Research on Flow Uniformity in Multi-Channel Microfluidic ELISA
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Flow Uniformity Background and Objectives
Microfluidic Enzyme-Linked Immunosorbent Assay (ELISA) has emerged as a revolutionary technology in the field of biomedical diagnostics, offering significant advantages over conventional ELISA methods. The evolution of this technology began in the late 1990s with the initial integration of microfluidic principles into immunoassay platforms, followed by rapid development in the early 2000s as microfabrication techniques matured.
Flow uniformity represents one of the most critical parameters in multi-channel microfluidic ELISA systems, directly impacting assay sensitivity, reproducibility, and overall diagnostic reliability. Historical data indicates that flow non-uniformities can lead to coefficient of variation (CV) values exceeding 20% in assay results, significantly undermining clinical utility.
The technological progression in this field has been marked by several key milestones, including the transition from single-channel to multi-channel architectures, the development of passive flow control mechanisms, and most recently, the integration of active flow regulation systems. Each advancement has incrementally addressed flow uniformity challenges, yet substantial limitations persist in current implementations.
Current research trends indicate growing interest in computational fluid dynamics (CFD) modeling to predict and optimize flow behavior, alongside experimental validation using advanced flow visualization techniques such as micro-particle image velocimetry (μPIV). These approaches represent complementary strategies for addressing the fundamental physics of microfluidic flow distribution.
The primary technical objective of this research is to achieve flow rate variations of less than 5% across all channels in multi-channel microfluidic ELISA systems, while maintaining compatibility with existing fabrication processes and materials. Secondary objectives include reducing the sensitivity of flow uniformity to manufacturing tolerances and developing robust design principles that can be applied across different microfluidic ELISA architectures.
From a broader perspective, this research aims to establish standardized methodologies for characterizing and optimizing flow uniformity in microfluidic diagnostic platforms. Such standardization would significantly accelerate the translation of laboratory prototypes into clinically viable diagnostic tools, particularly for point-of-care applications in resource-limited settings.
The ultimate goal extends beyond mere technical optimization to enabling a new generation of highly reliable, automated microfluidic ELISA systems capable of delivering laboratory-quality results in decentralized healthcare environments. This aligns with the global trend toward personalized medicine and distributed healthcare delivery models, where rapid, accurate diagnostic capabilities at the point of need represent a critical enabling technology.
Flow uniformity represents one of the most critical parameters in multi-channel microfluidic ELISA systems, directly impacting assay sensitivity, reproducibility, and overall diagnostic reliability. Historical data indicates that flow non-uniformities can lead to coefficient of variation (CV) values exceeding 20% in assay results, significantly undermining clinical utility.
The technological progression in this field has been marked by several key milestones, including the transition from single-channel to multi-channel architectures, the development of passive flow control mechanisms, and most recently, the integration of active flow regulation systems. Each advancement has incrementally addressed flow uniformity challenges, yet substantial limitations persist in current implementations.
Current research trends indicate growing interest in computational fluid dynamics (CFD) modeling to predict and optimize flow behavior, alongside experimental validation using advanced flow visualization techniques such as micro-particle image velocimetry (μPIV). These approaches represent complementary strategies for addressing the fundamental physics of microfluidic flow distribution.
The primary technical objective of this research is to achieve flow rate variations of less than 5% across all channels in multi-channel microfluidic ELISA systems, while maintaining compatibility with existing fabrication processes and materials. Secondary objectives include reducing the sensitivity of flow uniformity to manufacturing tolerances and developing robust design principles that can be applied across different microfluidic ELISA architectures.
From a broader perspective, this research aims to establish standardized methodologies for characterizing and optimizing flow uniformity in microfluidic diagnostic platforms. Such standardization would significantly accelerate the translation of laboratory prototypes into clinically viable diagnostic tools, particularly for point-of-care applications in resource-limited settings.
The ultimate goal extends beyond mere technical optimization to enabling a new generation of highly reliable, automated microfluidic ELISA systems capable of delivering laboratory-quality results in decentralized healthcare environments. This aligns with the global trend toward personalized medicine and distributed healthcare delivery models, where rapid, accurate diagnostic capabilities at the point of need represent a critical enabling technology.
Market Analysis for Multi-Channel Microfluidic ELISA Systems
The global market for multi-channel microfluidic ELISA systems is experiencing robust growth, driven by increasing demand for high-throughput diagnostic solutions across healthcare, pharmaceutical research, and life sciences sectors. Current market valuations indicate that the microfluidic-based diagnostic segment reached approximately 4.5 billion USD in 2022, with multi-channel ELISA applications representing a significant growth segment projected to expand at a compound annual growth rate of 12.3% through 2028.
The clinical diagnostics sector remains the largest consumer of these technologies, accounting for nearly 45% of market share. This dominance stems from the critical need for rapid, accurate, and multiplexed testing capabilities in hospital laboratories, reference laboratories, and point-of-care settings. Flow uniformity innovations directly address key pain points in this sector, including test reliability, reproducibility, and throughput capacity.
Pharmaceutical and biotechnology research represents the fastest-growing application segment, with projected growth rates exceeding 15% annually. This acceleration is fueled by intensified drug discovery efforts and the rising adoption of high-throughput screening methodologies. Companies developing multi-channel systems with superior flow uniformity can command premium pricing in this segment, where precision and reliability outweigh cost considerations.
Geographically, North America maintains market leadership with approximately 38% market share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region demonstrates the highest growth trajectory, with China and India emerging as particularly dynamic markets due to expanding healthcare infrastructure and increasing R&D investments.
The competitive landscape features both established diagnostic equipment manufacturers and specialized microfluidic technology startups. Major players include Thermo Fisher Scientific, Bio-Rad Laboratories, and Abbott Laboratories, who collectively control approximately 45% of the market. These companies are increasingly focusing on flow uniformity as a key differentiator in their product development roadmaps.
End-user feedback indicates that flow uniformity represents a critical purchase consideration, with 78% of laboratory managers citing inconsistent flow as a major limitation in current systems. This technical challenge directly impacts test sensitivity, specificity, and reproducibility—metrics that drive purchasing decisions in regulated environments.
Market analysis reveals a significant price premium potential for systems demonstrating superior flow uniformity characteristics. Products with documented performance improvements in this area command 15-25% higher prices compared to standard offerings, highlighting the commercial value of technical advances in this domain.
The clinical diagnostics sector remains the largest consumer of these technologies, accounting for nearly 45% of market share. This dominance stems from the critical need for rapid, accurate, and multiplexed testing capabilities in hospital laboratories, reference laboratories, and point-of-care settings. Flow uniformity innovations directly address key pain points in this sector, including test reliability, reproducibility, and throughput capacity.
Pharmaceutical and biotechnology research represents the fastest-growing application segment, with projected growth rates exceeding 15% annually. This acceleration is fueled by intensified drug discovery efforts and the rising adoption of high-throughput screening methodologies. Companies developing multi-channel systems with superior flow uniformity can command premium pricing in this segment, where precision and reliability outweigh cost considerations.
Geographically, North America maintains market leadership with approximately 38% market share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region demonstrates the highest growth trajectory, with China and India emerging as particularly dynamic markets due to expanding healthcare infrastructure and increasing R&D investments.
The competitive landscape features both established diagnostic equipment manufacturers and specialized microfluidic technology startups. Major players include Thermo Fisher Scientific, Bio-Rad Laboratories, and Abbott Laboratories, who collectively control approximately 45% of the market. These companies are increasingly focusing on flow uniformity as a key differentiator in their product development roadmaps.
End-user feedback indicates that flow uniformity represents a critical purchase consideration, with 78% of laboratory managers citing inconsistent flow as a major limitation in current systems. This technical challenge directly impacts test sensitivity, specificity, and reproducibility—metrics that drive purchasing decisions in regulated environments.
Market analysis reveals a significant price premium potential for systems demonstrating superior flow uniformity characteristics. Products with documented performance improvements in this area command 15-25% higher prices compared to standard offerings, highlighting the commercial value of technical advances in this domain.
Current Challenges in Multi-Channel Flow Uniformity
Despite significant advancements in microfluidic ELISA technology, achieving uniform flow distribution across multiple microchannels remains a persistent challenge that impedes the reliability and reproducibility of assay results. The primary obstacle stems from the inherent physical properties of fluid dynamics at the microscale, where surface tension, viscous forces, and capillary effects dominate over inertial forces. These phenomena create non-linear flow behaviors that are difficult to predict and control across parallel channel networks.
Channel geometry variations, even at the submicron level, significantly impact flow distribution. Manufacturing tolerances in microfabrication processes inevitably introduce slight dimensional inconsistencies between channels intended to be identical. These variations, though minimal, create preferential flow paths that result in uneven reagent distribution, varying residence times, and ultimately inconsistent assay performance across different channels.
Surface chemistry heterogeneity presents another substantial challenge. Variations in surface properties across different channels can alter the fluid-wall interactions, creating differences in flow resistance. This is particularly problematic in ELISA applications where biomolecule adsorption to channel surfaces can progressively alter flow characteristics during the assay, leading to time-dependent flow non-uniformities.
Bubble formation and entrapment represent critical operational challenges that disrupt flow uniformity. Air bubbles can block channels, redirect flow, or create pressure fluctuations that propagate throughout the system. In multi-channel configurations, these effects become more pronounced as bubbles can differentially affect individual channels, creating unpredictable flow patterns across the device.
External pressure control systems often lack the precision required for maintaining uniform flow across multiple microchannels simultaneously. Pressure fluctuations, pump pulsations, or inconsistent syringe drive mechanisms introduce temporal variations in flow rates that are difficult to synchronize across all channels. These fluctuations are particularly problematic during critical ELISA steps such as antibody binding or washing procedures.
Temperature gradients across the microfluidic chip can induce viscosity variations that affect flow uniformity. Even slight temperature differences can create significant viscosity changes in biological samples, leading to non-uniform flow distribution. This effect is amplified in larger multi-channel arrays where maintaining thermal uniformity becomes increasingly difficult.
The integration of detection systems with multi-channel microfluidic ELISA introduces additional complexities. Optical or electrochemical detection components may create physical constraints that affect channel design and consequently flow uniformity. The need to incorporate these elements while maintaining consistent fluidic performance represents a significant engineering challenge that has not been fully resolved in current systems.
Channel geometry variations, even at the submicron level, significantly impact flow distribution. Manufacturing tolerances in microfabrication processes inevitably introduce slight dimensional inconsistencies between channels intended to be identical. These variations, though minimal, create preferential flow paths that result in uneven reagent distribution, varying residence times, and ultimately inconsistent assay performance across different channels.
Surface chemistry heterogeneity presents another substantial challenge. Variations in surface properties across different channels can alter the fluid-wall interactions, creating differences in flow resistance. This is particularly problematic in ELISA applications where biomolecule adsorption to channel surfaces can progressively alter flow characteristics during the assay, leading to time-dependent flow non-uniformities.
Bubble formation and entrapment represent critical operational challenges that disrupt flow uniformity. Air bubbles can block channels, redirect flow, or create pressure fluctuations that propagate throughout the system. In multi-channel configurations, these effects become more pronounced as bubbles can differentially affect individual channels, creating unpredictable flow patterns across the device.
External pressure control systems often lack the precision required for maintaining uniform flow across multiple microchannels simultaneously. Pressure fluctuations, pump pulsations, or inconsistent syringe drive mechanisms introduce temporal variations in flow rates that are difficult to synchronize across all channels. These fluctuations are particularly problematic during critical ELISA steps such as antibody binding or washing procedures.
Temperature gradients across the microfluidic chip can induce viscosity variations that affect flow uniformity. Even slight temperature differences can create significant viscosity changes in biological samples, leading to non-uniform flow distribution. This effect is amplified in larger multi-channel arrays where maintaining thermal uniformity becomes increasingly difficult.
The integration of detection systems with multi-channel microfluidic ELISA introduces additional complexities. Optical or electrochemical detection components may create physical constraints that affect channel design and consequently flow uniformity. The need to incorporate these elements while maintaining consistent fluidic performance represents a significant engineering challenge that has not been fully resolved in current systems.
Current Flow Control Solutions for Microfluidic ELISA
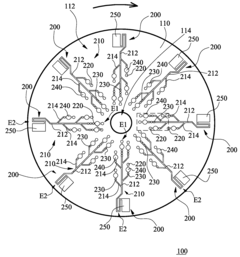
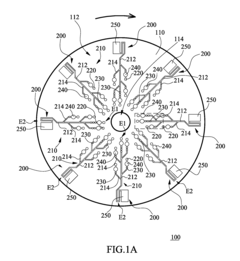
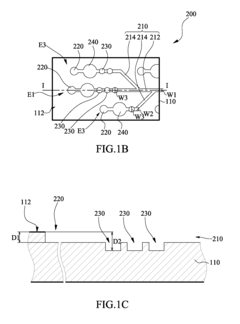
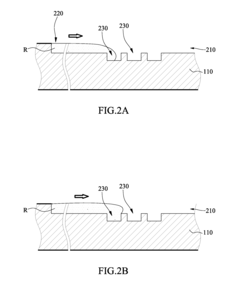
01 Channel design for uniform flow distribution
Specific channel designs can be implemented to ensure uniform flow distribution in multi-channel microfluidic ELISA systems. These designs include symmetric branching structures, equal-length channels, and optimized cross-sectional geometries that help maintain consistent flow rates across all channels. Such uniform flow distribution is critical for ensuring reproducible ELISA results by providing consistent reaction conditions throughout the microfluidic device.- Channel design for flow uniformity: Specific channel designs in microfluidic ELISA systems can significantly improve flow uniformity across multiple channels. These designs include symmetric channel layouts, equal path lengths, and optimized channel widths and depths. By ensuring geometric consistency across all channels, pressure drops remain uniform, resulting in consistent flow rates. Some designs incorporate flow distributors or manifolds at channel inlets to evenly distribute fluid before it enters the parallel channels.
- Flow control mechanisms: Various flow control mechanisms can be implemented to maintain uniform flow in multi-channel microfluidic ELISA systems. These include integrated micropumps, pressure regulators, and flow restrictors that actively adjust flow rates across channels. Some systems employ feedback control loops with flow sensors to detect and correct flow imbalances in real-time. Passive flow control elements such as resistance balancing structures can also be incorporated to naturally equalize flow without external intervention.
- Surface treatment and modification: Surface treatments and modifications of microfluidic channels can enhance flow uniformity in ELISA applications. Hydrophilic or hydrophobic coatings can be applied to channel walls to control surface tension effects and reduce flow variations. Some approaches involve plasma treatment or chemical functionalization to create consistent surface properties across all channels. These modifications help minimize protein adsorption and air bubble formation that could otherwise disrupt uniform flow distribution.
- Integrated mixing and distribution structures: Specialized mixing and distribution structures can be integrated into multi-channel microfluidic ELISA systems to ensure uniform flow. These include herringbone mixers, serpentine channels, and gradient generators that promote even distribution of reagents across parallel channels. Some designs incorporate expansion chambers or bifurcation structures at critical junctions to equalize pressure and flow. These integrated structures help maintain consistent ELISA reaction conditions across all channels.
- Monitoring and validation methods: Various monitoring and validation methods can be employed to assess and maintain flow uniformity in multi-channel microfluidic ELISA systems. These include integrated optical sensors, fluorescent tracers, and image analysis techniques to visualize and quantify flow patterns. Some systems incorporate computational fluid dynamics modeling to predict and optimize flow behavior before fabrication. Real-time monitoring allows for detection of channel blockages or flow imbalances that could affect ELISA results.
02 Flow control mechanisms for ELISA uniformity
Various flow control mechanisms can be incorporated into microfluidic ELISA systems to achieve uniform flow. These include integrated valves, pumps, pressure regulators, and flow restrictors that actively manage fluid movement through multiple channels. Advanced control systems can dynamically adjust flow rates to compensate for variations, ensuring that reagents and samples are distributed evenly across all reaction chambers for consistent assay performance.Expand Specific Solutions03 Surface treatment techniques for flow uniformity
Surface modifications and treatments can significantly improve flow uniformity in microfluidic ELISA channels. Techniques include hydrophilic or hydrophobic coatings, surface functionalization, and the creation of specific surface energies that control fluid behavior. These treatments help prevent uneven wetting, bubble formation, and protein adsorption issues that could otherwise lead to flow irregularities and compromise assay performance.Expand Specific Solutions04 Integrated sensors for real-time flow monitoring
Incorporating sensors into multi-channel microfluidic ELISA systems enables real-time monitoring and adjustment of flow conditions. These sensors can detect flow rates, pressure differences, or fluid properties across different channels, providing feedback for automated control systems. This monitoring capability ensures that flow uniformity is maintained throughout the assay process, allowing for correction of any deviations that might affect test results.Expand Specific Solutions05 Parallel channel optimization for high-throughput ELISA
Optimizing parallel channel configurations is essential for high-throughput microfluidic ELISA applications while maintaining flow uniformity. This involves careful consideration of channel dimensions, spacing, and interconnections to prevent cross-channel interference. Advanced designs incorporate gradient generators, multiplexed sample handling, and balanced resistance networks to ensure that each parallel channel experiences identical flow conditions despite the increased complexity of the system.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Microfluidics
The microfluidic ELISA market is currently in a growth phase, with increasing demand for point-of-care diagnostics driving innovation in flow uniformity technologies. The global market size for microfluidic immunoassays is expanding rapidly, projected to reach significant valuation as healthcare systems seek more efficient diagnostic solutions. Technical maturity varies across competitors, with established players like Revvity Health Sciences and Koninklijke Philips leading commercial development, while research institutions such as CNRS and Harvard College focus on fundamental innovations. Companies like Boehringer Ingelheim microParts and Cellix Ltd. have developed specialized expertise in channel design and flow control, while emerging players from Asia, including Fudan University and KIST, are making notable advances in novel microfluidic architectures that address flow uniformity challenges.
Boehringer Ingelheim microParts GmbH
Technical Solution: Boehringer Ingelheim microParts has developed a sophisticated microfluidic ELISA platform that addresses flow uniformity challenges through precision engineering and advanced materials science. Their system utilizes a proprietary "FlowEqualize" technology that incorporates precisely machined distribution manifolds with computational fluid dynamics-optimized geometries to ensure equal pressure distribution across all channels. The platform features microchannels fabricated with sub-micron precision using their specialized injection molding techniques, ensuring identical cross-sectional areas and surface properties across all channels. Their approach incorporates passive flow restrictors at channel inlets that automatically compensate for pressure variations, maintaining uniform flow without active control systems. The company has developed specialized surface modification techniques that create consistent hydrophilic coatings across all channels, preventing preferential flow paths. Their technology demonstrates flow rate variations of less than 2.5% across 24 parallel channels under standard operating conditions. Additionally, they've implemented specialized bubble traps and degassing systems that prevent air bubbles from disrupting flow uniformity, a common challenge in microfluidic ELISA systems.
Strengths: Exceptional manufacturing precision with industry-leading quality control; excellent reproducibility between production batches; robust design suitable for point-of-care applications. Weaknesses: Less flexibility for custom configurations; higher initial investment costs; limited compatibility with certain biological samples requiring specialized surface treatments.
President & Fellows of Harvard College
Technical Solution: Harvard has developed advanced microfluidic ELISA platforms that address flow uniformity challenges through innovative channel design and flow control mechanisms. Their technology utilizes deterministic lateral displacement (DLD) arrays integrated with precisely engineered microchannels to ensure uniform flow distribution across multiple detection chambers. The system incorporates pressure-balanced manifolds with equal hydraulic resistance paths to maintain consistent flow rates across parallel channels. Harvard researchers have implemented computational fluid dynamics (CFD) modeling to optimize channel geometries, resulting in less than 5% flow variation between channels. Their platforms also feature integrated on-chip valves and pumps that dynamically adjust flow rates in real-time, compensating for pressure fluctuations that could disrupt uniformity. Additionally, they've developed surface modification techniques that reduce protein adsorption and prevent channel clogging, which can cause flow irregularities.
Strengths: Superior precision in flow control through advanced microfluidics engineering; comprehensive integration of computational modeling with experimental validation; excellent reproducibility across multiple channels. Weaknesses: Higher manufacturing complexity requiring specialized fabrication facilities; more expensive than conventional systems; requires sophisticated control systems for operation.
Key Patents and Research on Multi-Channel Flow Optimization
Microfluidic chip
PatentInactiveUS20100304470A1
Innovation
- A microfluidic chip with a substrate and channel sets, including filler and well fillisters that function as valves to control fluid flow, allowing for automated and controlled fluid handling, adaptable for ELISA and other biological or chemical applications.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Materials Science Advancements for Microfluidic Channels
The evolution of materials science has been pivotal in addressing flow uniformity challenges in multi-channel microfluidic ELISA systems. Traditional materials like polydimethylsiloxane (PDMS) and glass have dominated microfluidic fabrication due to their optical transparency and biocompatibility. However, these materials present limitations in surface properties that affect flow dynamics and protein adsorption, compromising ELISA performance.
Recent advancements have introduced surface-modified polymers with enhanced hydrophilicity to reduce non-specific protein binding and improve flow characteristics. Cyclic olefin copolymers (COC) and cyclic olefin polymers (COP) have emerged as superior alternatives, offering excellent chemical resistance, minimal autofluorescence, and reduced biomolecule adsorption—critical factors for maintaining uniform flow across multiple channels.
Nanomaterial integration represents another significant breakthrough. Graphene oxide and carbon nanotubes incorporated into channel walls have demonstrated remarkable capabilities in controlling fluid dynamics at the microscale. These materials create more predictable flow patterns by modifying surface energy and reducing the boundary layer effects that typically cause flow discrepancies between channels.
Smart responsive materials constitute a promising frontier for next-generation microfluidic ELISA platforms. Stimuli-responsive polymers that alter their properties in response to temperature, pH, or electrical signals enable dynamic flow control without mechanical valves. This advancement addresses the challenge of maintaining consistent flow rates across parallel channels during different ELISA stages.
3D-printable biocompatible resins have revolutionized microfluidic channel fabrication. These materials allow for complex channel geometries with precisely controlled dimensions that were previously unattainable. The ability to create identical channel cross-sections with minimal manufacturing variability directly contributes to flow uniformity across multiple channels.
Anti-fouling coatings derived from zwitterionic materials and polyethylene glycol (PEG) derivatives have significantly reduced protein adsorption on channel surfaces. This reduction in biofouling maintains consistent channel dimensions throughout extended ELISA procedures, preventing progressive flow restriction that typically leads to channel-to-channel variations.
The integration of these material advancements with computational fluid dynamics modeling has enabled predictive design of channel geometries and surface properties. This synergistic approach allows researchers to optimize material selection and surface modifications specifically for achieving uniform flow distribution in multi-channel microfluidic ELISA systems, representing a holistic materials science solution to this complex engineering challenge.
Recent advancements have introduced surface-modified polymers with enhanced hydrophilicity to reduce non-specific protein binding and improve flow characteristics. Cyclic olefin copolymers (COC) and cyclic olefin polymers (COP) have emerged as superior alternatives, offering excellent chemical resistance, minimal autofluorescence, and reduced biomolecule adsorption—critical factors for maintaining uniform flow across multiple channels.
Nanomaterial integration represents another significant breakthrough. Graphene oxide and carbon nanotubes incorporated into channel walls have demonstrated remarkable capabilities in controlling fluid dynamics at the microscale. These materials create more predictable flow patterns by modifying surface energy and reducing the boundary layer effects that typically cause flow discrepancies between channels.
Smart responsive materials constitute a promising frontier for next-generation microfluidic ELISA platforms. Stimuli-responsive polymers that alter their properties in response to temperature, pH, or electrical signals enable dynamic flow control without mechanical valves. This advancement addresses the challenge of maintaining consistent flow rates across parallel channels during different ELISA stages.
3D-printable biocompatible resins have revolutionized microfluidic channel fabrication. These materials allow for complex channel geometries with precisely controlled dimensions that were previously unattainable. The ability to create identical channel cross-sections with minimal manufacturing variability directly contributes to flow uniformity across multiple channels.
Anti-fouling coatings derived from zwitterionic materials and polyethylene glycol (PEG) derivatives have significantly reduced protein adsorption on channel surfaces. This reduction in biofouling maintains consistent channel dimensions throughout extended ELISA procedures, preventing progressive flow restriction that typically leads to channel-to-channel variations.
The integration of these material advancements with computational fluid dynamics modeling has enabled predictive design of channel geometries and surface properties. This synergistic approach allows researchers to optimize material selection and surface modifications specifically for achieving uniform flow distribution in multi-channel microfluidic ELISA systems, representing a holistic materials science solution to this complex engineering challenge.
Standardization and Quality Control Protocols
Standardization of microfluidic ELISA protocols is essential for ensuring reliable and reproducible results across different laboratories and experimental setups. The development of comprehensive quality control protocols specifically tailored for multi-channel microfluidic ELISA systems represents a critical advancement in this field. These protocols must address the unique challenges posed by flow uniformity variations that can significantly impact assay performance.
A fundamental component of standardization involves the establishment of calibration procedures for flow rate verification across all microchannels. This requires the development of reference standards and calibration materials that can be universally applied. Current approaches include the use of fluorescent dye solutions with known concentration gradients to validate flow consistency, with measurement precision typically achieving coefficients of variation below 5% in optimized systems.
Quality control measures must incorporate regular assessment of channel-to-channel variability through statistical analysis of flow parameters. This includes monitoring pressure differentials, flow rates, and residence times across all channels simultaneously. Advanced systems now implement automated quality control modules that continuously monitor these parameters during operation, flagging deviations that exceed predetermined thresholds.
Documentation standards represent another crucial aspect of quality control protocols. Comprehensive records must be maintained regarding system calibration, maintenance procedures, and performance verification tests. This documentation should follow internationally recognized formats such as those outlined by ISO standards for medical devices and diagnostic systems, particularly ISO 13485 which addresses quality management systems for medical devices.
Validation procedures for multi-channel microfluidic ELISA systems must include robustness testing under various operational conditions. This involves challenging the system with deliberate variations in sample viscosity, temperature fluctuations, and partial channel blockages to assess system resilience. Performance criteria typically specify that flow uniformity must be maintained within ±3% across all channels under these stress conditions.
Inter-laboratory comparison studies have emerged as a valuable tool for validating standardization protocols. These collaborative efforts involve multiple research groups performing identical assays using standardized reagents and protocols, with results analyzed for consistency and reproducibility. Recent studies have demonstrated that implementation of rigorous standardization protocols can reduce inter-laboratory variability from over 30% to less than 10% in quantitative ELISA results.
The integration of internal control channels within microfluidic devices provides continuous quality monitoring during actual assay performance. These dedicated channels process control samples in parallel with test samples, allowing real-time verification of system performance and immediate identification of potential flow uniformity issues that might compromise assay results.
A fundamental component of standardization involves the establishment of calibration procedures for flow rate verification across all microchannels. This requires the development of reference standards and calibration materials that can be universally applied. Current approaches include the use of fluorescent dye solutions with known concentration gradients to validate flow consistency, with measurement precision typically achieving coefficients of variation below 5% in optimized systems.
Quality control measures must incorporate regular assessment of channel-to-channel variability through statistical analysis of flow parameters. This includes monitoring pressure differentials, flow rates, and residence times across all channels simultaneously. Advanced systems now implement automated quality control modules that continuously monitor these parameters during operation, flagging deviations that exceed predetermined thresholds.
Documentation standards represent another crucial aspect of quality control protocols. Comprehensive records must be maintained regarding system calibration, maintenance procedures, and performance verification tests. This documentation should follow internationally recognized formats such as those outlined by ISO standards for medical devices and diagnostic systems, particularly ISO 13485 which addresses quality management systems for medical devices.
Validation procedures for multi-channel microfluidic ELISA systems must include robustness testing under various operational conditions. This involves challenging the system with deliberate variations in sample viscosity, temperature fluctuations, and partial channel blockages to assess system resilience. Performance criteria typically specify that flow uniformity must be maintained within ±3% across all channels under these stress conditions.
Inter-laboratory comparison studies have emerged as a valuable tool for validating standardization protocols. These collaborative efforts involve multiple research groups performing identical assays using standardized reagents and protocols, with results analyzed for consistency and reproducibility. Recent studies have demonstrated that implementation of rigorous standardization protocols can reduce inter-laboratory variability from over 30% to less than 10% in quantitative ELISA results.
The integration of internal control channels within microfluidic devices provides continuous quality monitoring during actual assay performance. These dedicated channels process control samples in parallel with test samples, allowing real-time verification of system performance and immediate identification of potential flow uniformity issues that might compromise assay results.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!