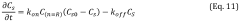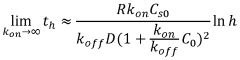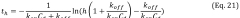Microfluidic ELISA for Point-of-Care Medical Diagnostics
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Background and Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has been a cornerstone of clinical diagnostics since its development in the 1970s, providing high sensitivity and specificity for detecting various biomarkers. Traditional ELISA techniques, however, require laboratory settings, trained personnel, and lengthy processing times, limiting their utility in resource-constrained environments. The integration of microfluidic technology with ELISA represents a significant advancement in point-of-care (POC) diagnostics, addressing these limitations through miniaturization and automation.
The evolution of microfluidic ELISA has been marked by progressive innovations in materials science, microfabrication techniques, and detection methodologies. Early developments focused on simple channel designs using polydimethylsiloxane (PDMS), while recent advancements incorporate sophisticated paper-based platforms, digital microfluidics, and centrifugal microfluidic systems. This technological progression has been driven by the growing demand for accessible healthcare diagnostics in both developed and developing regions.
The primary objective of microfluidic ELISA development is to create robust, user-friendly diagnostic platforms that maintain the analytical performance of conventional ELISA while enabling rapid testing at the point of care. These systems aim to reduce sample and reagent volumes, decrease analysis time from hours to minutes, and eliminate the need for specialized equipment and expertise, all while maintaining or improving detection sensitivity and specificity.
Current research trends focus on enhancing system integration, improving detection limits, expanding multiplexing capabilities, and developing smartphone-compatible readout systems. The field is moving toward fully automated sample-to-answer platforms that can process whole blood or other complex biological samples with minimal user intervention, making sophisticated diagnostics accessible in primary care settings, remote locations, and home environments.
The convergence of microfluidic ELISA with emerging technologies such as nanomaterials, artificial intelligence, and wireless connectivity is expected to further revolutionize POC diagnostics. These innovations promise to enable real-time disease monitoring, personalized medicine approaches, and integration with telehealth systems, potentially transforming healthcare delivery models globally.
The ultimate goal of microfluidic ELISA technology development is to democratize access to high-quality diagnostics, reducing healthcare disparities and enabling earlier disease detection and intervention. This aligns with broader global health initiatives focused on universal healthcare access and preparedness for emerging infectious diseases, positioning microfluidic ELISA as a critical technology for addressing both current and future diagnostic challenges.
The evolution of microfluidic ELISA has been marked by progressive innovations in materials science, microfabrication techniques, and detection methodologies. Early developments focused on simple channel designs using polydimethylsiloxane (PDMS), while recent advancements incorporate sophisticated paper-based platforms, digital microfluidics, and centrifugal microfluidic systems. This technological progression has been driven by the growing demand for accessible healthcare diagnostics in both developed and developing regions.
The primary objective of microfluidic ELISA development is to create robust, user-friendly diagnostic platforms that maintain the analytical performance of conventional ELISA while enabling rapid testing at the point of care. These systems aim to reduce sample and reagent volumes, decrease analysis time from hours to minutes, and eliminate the need for specialized equipment and expertise, all while maintaining or improving detection sensitivity and specificity.
Current research trends focus on enhancing system integration, improving detection limits, expanding multiplexing capabilities, and developing smartphone-compatible readout systems. The field is moving toward fully automated sample-to-answer platforms that can process whole blood or other complex biological samples with minimal user intervention, making sophisticated diagnostics accessible in primary care settings, remote locations, and home environments.
The convergence of microfluidic ELISA with emerging technologies such as nanomaterials, artificial intelligence, and wireless connectivity is expected to further revolutionize POC diagnostics. These innovations promise to enable real-time disease monitoring, personalized medicine approaches, and integration with telehealth systems, potentially transforming healthcare delivery models globally.
The ultimate goal of microfluidic ELISA technology development is to democratize access to high-quality diagnostics, reducing healthcare disparities and enabling earlier disease detection and intervention. This aligns with broader global health initiatives focused on universal healthcare access and preparedness for emerging infectious diseases, positioning microfluidic ELISA as a critical technology for addressing both current and future diagnostic challenges.
Point-of-Care Diagnostics Market Analysis
The Point-of-Care (POC) diagnostics market has experienced substantial growth in recent years, driven by increasing demand for rapid, accessible, and cost-effective diagnostic solutions. The global POC diagnostics market was valued at approximately 29.7 billion USD in 2022 and is projected to reach 51.4 billion USD by 2028, representing a compound annual growth rate (CAGR) of 9.6% during the forecast period.
Microfluidic ELISA technology has emerged as a particularly promising segment within this market due to its ability to miniaturize conventional laboratory procedures while maintaining high sensitivity and specificity. The technology addresses critical market needs for decentralized testing capabilities, especially in resource-limited settings where traditional laboratory infrastructure is unavailable.
Market research indicates that infectious disease diagnostics currently represents the largest application segment for POC testing, accounting for approximately 32% of the market share. This is followed by glucose monitoring (28%), cardiometabolic testing (15%), and pregnancy and fertility testing (10%). Microfluidic ELISA platforms are particularly well-positioned to capture market share in infectious disease diagnostics due to their ability to detect multiple biomarkers simultaneously with high sensitivity.
Geographically, North America dominates the POC diagnostics market with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the highest growth rates are being observed in emerging markets across Asia-Pacific and Latin America, where healthcare infrastructure development and increasing healthcare expenditure are creating new opportunities for POC diagnostic technologies.
Key market drivers include the aging global population, rising incidence of chronic and infectious diseases, increasing patient preference for home-based testing, and growing pressure to reduce healthcare costs through early diagnosis. The COVID-19 pandemic has significantly accelerated market growth by highlighting the importance of rapid, decentralized testing capabilities during public health emergencies.
Reimbursement policies remain a critical factor influencing market adoption. Countries with favorable reimbursement structures for POC testing have demonstrated faster market penetration. Additionally, regulatory pathways for novel POC technologies are evolving, with many countries establishing expedited review processes for innovative diagnostic solutions that address unmet clinical needs.
Market challenges include quality control concerns in non-laboratory settings, integration with existing healthcare IT infrastructure, and price sensitivity in low-resource markets. Despite these challenges, the convergence of microfluidics, biosensors, and mobile technology is expected to drive continued innovation and market expansion for microfluidic ELISA-based POC diagnostics over the next decade.
Microfluidic ELISA technology has emerged as a particularly promising segment within this market due to its ability to miniaturize conventional laboratory procedures while maintaining high sensitivity and specificity. The technology addresses critical market needs for decentralized testing capabilities, especially in resource-limited settings where traditional laboratory infrastructure is unavailable.
Market research indicates that infectious disease diagnostics currently represents the largest application segment for POC testing, accounting for approximately 32% of the market share. This is followed by glucose monitoring (28%), cardiometabolic testing (15%), and pregnancy and fertility testing (10%). Microfluidic ELISA platforms are particularly well-positioned to capture market share in infectious disease diagnostics due to their ability to detect multiple biomarkers simultaneously with high sensitivity.
Geographically, North America dominates the POC diagnostics market with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the highest growth rates are being observed in emerging markets across Asia-Pacific and Latin America, where healthcare infrastructure development and increasing healthcare expenditure are creating new opportunities for POC diagnostic technologies.
Key market drivers include the aging global population, rising incidence of chronic and infectious diseases, increasing patient preference for home-based testing, and growing pressure to reduce healthcare costs through early diagnosis. The COVID-19 pandemic has significantly accelerated market growth by highlighting the importance of rapid, decentralized testing capabilities during public health emergencies.
Reimbursement policies remain a critical factor influencing market adoption. Countries with favorable reimbursement structures for POC testing have demonstrated faster market penetration. Additionally, regulatory pathways for novel POC technologies are evolving, with many countries establishing expedited review processes for innovative diagnostic solutions that address unmet clinical needs.
Market challenges include quality control concerns in non-laboratory settings, integration with existing healthcare IT infrastructure, and price sensitivity in low-resource markets. Despite these challenges, the convergence of microfluidics, biosensors, and mobile technology is expected to drive continued innovation and market expansion for microfluidic ELISA-based POC diagnostics over the next decade.
Current Microfluidic ELISA Challenges
Despite significant advancements in microfluidic ELISA technologies for point-of-care diagnostics, several critical challenges continue to impede widespread clinical adoption. The miniaturization of conventional ELISA onto microfluidic platforms introduces unique fluid dynamics issues that affect assay performance. Surface tension effects and capillary forces become dominant at the microscale, creating challenges in achieving consistent flow rates and uniform reagent distribution across detection chambers.
Sample preparation remains a significant bottleneck in microfluidic ELISA systems. Complex biological samples such as whole blood, saliva, or urine contain numerous interfering substances that can compromise assay sensitivity and specificity. Current on-chip sample preparation techniques often lack the sophistication to effectively isolate target analytes from these complex matrices without additional external equipment, limiting true point-of-care functionality.
Sensitivity and detection limits present ongoing challenges, particularly for early disease biomarkers present at extremely low concentrations. While conventional laboratory ELISA can achieve femtomolar detection limits under optimal conditions, translating this sensitivity to microfluidic platforms remains difficult due to reduced reaction volumes, shorter diffusion distances, and limited signal amplification capabilities within integrated systems.
Integration of multiple assay steps poses substantial engineering challenges. A complete ELISA workflow requires precise sequential delivery of samples and reagents, controlled incubation periods, effective washing steps, and sensitive detection mechanisms. Orchestrating these processes in a self-contained, automated microfluidic device without external instrumentation has proven exceptionally difficult, particularly when maintaining quantitative performance comparable to laboratory standards.
Manufacturing scalability and reproducibility issues persist across the field. Many microfluidic ELISA platforms rely on complex fabrication techniques that are difficult to translate to mass production. Variations in channel dimensions, surface properties, and reagent deposition during manufacturing can lead to device-to-device performance inconsistencies, undermining clinical reliability.
Storage stability represents another critical challenge. Microfluidic ELISA devices must maintain reagent viability during transportation and storage, often in environments without refrigeration. Current approaches to on-chip reagent preservation, such as lyophilization or encapsulation, frequently result in reduced assay performance compared to freshly prepared reagents.
Regulatory hurdles further complicate commercialization efforts. The novel nature of integrated microfluidic diagnostic platforms creates uncertainty in validation protocols and quality control standards. Establishing appropriate performance benchmarks and reference methods for these hybrid technologies has proven challenging within existing regulatory frameworks.
Sample preparation remains a significant bottleneck in microfluidic ELISA systems. Complex biological samples such as whole blood, saliva, or urine contain numerous interfering substances that can compromise assay sensitivity and specificity. Current on-chip sample preparation techniques often lack the sophistication to effectively isolate target analytes from these complex matrices without additional external equipment, limiting true point-of-care functionality.
Sensitivity and detection limits present ongoing challenges, particularly for early disease biomarkers present at extremely low concentrations. While conventional laboratory ELISA can achieve femtomolar detection limits under optimal conditions, translating this sensitivity to microfluidic platforms remains difficult due to reduced reaction volumes, shorter diffusion distances, and limited signal amplification capabilities within integrated systems.
Integration of multiple assay steps poses substantial engineering challenges. A complete ELISA workflow requires precise sequential delivery of samples and reagents, controlled incubation periods, effective washing steps, and sensitive detection mechanisms. Orchestrating these processes in a self-contained, automated microfluidic device without external instrumentation has proven exceptionally difficult, particularly when maintaining quantitative performance comparable to laboratory standards.
Manufacturing scalability and reproducibility issues persist across the field. Many microfluidic ELISA platforms rely on complex fabrication techniques that are difficult to translate to mass production. Variations in channel dimensions, surface properties, and reagent deposition during manufacturing can lead to device-to-device performance inconsistencies, undermining clinical reliability.
Storage stability represents another critical challenge. Microfluidic ELISA devices must maintain reagent viability during transportation and storage, often in environments without refrigeration. Current approaches to on-chip reagent preservation, such as lyophilization or encapsulation, frequently result in reduced assay performance compared to freshly prepared reagents.
Regulatory hurdles further complicate commercialization efforts. The novel nature of integrated microfluidic diagnostic platforms creates uncertainty in validation protocols and quality control standards. Establishing appropriate performance benchmarks and reference methods for these hybrid technologies has proven challenging within existing regulatory frameworks.
Current Microfluidic ELISA Implementation Approaches
01 Microfluidic chip designs for ELISA
Various microfluidic chip designs have been developed specifically for ELISA applications. These designs incorporate channels, chambers, and valves to facilitate the sequential steps of ELISA reactions in a miniaturized format. The chips are typically fabricated using materials such as PDMS, glass, or polymers, and may include features for sample preparation, reagent mixing, incubation, and detection. These designs aim to reduce sample volume requirements while maintaining or improving sensitivity compared to conventional ELISA methods.- Microfluidic chip designs for ELISA: Various microfluidic chip designs have been developed specifically for ELISA applications. These designs incorporate channels, chambers, and reaction zones optimized for the sequential steps of ELISA protocols. The miniaturized platforms enable precise control of fluid flow, reduced sample volumes, and enhanced sensitivity compared to conventional ELISA methods. These chip designs often include features for sample preparation, reagent mixing, incubation, washing, and detection within a single integrated device.
- Automated microfluidic ELISA systems: Automated systems for microfluidic ELISA have been developed to improve efficiency and reproducibility. These systems integrate pumping mechanisms, valves, and control systems to automate the sequential steps of ELISA without manual intervention. The automation reduces human error, increases throughput, and enables standardized testing protocols. Some systems incorporate programmable interfaces that allow users to customize assay parameters and monitor the progress of the analysis in real-time.
- Detection methods in microfluidic ELISA: Various detection methods have been integrated into microfluidic ELISA platforms to enhance sensitivity and specificity. These include optical detection (fluorescence, chemiluminescence, colorimetric), electrochemical detection, and surface plasmon resonance. The miniaturized format of microfluidic devices allows for concentrated detection zones, resulting in improved signal-to-noise ratios. Some platforms incorporate multiple detection modalities to provide complementary information or to accommodate different types of biomarkers within a single assay.
- Surface modification for antibody immobilization: Surface modification techniques have been developed to optimize antibody immobilization in microfluidic ELISA devices. These techniques include chemical functionalization, polymer coatings, and nanomaterial-based approaches that enhance antibody orientation, density, and stability. Properly immobilized antibodies maintain their binding capacity and specificity, leading to improved assay performance. Some modifications also reduce non-specific binding, which decreases background signals and improves the overall sensitivity of the assay.
- Point-of-care microfluidic ELISA applications: Microfluidic ELISA technologies have been adapted for point-of-care applications, enabling diagnostic testing outside of traditional laboratory settings. These portable systems are designed to be user-friendly, rapid, and cost-effective while maintaining analytical performance comparable to laboratory-based methods. Some devices integrate sample preparation, assay execution, and result interpretation into a single platform suitable for use in resource-limited settings. Applications include infectious disease diagnosis, monitoring of chronic conditions, and environmental testing.
02 Integration of detection systems in microfluidic ELISA
Microfluidic ELISA platforms incorporate various detection systems to quantify the results of immunoassays. These systems include optical detection methods such as fluorescence, chemiluminescence, and colorimetric detection, as well as electrochemical detection approaches. The integration of these detection systems with microfluidic channels allows for real-time monitoring of ELISA reactions and can significantly improve sensitivity and detection limits compared to traditional plate-based methods.Expand Specific Solutions03 Automation and multiplexing in microfluidic ELISA
Advanced microfluidic ELISA systems incorporate automation and multiplexing capabilities to increase throughput and efficiency. These systems may include integrated pumps, valves, and control systems that automate the sequential steps of ELISA reactions. Multiplexing features allow for the simultaneous detection of multiple analytes from a single sample, significantly increasing the information obtained from limited sample volumes. This approach is particularly valuable for diagnostic applications where sample availability may be limited.Expand Specific Solutions04 Novel substrate materials and fabrication methods
Innovations in substrate materials and fabrication methods have enhanced the performance of microfluidic ELISA devices. These include the use of paper-based microfluidics, 3D-printed structures, and hybrid material systems that combine the advantages of different substrates. Novel fabrication techniques such as laser cutting, soft lithography, and injection molding have been developed to create complex microfluidic structures with precise dimensions. These advances have led to more cost-effective, sensitive, and user-friendly microfluidic ELISA platforms.Expand Specific Solutions05 Point-of-care applications of microfluidic ELISA
Microfluidic ELISA technologies have been adapted for point-of-care diagnostic applications, enabling rapid testing in resource-limited settings. These systems are designed to be portable, user-friendly, and capable of providing results without sophisticated laboratory equipment. They often incorporate simplified sample preparation steps and integrated detection systems that can be read with minimal instrumentation. Some designs include smartphone-based detection methods, making them particularly suitable for field use and remote healthcare settings.Expand Specific Solutions
Key Industry Players in POC Diagnostics
Microfluidic ELISA for Point-of-Care diagnostics is currently in the growth phase, with the global market expected to reach $1.2 billion by 2026, driven by increasing demand for rapid, decentralized testing solutions. The technology has reached moderate maturity, with key players demonstrating viable commercial applications. Companies like Becton Dickinson, Revvity Health Sciences, and Beijing Wantai Biological are leading commercial development, while academic institutions including Cornell University, KAIST, and EPFL are advancing fundamental research. Collaborations between industry leaders and research institutions, such as Mayo Foundation and Brigham & Women's Hospital, are accelerating innovation in miniaturized, automated diagnostic platforms that combine high sensitivity with point-of-care convenience.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences (formerly PerkinElmer) has pioneered microfluidic ELISA technology through their Laminar Flow Assisted Microfluidic ELISA (LF-μELISA) platform. This system utilizes laminar flow properties within microchannels to create stable liquid interfaces, enabling precise control over reagent interactions without physical barriers. Their technology incorporates specialized surface chemistry modifications that enhance protein binding while minimizing non-specific interactions, significantly improving signal-to-noise ratios. Revvity's platform features integrated sample preparation modules that can process whole blood directly, eliminating the need for centrifugation or manual separation steps. The company has developed proprietary detection methods combining chemiluminescence with microfluidic optical pathways, achieving detection limits in the picogram range while maintaining the compact form factor necessary for point-of-care applications.
Strengths: Superior analytical sensitivity approaching laboratory-grade ELISA performance; versatile platform adaptable to multiple biomarker types; strong intellectual property portfolio protecting core technologies. Weaknesses: More complex manufacturing requirements increase unit costs; requires more specialized training for operators compared to simpler POC tests; higher power requirements may limit deployment in resource-limited settings.
Cornell University
Technical Solution: Cornell University has developed cutting-edge microfluidic ELISA technologies through their Biomedical Engineering Department. Their platform utilizes digital microfluidics (DMF), where discrete droplets are manipulated on an array of electrodes using electrowetting principles, eliminating the need for conventional microchannels and pumps. This approach enables unprecedented flexibility in assay protocols, as reagent additions and washing steps can be programmatically controlled at the individual droplet level. Cornell researchers have pioneered specialized surface coatings that maintain hydrophobicity while preventing protein adsorption, a critical requirement for reliable DMF operation with biological samples. Their system incorporates impedance-based sensing elements that can detect droplet position and composition in real-time, enabling closed-loop control of the assay process. The Cornell platform also features innovative sample preparation modules that can perform cell lysis, protein extraction, and target enrichment directly on the microfluidic chip, creating a sample-to-answer workflow suitable for point-of-care applications.
Strengths: Exceptional flexibility allows rapid protocol optimization without hardware changes; minimal sample volume requirements (nanoliter scale) conserve precious clinical specimens; digital approach enables multiplexed testing within a single device. Weaknesses: More complex electronic control systems increase device cost; current implementations have lower throughput than traditional microfluidic approaches; technology remains primarily in research phase with limited commercial development.
Core Patents and Innovations in Microfluidic Immunoassays
System and method for picoliter volume microfluidic diagnotics
PatentWO2014107698A1
Innovation
- A droplet microfluidics-based platform for picoliter volume bio-analyte detection and quantification using microspheres and fluorescently labeled antibodies, which reduces reagent volume by four orders of magnitude and enhances reaction rates, enabling rapid and low-cost analysis.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Regulatory Pathway for POC Diagnostic Devices
The regulatory landscape for Point-of-Care (POC) diagnostic devices, particularly those utilizing microfluidic ELISA technology, presents a complex pathway that manufacturers must navigate to bring products to market. In the United States, the Food and Drug Administration (FDA) classifies most POC diagnostic devices as Class II medical devices, requiring a 510(k) premarket notification unless the device presents novel technology, in which case a De Novo classification request or Premarket Approval (PMA) may be necessary.
For microfluidic ELISA POC diagnostics, the regulatory process typically begins with analytical validation studies demonstrating the device's precision, accuracy, linearity, and detection limits. Clinical validation follows, requiring evidence that the device can effectively detect the target biomarkers in the intended patient population. The FDA's Center for Devices and Radiological Health (CDRH) has established specific guidance documents for POC testing devices that outline the expected performance characteristics and validation requirements.
In the European Union, the regulatory framework has undergone significant changes with the implementation of the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous In Vitro Diagnostic Directive (IVDD). The IVDR introduces a risk-based classification system that places most POC diagnostic devices in higher risk classes, requiring more stringent conformity assessment procedures and involvement of Notified Bodies.
For emerging markets, regulatory requirements vary significantly. China's National Medical Products Administration (NMPA) has established specific pathways for innovative medical devices, while India's Central Drugs Standard Control Organization (CDSCO) has developed regulations specifically for in vitro diagnostic devices. These regional variations necessitate tailored regulatory strategies for global market access.
Quality Management Systems (QMS) compliance represents another critical regulatory component. Manufacturers must establish and maintain QMS that conform to ISO 13485 standards, ensuring consistent production of safe and effective devices. For microfluidic ELISA POC diagnostics, additional considerations include biocompatibility testing of materials, shelf-life stability studies, and user interface validation to ensure usability in POC settings.
Post-market surveillance requirements have become increasingly stringent globally, requiring manufacturers to implement systems for collecting and analyzing real-world performance data. This includes adverse event reporting, periodic safety update reports, and post-market clinical follow-up studies to identify emerging risks or performance issues.
Navigating these regulatory pathways requires strategic planning from early development stages. Manufacturers should engage with regulatory authorities through pre-submission consultations to clarify specific requirements for their microfluidic ELISA POC diagnostic devices, potentially accelerating the approval process while ensuring compliance with all applicable regulations.
For microfluidic ELISA POC diagnostics, the regulatory process typically begins with analytical validation studies demonstrating the device's precision, accuracy, linearity, and detection limits. Clinical validation follows, requiring evidence that the device can effectively detect the target biomarkers in the intended patient population. The FDA's Center for Devices and Radiological Health (CDRH) has established specific guidance documents for POC testing devices that outline the expected performance characteristics and validation requirements.
In the European Union, the regulatory framework has undergone significant changes with the implementation of the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous In Vitro Diagnostic Directive (IVDD). The IVDR introduces a risk-based classification system that places most POC diagnostic devices in higher risk classes, requiring more stringent conformity assessment procedures and involvement of Notified Bodies.
For emerging markets, regulatory requirements vary significantly. China's National Medical Products Administration (NMPA) has established specific pathways for innovative medical devices, while India's Central Drugs Standard Control Organization (CDSCO) has developed regulations specifically for in vitro diagnostic devices. These regional variations necessitate tailored regulatory strategies for global market access.
Quality Management Systems (QMS) compliance represents another critical regulatory component. Manufacturers must establish and maintain QMS that conform to ISO 13485 standards, ensuring consistent production of safe and effective devices. For microfluidic ELISA POC diagnostics, additional considerations include biocompatibility testing of materials, shelf-life stability studies, and user interface validation to ensure usability in POC settings.
Post-market surveillance requirements have become increasingly stringent globally, requiring manufacturers to implement systems for collecting and analyzing real-world performance data. This includes adverse event reporting, periodic safety update reports, and post-market clinical follow-up studies to identify emerging risks or performance issues.
Navigating these regulatory pathways requires strategic planning from early development stages. Manufacturers should engage with regulatory authorities through pre-submission consultations to clarify specific requirements for their microfluidic ELISA POC diagnostic devices, potentially accelerating the approval process while ensuring compliance with all applicable regulations.
Cost-Effectiveness Analysis of Microfluidic ELISA Solutions
The economic viability of microfluidic ELISA systems represents a critical factor in their adoption for point-of-care diagnostics. Traditional laboratory-based ELISA testing involves substantial costs related to specialized equipment, trained personnel, and centralized facilities. In contrast, microfluidic ELISA platforms offer potential cost advantages through reduced reagent consumption, decreased labor requirements, and elimination of expensive laboratory infrastructure.
Initial investment costs for microfluidic ELISA systems vary significantly based on design complexity and manufacturing approach. Single-use disposable devices typically range from $5-20 per unit when mass-produced, while reusable platforms with electronic components may cost $500-2,000 for the base unit with per-test costs of $3-10. These figures compare favorably against traditional ELISA testing, which averages $25-75 per test when accounting for all associated expenses.
Material selection significantly impacts the cost structure of microfluidic ELISA solutions. Polymer-based devices utilizing PDMS, PMMA, or COC offer economical manufacturing through injection molding or hot embossing, with unit costs potentially below $1 at scale. Paper-based microfluidic platforms present even lower material costs (approximately $0.10-0.50 per device) but may sacrifice sensitivity or reproducibility compared to polymer alternatives.
Operational expenses must be evaluated alongside capital investments. Microfluidic ELISA platforms reduce reagent consumption by 80-95% compared to conventional methods, translating to savings of $3-8 per test. Additionally, these systems typically reduce testing time from 3-4 hours to 15-45 minutes, decreasing labor costs by approximately 70-85% per sample analyzed.
Healthcare economic modeling demonstrates that point-of-care microfluidic ELISA implementation can reduce overall diagnostic expenses by 30-60% when considering the complete testing workflow. This analysis incorporates reduced sample transportation costs, decreased result reporting delays, and minimized patient follow-up requirements. For resource-limited settings, these systems eliminate the need for $50,000-200,000 in laboratory infrastructure investments.
Long-term cost-effectiveness analysis reveals that microfluidic ELISA solutions become increasingly economical at higher testing volumes. The break-even point typically occurs at 500-2,000 tests for reusable platforms, after which the per-test cost advantage becomes substantial. For healthcare systems processing over 10,000 tests annually, the cumulative savings can exceed $150,000-500,000 compared to conventional laboratory methods.
Initial investment costs for microfluidic ELISA systems vary significantly based on design complexity and manufacturing approach. Single-use disposable devices typically range from $5-20 per unit when mass-produced, while reusable platforms with electronic components may cost $500-2,000 for the base unit with per-test costs of $3-10. These figures compare favorably against traditional ELISA testing, which averages $25-75 per test when accounting for all associated expenses.
Material selection significantly impacts the cost structure of microfluidic ELISA solutions. Polymer-based devices utilizing PDMS, PMMA, or COC offer economical manufacturing through injection molding or hot embossing, with unit costs potentially below $1 at scale. Paper-based microfluidic platforms present even lower material costs (approximately $0.10-0.50 per device) but may sacrifice sensitivity or reproducibility compared to polymer alternatives.
Operational expenses must be evaluated alongside capital investments. Microfluidic ELISA platforms reduce reagent consumption by 80-95% compared to conventional methods, translating to savings of $3-8 per test. Additionally, these systems typically reduce testing time from 3-4 hours to 15-45 minutes, decreasing labor costs by approximately 70-85% per sample analyzed.
Healthcare economic modeling demonstrates that point-of-care microfluidic ELISA implementation can reduce overall diagnostic expenses by 30-60% when considering the complete testing workflow. This analysis incorporates reduced sample transportation costs, decreased result reporting delays, and minimized patient follow-up requirements. For resource-limited settings, these systems eliminate the need for $50,000-200,000 in laboratory infrastructure investments.
Long-term cost-effectiveness analysis reveals that microfluidic ELISA solutions become increasingly economical at higher testing volumes. The break-even point typically occurs at 500-2,000 tests for reusable platforms, after which the per-test cost advantage becomes substantial. For healthcare systems processing over 10,000 tests annually, the cumulative savings can exceed $150,000-500,000 compared to conventional laboratory methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!