speed, sensitivity, or point-of-care applicability.
Clinical validation requirements for sepsis diagnostics are particularly stringent due to the condition's complexity and heterogeneity. Manufacturers must demonstrate not only analytical validity (precision, accuracy, limit of detection) but also clinical validity through prospective studies with diverse patient populations. The FDA typically requires evidence that the device can effectively identify sepsis biomarkers at clinically relevant concentrations and timeframes that enable meaningful intervention.
In the European market, the transition from the Medical Device Directive (MDD) to the more stringent Medical Device Regulation (MDR) has significant implications for sepsis diagnostics. Under MDR, these devices are typically classified as Class C under the In Vitro Diagnostic Regulation (IVDR), requiring conformity assessment by a Notified Body and more comprehensive clinical evidence than previously required.
Regulatory considerations specific to microfluidic platforms include validation of sample preparation methods, demonstration of consistent flow dynamics, and verification of compatibility with clinical workflows. Manufacturers must address potential interference factors and establish robust quality control measures for these miniaturized systems.
Reimbursement pathways represent another critical regulatory consideration. In the US, obtaining a CPT code and favorable coverage determinations from the Centers for Medicare & Medicaid Services (CMS) and private insurers requires demonstration of clinical utility – evidence that test results meaningfully impact patient management and outcomes. This often necessitates additional studies beyond those required for regulatory approval.
Global harmonization efforts, including the Medical Device Single Audit Program (MDSAP), can streamline regulatory processes across multiple jurisdictions, though country-specific requirements for sepsis diagnostics still exist, particularly in emerging markets with evolving regulatory frameworks.
For these innovative diagnostic platforms, the FDA's Breakthrough Devices Program offers potential advantages, including prioritized review and enhanced communication channels with regulatory officials. This pathway is particularly relevant for microfluidic ELISA technologies that demonstrate significant advantages over conventional methods in terms of detection speed, sensitivity, or point-of-care applicability.
Clinical validation requirements for sepsis diagnostics are particularly stringent due to the condition's complexity and heterogeneity. Manufacturers must demonstrate not only analytical validity (precision, accuracy, limit of detection) but also clinical validity through prospective studies with diverse patient populations. The FDA typically requires evidence that the device can effectively identify sepsis biomarkers at clinically relevant concentrations and timeframes that enable meaningful intervention.
In the European market, the transition from the Medical Device Directive (MDD) to the more stringent Medical Device Regulation (MDR) has significant implications for sepsis diagnostics. Under MDR, these devices are typically classified as Class C under the In Vitro Diagnostic Regulation (IVDR), requiring conformity assessment by a Notified Body and more comprehensive clinical evidence than previously required.
Regulatory considerations specific to microfluidic platforms include validation of sample preparation methods, demonstration of consistent flow dynamics, and verification of compatibility with clinical workflows. Manufacturers must address potential interference factors and establish robust quality control measures for these miniaturized systems.
Reimbursement pathways represent another critical regulatory consideration. In the US, obtaining a CPT code and favorable coverage determinations from the Centers for Medicare & Medicaid Services (CMS) and private insurers requires demonstration of clinical utility – evidence that test results meaningfully impact patient management and outcomes. This often necessitates additional studies beyond those required for regulatory approval.
Global harmonization efforts, including the Medical Device Single Audit Program (MDSAP), can streamline regulatory processes across multiple jurisdictions, though country-specific requirements for sepsis diagnostics still exist, particularly in emerging markets with evolving regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!