Microfluidic ELISA for Trace Contaminant Quantification
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Background and Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has been a cornerstone analytical technique in biomedical research and clinical diagnostics since its development in the 1970s. This highly sensitive immunoassay has evolved significantly over decades, transitioning from traditional plate-based formats to advanced microfluidic platforms. The integration of microfluidics with ELISA represents a transformative advancement in analytical capabilities, particularly for environmental monitoring, food safety testing, and clinical diagnostics where trace contaminant detection is critical.
Microfluidic ELISA leverages the principles of fluid manipulation at the microscale to enhance traditional ELISA performance metrics. The historical trajectory shows a progressive miniaturization of analytical systems, moving from macro to micro dimensions, with corresponding improvements in sensitivity, reagent consumption, and analysis time. This evolution has been driven by increasing demands for portable, rapid, and highly sensitive detection systems capable of field deployment.
The current technological landscape demonstrates significant advancements in microfluidic fabrication techniques, surface chemistry modifications, and detection methodologies. Materials science innovations have enabled the development of various substrate platforms including glass, polymers (PDMS, PMMA), and paper-based systems, each offering distinct advantages for specific applications in trace contaminant quantification.
Recent developments in microfluidic ELISA have focused on addressing key limitations of conventional ELISA, particularly regarding sensitivity thresholds, analysis time, and automation capabilities. The integration of novel signal amplification strategies, including nanomaterials and enzymatic cascades, has pushed detection limits into the femtomolar range for certain analytes, representing orders of magnitude improvement over traditional methods.
The primary objective of advancing microfluidic ELISA technology for trace contaminant quantification is to develop robust, sensitive, and field-deployable analytical platforms capable of detecting environmental pollutants, food contaminants, and clinical biomarkers at increasingly lower concentrations. This includes achieving sub-picomolar sensitivity while maintaining assay reproducibility and reliability across diverse sample matrices.
Additional technical goals include reducing total analysis time from hours to minutes, minimizing sample and reagent volumes to microliters or less, enhancing multiplexing capabilities for simultaneous multi-analyte detection, and developing integrated sample preparation modules to enable true sample-to-answer functionality. The ultimate aim is to democratize advanced analytical capabilities by creating accessible, user-friendly platforms that require minimal technical expertise to operate.
The convergence of microfluidics, immunoassay chemistry, and advanced detection technologies presents a promising frontier for next-generation analytical systems. As environmental and health regulations continue to demand lower detection limits and faster analytical turnaround times, microfluidic ELISA stands poised to address these challenges through continued innovation and interdisciplinary collaboration.
Microfluidic ELISA leverages the principles of fluid manipulation at the microscale to enhance traditional ELISA performance metrics. The historical trajectory shows a progressive miniaturization of analytical systems, moving from macro to micro dimensions, with corresponding improvements in sensitivity, reagent consumption, and analysis time. This evolution has been driven by increasing demands for portable, rapid, and highly sensitive detection systems capable of field deployment.
The current technological landscape demonstrates significant advancements in microfluidic fabrication techniques, surface chemistry modifications, and detection methodologies. Materials science innovations have enabled the development of various substrate platforms including glass, polymers (PDMS, PMMA), and paper-based systems, each offering distinct advantages for specific applications in trace contaminant quantification.
Recent developments in microfluidic ELISA have focused on addressing key limitations of conventional ELISA, particularly regarding sensitivity thresholds, analysis time, and automation capabilities. The integration of novel signal amplification strategies, including nanomaterials and enzymatic cascades, has pushed detection limits into the femtomolar range for certain analytes, representing orders of magnitude improvement over traditional methods.
The primary objective of advancing microfluidic ELISA technology for trace contaminant quantification is to develop robust, sensitive, and field-deployable analytical platforms capable of detecting environmental pollutants, food contaminants, and clinical biomarkers at increasingly lower concentrations. This includes achieving sub-picomolar sensitivity while maintaining assay reproducibility and reliability across diverse sample matrices.
Additional technical goals include reducing total analysis time from hours to minutes, minimizing sample and reagent volumes to microliters or less, enhancing multiplexing capabilities for simultaneous multi-analyte detection, and developing integrated sample preparation modules to enable true sample-to-answer functionality. The ultimate aim is to democratize advanced analytical capabilities by creating accessible, user-friendly platforms that require minimal technical expertise to operate.
The convergence of microfluidics, immunoassay chemistry, and advanced detection technologies presents a promising frontier for next-generation analytical systems. As environmental and health regulations continue to demand lower detection limits and faster analytical turnaround times, microfluidic ELISA stands poised to address these challenges through continued innovation and interdisciplinary collaboration.
Market Demand for Trace Contaminant Detection
The global market for trace contaminant detection has been experiencing robust growth, driven by increasing regulatory requirements and growing public awareness about environmental and health safety. The demand for microfluidic ELISA technologies specifically for trace contaminant quantification is expanding across multiple sectors including environmental monitoring, food safety, pharmaceutical quality control, and clinical diagnostics.
Environmental monitoring represents one of the largest market segments, with government agencies worldwide implementing stricter regulations for water, soil, and air quality assessment. The EPA in the United States, the European Environment Agency, and similar organizations in Asia have established increasingly stringent thresholds for contaminants, creating substantial demand for sensitive detection technologies. The global environmental testing market was valued at $11.5 billion in 2022 and is projected to reach $15.3 billion by 2027, with trace contaminant detection comprising approximately 30% of this market.
Food safety testing represents another critical market driver, particularly with growing concerns about pesticide residues, antibiotics, hormones, and other chemical contaminants in food products. The global food safety testing market is expected to grow at a CAGR of 7.9% through 2028, with trace contaminant detection technologies being a key component of this growth trajectory.
Pharmaceutical and biopharmaceutical industries require ultra-sensitive detection methods for quality control and regulatory compliance. The need to detect minute quantities of impurities in drug formulations has created significant demand for advanced microfluidic ELISA platforms that can provide accurate quantification at parts-per-billion levels.
Clinical diagnostics represents an emerging application area, particularly for detecting biomarkers at ultra-low concentrations for early disease detection. The ability to quantify trace levels of specific proteins, hormones, or metabolites can enable earlier intervention and improved patient outcomes.
End-users across these sectors are increasingly demanding detection systems with several key characteristics: higher sensitivity (detection limits in the picogram to femtogram range), faster analysis times (minutes rather than hours), reduced sample volume requirements, multiplexing capabilities, automation, and reduced costs per test. Portable and field-deployable systems are gaining particular traction, with the point-of-need testing market growing at nearly twice the rate of laboratory-based testing.
Regional analysis indicates that North America currently holds the largest market share for trace contaminant detection technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by rapid industrialization, increasing environmental concerns, and strengthening regulatory frameworks in countries like China and India.
Environmental monitoring represents one of the largest market segments, with government agencies worldwide implementing stricter regulations for water, soil, and air quality assessment. The EPA in the United States, the European Environment Agency, and similar organizations in Asia have established increasingly stringent thresholds for contaminants, creating substantial demand for sensitive detection technologies. The global environmental testing market was valued at $11.5 billion in 2022 and is projected to reach $15.3 billion by 2027, with trace contaminant detection comprising approximately 30% of this market.
Food safety testing represents another critical market driver, particularly with growing concerns about pesticide residues, antibiotics, hormones, and other chemical contaminants in food products. The global food safety testing market is expected to grow at a CAGR of 7.9% through 2028, with trace contaminant detection technologies being a key component of this growth trajectory.
Pharmaceutical and biopharmaceutical industries require ultra-sensitive detection methods for quality control and regulatory compliance. The need to detect minute quantities of impurities in drug formulations has created significant demand for advanced microfluidic ELISA platforms that can provide accurate quantification at parts-per-billion levels.
Clinical diagnostics represents an emerging application area, particularly for detecting biomarkers at ultra-low concentrations for early disease detection. The ability to quantify trace levels of specific proteins, hormones, or metabolites can enable earlier intervention and improved patient outcomes.
End-users across these sectors are increasingly demanding detection systems with several key characteristics: higher sensitivity (detection limits in the picogram to femtogram range), faster analysis times (minutes rather than hours), reduced sample volume requirements, multiplexing capabilities, automation, and reduced costs per test. Portable and field-deployable systems are gaining particular traction, with the point-of-need testing market growing at nearly twice the rate of laboratory-based testing.
Regional analysis indicates that North America currently holds the largest market share for trace contaminant detection technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by rapid industrialization, increasing environmental concerns, and strengthening regulatory frameworks in countries like China and India.
Technical Challenges in Microfluidic ELISA Development
Despite the significant advancements in microfluidic ELISA technology for trace contaminant detection, several technical challenges continue to impede its widespread implementation and optimal performance. The miniaturization of conventional ELISA protocols into microfluidic platforms introduces unique obstacles that require innovative solutions.
Surface chemistry optimization remains a critical challenge in microfluidic ELISA development. The high surface-to-volume ratio in microchannels significantly increases non-specific binding, which can lead to elevated background signals and reduced sensitivity. Researchers struggle to develop surface treatments that effectively minimize protein adsorption while maintaining the specific binding capacity required for target analyte detection.
Fluid handling presents another substantial hurdle. Precise control of nanoliter to picoliter volumes demands sophisticated pumping mechanisms and valve systems. Capillary forces, surface tension effects, and laminar flow characteristics in microchannels complicate the predictable movement of reagents, often resulting in uneven distribution and inconsistent reaction kinetics across the detection area.
Integration of multiple assay steps poses significant engineering challenges. Traditional ELISA protocols involve numerous washing, reagent addition, and incubation steps that must be seamlessly incorporated into a microfluidic format. Achieving this integration while maintaining assay performance and minimizing cross-contamination requires complex microfluidic architectures and precise timing control mechanisms.
Detection sensitivity limitations represent a persistent challenge, particularly for ultra-trace contaminant quantification. While microfluidic platforms theoretically offer enhanced sensitivity due to reduced diffusion distances, practical implementation often falls short of theoretical predictions. Signal amplification strategies compatible with microfluidic constraints are needed to achieve the required detection limits for environmental and food safety applications.
Manufacturing scalability and reproducibility issues hinder commercial translation. Current fabrication methods for high-performance microfluidic ELISA devices often involve complex, multi-step processes that are difficult to scale for mass production. Variations in channel dimensions, surface properties, and reagent deposition lead to device-to-device performance inconsistencies that undermine reliability in field applications.
Sample preparation integration remains underdeveloped in current systems. Real-world samples typically require filtration, concentration, or other pretreatment steps before analysis. Incorporating these functions into microfluidic ELISA platforms without compromising overall system performance represents a significant technical challenge that few research groups have successfully addressed.
Cross-platform standardization is notably absent in the field. The lack of standardized protocols, materials, and design principles makes it difficult to compare results across different microfluidic ELISA platforms and establish reliable benchmarks for performance evaluation. This fragmentation impedes knowledge transfer and slows the overall pace of technological advancement.
Surface chemistry optimization remains a critical challenge in microfluidic ELISA development. The high surface-to-volume ratio in microchannels significantly increases non-specific binding, which can lead to elevated background signals and reduced sensitivity. Researchers struggle to develop surface treatments that effectively minimize protein adsorption while maintaining the specific binding capacity required for target analyte detection.
Fluid handling presents another substantial hurdle. Precise control of nanoliter to picoliter volumes demands sophisticated pumping mechanisms and valve systems. Capillary forces, surface tension effects, and laminar flow characteristics in microchannels complicate the predictable movement of reagents, often resulting in uneven distribution and inconsistent reaction kinetics across the detection area.
Integration of multiple assay steps poses significant engineering challenges. Traditional ELISA protocols involve numerous washing, reagent addition, and incubation steps that must be seamlessly incorporated into a microfluidic format. Achieving this integration while maintaining assay performance and minimizing cross-contamination requires complex microfluidic architectures and precise timing control mechanisms.
Detection sensitivity limitations represent a persistent challenge, particularly for ultra-trace contaminant quantification. While microfluidic platforms theoretically offer enhanced sensitivity due to reduced diffusion distances, practical implementation often falls short of theoretical predictions. Signal amplification strategies compatible with microfluidic constraints are needed to achieve the required detection limits for environmental and food safety applications.
Manufacturing scalability and reproducibility issues hinder commercial translation. Current fabrication methods for high-performance microfluidic ELISA devices often involve complex, multi-step processes that are difficult to scale for mass production. Variations in channel dimensions, surface properties, and reagent deposition lead to device-to-device performance inconsistencies that undermine reliability in field applications.
Sample preparation integration remains underdeveloped in current systems. Real-world samples typically require filtration, concentration, or other pretreatment steps before analysis. Incorporating these functions into microfluidic ELISA platforms without compromising overall system performance represents a significant technical challenge that few research groups have successfully addressed.
Cross-platform standardization is notably absent in the field. The lack of standardized protocols, materials, and design principles makes it difficult to compare results across different microfluidic ELISA platforms and establish reliable benchmarks for performance evaluation. This fragmentation impedes knowledge transfer and slows the overall pace of technological advancement.
Current Microfluidic ELISA Implementation Approaches
01 Microfluidic ELISA platforms for trace contaminant detection
Microfluidic platforms specifically designed for ELISA-based detection of trace contaminants offer advantages in sensitivity and sample volume requirements. These systems integrate sample preparation, reagent handling, and detection in a single miniaturized device, allowing for efficient quantification of contaminants at very low concentrations. The microfluidic architecture enables precise control of fluid flow and reaction conditions, enhancing the performance of traditional ELISA methods for environmental and food safety applications.- Microfluidic ELISA platforms for trace contaminant detection: Microfluidic platforms specifically designed for ELISA-based detection of trace contaminants offer advantages in terms of sensitivity and sample volume requirements. These systems integrate various components such as sample preparation, reagent mixing, and detection zones within a single microfluidic chip. The miniaturized format allows for enhanced detection limits for trace contaminants while reducing reagent consumption and analysis time compared to conventional ELISA methods.
- Novel detection methods for enhanced sensitivity in microfluidic ELISA: Advanced detection methods incorporated into microfluidic ELISA systems enable quantification of extremely low concentrations of contaminants. These include electrochemical detection, fluorescence-based techniques, chemiluminescence, and optical sensing approaches. Such detection methods can be integrated with microfluidic channels to achieve real-time monitoring of trace contaminants with improved sensitivity and specificity compared to traditional detection methods.
- Automated sample processing for trace contaminant analysis: Automated sample processing systems integrated with microfluidic ELISA platforms enable efficient handling of multiple samples for trace contaminant quantification. These systems incorporate automated sample loading, reagent dispensing, incubation, washing steps, and detection processes. Automation reduces human error, improves reproducibility, and enables high-throughput screening of environmental, food, or biological samples for trace contaminants.
- Multiplexed detection of trace contaminants: Multiplexed microfluidic ELISA systems allow for simultaneous detection of multiple trace contaminants in a single sample. These platforms incorporate multiple detection zones or channels with different antibodies or recognition elements specific to various target contaminants. Multiplexing capabilities significantly increase the throughput and efficiency of trace contaminant analysis while reducing the sample volume and analysis time required.
- Portable microfluidic ELISA devices for field-based contaminant testing: Portable microfluidic ELISA devices enable on-site quantification of trace contaminants without the need for sophisticated laboratory equipment. These compact systems integrate sample preparation, microfluidic ELISA reactions, and detection components into portable, user-friendly devices. The portability allows for rapid field testing of environmental samples, food products, or water sources for trace contaminants, facilitating immediate decision-making and intervention when necessary.
02 Enhanced sensitivity techniques for trace contaminant quantification
Advanced techniques to enhance the sensitivity of microfluidic ELISA systems for trace contaminant detection incorporate signal amplification strategies, novel detection methods, and optimized surface chemistry. These approaches may include electrochemical detection, fluorescence enhancement, nanoparticle-based signal amplification, and specialized surface modifications to improve antibody binding and reduce non-specific interactions. Such enhancements enable detection of contaminants at parts-per-billion or even parts-per-trillion levels, critical for applications in environmental monitoring and food safety.Expand Specific Solutions03 Automated sample processing for high-throughput analysis
Automated sample processing systems integrated with microfluidic ELISA platforms enable high-throughput analysis of multiple samples for trace contaminant quantification. These systems incorporate automated sample loading, reagent dispensing, incubation control, and washing steps to minimize human intervention and reduce variability. The automation allows for standardized testing protocols, improved reproducibility, and increased sample throughput, making them suitable for routine monitoring applications in environmental testing, food safety, and quality control.Expand Specific Solutions04 Multiplexed detection of multiple contaminants
Multiplexed microfluidic ELISA systems enable simultaneous detection and quantification of multiple trace contaminants in a single sample. These platforms incorporate spatially separated detection zones, differentially labeled antibodies, or array-based detection methods to distinguish between multiple analytes. Multiplexing capabilities significantly increase analytical throughput and efficiency while reducing sample volume requirements and analysis time. This approach is particularly valuable for comprehensive environmental monitoring, food safety testing, and water quality assessment where multiple contaminants must be quantified.Expand Specific Solutions05 Portable and field-deployable microfluidic ELISA systems
Portable microfluidic ELISA systems designed for field deployment enable on-site quantification of trace contaminants without requiring sophisticated laboratory infrastructure. These compact devices integrate sample preparation, microfluidic ELISA reactions, and detection components in a user-friendly format suitable for non-specialists. They often incorporate smartphone-based detection or other portable readout methods, allowing for rapid analysis in remote locations. Such systems are valuable for environmental monitoring, food safety testing in production facilities, and point-of-need contaminant screening in resource-limited settings.Expand Specific Solutions
Key Industry Players in Microfluidic Diagnostics
The microfluidic ELISA market for trace contaminant quantification is in a growth phase, characterized by increasing adoption of miniaturized analytical platforms. The global market is expanding rapidly, driven by demands for portable, sensitive detection systems in environmental monitoring, food safety, and clinical diagnostics. Technology maturity varies across players, with companies like Boehringer Ingelheim microParts and Toshiba demonstrating advanced capabilities in microfluidic device manufacturing. Research institutions including University of Washington and KRIBB are pushing boundaries in biosensor integration, while specialized firms like FREDsense Technologies and Optofluidic Bioassay are commercializing innovative detection platforms. Established analytical instrumentation companies such as 3M and Revvity Health Sciences are leveraging their expertise to enter this emerging field, creating a competitive landscape balanced between established corporations and agile startups.
Optofluidic Bioassay LLC
Technical Solution: Optofluidic Bioassay has developed a proprietary microfluidic ELISA platform that integrates optical detection systems directly into microfluidic channels. Their technology utilizes a unique flow-through design with immobilized antibodies on microbeads packed within microchannels, allowing for enhanced surface-to-volume ratios and improved binding kinetics. The system incorporates real-time fluorescence detection with integrated photomultiplier tubes that can detect trace contaminants at concentrations as low as 0.1 pg/mL. Their platform reduces sample volumes to under 10 μL while maintaining high sensitivity through optimized flow rates that enhance antigen-antibody interactions. The company has also developed specialized surface chemistry for their microchannels that minimizes non-specific binding, thereby improving signal-to-noise ratios for trace contaminant detection.
Strengths: Superior detection limits compared to conventional ELISA; significantly reduced reagent consumption and analysis time (15-30 minutes versus hours); portable form factor suitable for field deployment. Weaknesses: Higher initial equipment costs; requires specialized training for operation; limited multiplexing capability compared to some newer technologies.
The Regents of the University of California
Technical Solution: The University of California has developed a comprehensive microfluidic ELISA platform for trace contaminant quantification that combines digital microfluidics with novel nanomaterial-enhanced detection methods. Their system utilizes a multilayer PDMS (polydimethylsiloxane) chip design with integrated pneumatic valves that enable precise fluid control and compartmentalization. The platform incorporates on-chip sample preparation modules including filtration and pre-concentration capabilities, allowing direct processing of complex environmental samples. For enhanced sensitivity in trace contaminant detection, UC researchers have implemented quantum dot-labeled secondary antibodies that provide significantly higher signal intensity and photostability compared to conventional fluorophores. The detection module employs a miniaturized fluorescence microscope with a high-sensitivity EMCCD camera, achieving detection limits in the femtomolar range for various environmental pollutants and biological toxins. The system also features an integrated smartphone-based readout option for field applications, with cloud-connected data analysis that enables real-time mapping of contamination levels across sampling locations.
Strengths: Exceptional sensitivity with detection limits 100-1000 times lower than conventional ELISA; versatile platform adaptable to various contaminant types; field-deployable with smartphone integration. Weaknesses: Complex fabrication process increases production costs; requires specialized expertise for assay development; limited commercial availability as primarily a research platform.
Critical Patents and Innovations in Microfluidic Detection
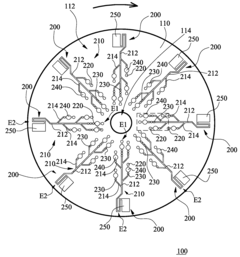
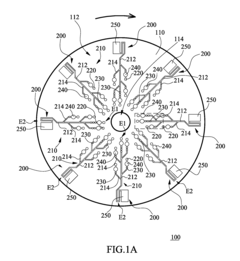
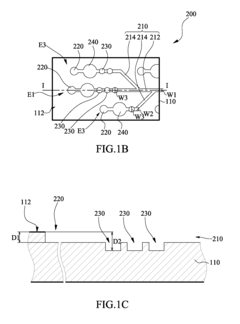
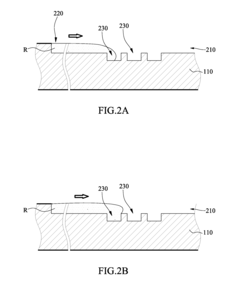
Microfluidic chip
PatentInactiveUS20100304470A1
Innovation
- A microfluidic chip with a substrate and channel sets, including filler and well fillisters that function as valves to control fluid flow, allowing for automated and controlled fluid handling, adaptable for ELISA and other biological or chemical applications.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Regulatory Standards for Environmental Contaminant Testing
Environmental contaminant testing is governed by a complex framework of regulatory standards that vary across regions and jurisdictions. For microfluidic ELISA applications in trace contaminant quantification, adherence to these standards is critical for ensuring the validity and acceptance of test results. The United States Environmental Protection Agency (EPA) has established Method 1694 for pharmaceuticals and personal care products in water, soil, sediment, and biosolids by HPLC/MS/MS, which provides reference points for sensitivity requirements applicable to microfluidic systems.
The European Union's Water Framework Directive (2000/60/EC) and its daughter directives set environmental quality standards for priority substances in surface waters, requiring detection limits in the low ng/L range for many contaminants. These standards directly impact the required performance specifications for microfluidic ELISA platforms targeting environmental monitoring applications.
ISO/IEC 17025 serves as the international standard for testing and calibration laboratories, establishing requirements for competence, impartiality, and consistent operation. Microfluidic ELISA systems must demonstrate compliance with these standards through validation studies that assess accuracy, precision, linearity, detection limits, and robustness across environmental matrices.
The Food and Drug Administration (FDA) guidelines for bioanalytical method validation (2018) provide additional frameworks applicable to trace contaminant detection, particularly for systems that may be used in food safety applications. These guidelines specify acceptance criteria for method performance that microfluidic platforms must meet to be considered reliable for regulatory purposes.
Emerging contaminants present particular regulatory challenges, as standards for many compounds of emerging concern (CECs) are still evolving. The NORMAN Network in Europe has developed a prioritization framework for these substances, which influences the development targets for new detection technologies including microfluidic ELISA.
Regulatory bodies increasingly recognize the need for field-deployable screening methods that complement laboratory-based confirmatory analysis. The EPA's Environmental Technology Verification (ETV) program provides a mechanism for evaluating innovative environmental technologies, including rapid detection methods like microfluidic ELISA, against established performance metrics.
For global market acceptance, microfluidic ELISA platforms must demonstrate equivalence to reference methods through interlaboratory comparison studies and proficiency testing schemes. The development of international standards specifically addressing microfluidic analytical systems is ongoing through organizations like ASTM International's Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products.
The European Union's Water Framework Directive (2000/60/EC) and its daughter directives set environmental quality standards for priority substances in surface waters, requiring detection limits in the low ng/L range for many contaminants. These standards directly impact the required performance specifications for microfluidic ELISA platforms targeting environmental monitoring applications.
ISO/IEC 17025 serves as the international standard for testing and calibration laboratories, establishing requirements for competence, impartiality, and consistent operation. Microfluidic ELISA systems must demonstrate compliance with these standards through validation studies that assess accuracy, precision, linearity, detection limits, and robustness across environmental matrices.
The Food and Drug Administration (FDA) guidelines for bioanalytical method validation (2018) provide additional frameworks applicable to trace contaminant detection, particularly for systems that may be used in food safety applications. These guidelines specify acceptance criteria for method performance that microfluidic platforms must meet to be considered reliable for regulatory purposes.
Emerging contaminants present particular regulatory challenges, as standards for many compounds of emerging concern (CECs) are still evolving. The NORMAN Network in Europe has developed a prioritization framework for these substances, which influences the development targets for new detection technologies including microfluidic ELISA.
Regulatory bodies increasingly recognize the need for field-deployable screening methods that complement laboratory-based confirmatory analysis. The EPA's Environmental Technology Verification (ETV) program provides a mechanism for evaluating innovative environmental technologies, including rapid detection methods like microfluidic ELISA, against established performance metrics.
For global market acceptance, microfluidic ELISA platforms must demonstrate equivalence to reference methods through interlaboratory comparison studies and proficiency testing schemes. The development of international standards specifically addressing microfluidic analytical systems is ongoing through organizations like ASTM International's Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products.
Cost-Benefit Analysis of Microfluidic vs. Traditional ELISA
When comparing microfluidic ELISA with traditional ELISA platforms for trace contaminant quantification, a comprehensive cost-benefit analysis reveals significant economic and operational differences. Traditional ELISA methods typically require substantial initial investments in laboratory equipment, including plate readers, washers, and incubators, with costs ranging from $20,000 to $50,000. In contrast, microfluidic ELISA systems often have lower initial capital requirements, with basic systems starting at $10,000 to $30,000, though advanced automated systems may reach comparable costs to traditional setups.
Operational expenses show even more dramatic differences. Traditional ELISA consumes reagents at volumes of 50-200 μL per well, resulting in higher per-test costs, typically $5-15 per sample. Microfluidic platforms significantly reduce reagent consumption to 1-10 μL per test, lowering per-sample costs to $1-5. This represents a 70-80% reduction in reagent expenses over time, particularly valuable for expensive antibodies and detection reagents.
Labor costs favor microfluidic systems due to higher automation potential and reduced hands-on time. Traditional ELISA protocols require 3-5 hours of technician involvement, while microfluidic systems often reduce this to 30-90 minutes. At average laboratory technician rates of $25-40 per hour, this translates to substantial labor savings in high-throughput environments.
Time-to-result metrics strongly favor microfluidic platforms, which typically deliver results in 30-60 minutes compared to 4-24 hours for traditional methods. This rapid turnaround enables more responsive decision-making in time-sensitive applications such as environmental monitoring or food safety testing.
Sensitivity considerations must be balanced against cost factors. While both methods can achieve similar detection limits (typically in the pg/mL range), microfluidic systems often demonstrate superior performance in trace contaminant detection due to optimized surface-to-volume ratios and more efficient binding kinetics. This enhanced sensitivity can justify the transition costs for applications requiring detection of ultra-low concentration contaminants.
Return on investment calculations indicate that microfluidic ELISA systems typically reach break-even points within 1-2 years for laboratories processing more than 1,000 samples annually. The economic advantage increases proportionally with testing volume, making microfluidic platforms particularly attractive for high-throughput operations.
Infrastructure requirements also differ significantly. Traditional ELISA demands dedicated laboratory space with controlled environmental conditions, while many microfluidic systems are portable and can operate in field conditions with minimal supporting infrastructure, enabling point-of-need testing capabilities that traditional methods cannot match.
Operational expenses show even more dramatic differences. Traditional ELISA consumes reagents at volumes of 50-200 μL per well, resulting in higher per-test costs, typically $5-15 per sample. Microfluidic platforms significantly reduce reagent consumption to 1-10 μL per test, lowering per-sample costs to $1-5. This represents a 70-80% reduction in reagent expenses over time, particularly valuable for expensive antibodies and detection reagents.
Labor costs favor microfluidic systems due to higher automation potential and reduced hands-on time. Traditional ELISA protocols require 3-5 hours of technician involvement, while microfluidic systems often reduce this to 30-90 minutes. At average laboratory technician rates of $25-40 per hour, this translates to substantial labor savings in high-throughput environments.
Time-to-result metrics strongly favor microfluidic platforms, which typically deliver results in 30-60 minutes compared to 4-24 hours for traditional methods. This rapid turnaround enables more responsive decision-making in time-sensitive applications such as environmental monitoring or food safety testing.
Sensitivity considerations must be balanced against cost factors. While both methods can achieve similar detection limits (typically in the pg/mL range), microfluidic systems often demonstrate superior performance in trace contaminant detection due to optimized surface-to-volume ratios and more efficient binding kinetics. This enhanced sensitivity can justify the transition costs for applications requiring detection of ultra-low concentration contaminants.
Return on investment calculations indicate that microfluidic ELISA systems typically reach break-even points within 1-2 years for laboratories processing more than 1,000 samples annually. The economic advantage increases proportionally with testing volume, making microfluidic platforms particularly attractive for high-throughput operations.
Infrastructure requirements also differ significantly. Traditional ELISA demands dedicated laboratory space with controlled environmental conditions, while many microfluidic systems are portable and can operate in field conditions with minimal supporting infrastructure, enabling point-of-need testing capabilities that traditional methods cannot match.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!