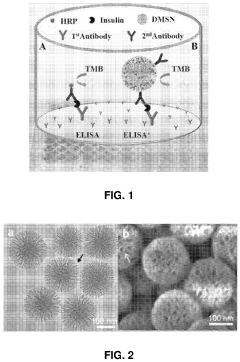
Nanozyme-Based Catalysis in Microfluidic ELISA Detection
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanozyme Catalysis Background and Objectives
Nanozymes represent a revolutionary class of artificial enzymes composed of nanomaterials that mimic natural enzyme activities. Since their discovery in the early 2000s, these synthetic catalysts have evolved from simple metal oxide nanoparticles to sophisticated engineered nanomaterials with tunable catalytic properties. The field has witnessed exponential growth over the past decade, with publications increasing from fewer than 50 in 2010 to over 500 annually by 2022, reflecting the scientific community's growing interest in this technology.
The integration of nanozymes into microfluidic ELISA (Enzyme-Linked Immunosorbent Assay) systems marks a significant advancement in biosensing technology. Traditional ELISA methods rely on natural enzymes like horseradish peroxidase (HRP) or alkaline phosphatase, which suffer from inherent limitations including poor stability, high production costs, and complex storage requirements. Nanozymes address these challenges by offering superior thermal stability, extended shelf life, and resistance to denaturation while maintaining comparable catalytic efficiency.
The technological evolution trajectory shows a clear progression from basic proof-of-concept demonstrations to increasingly sophisticated applications. Early nanozymes primarily focused on simple colorimetric detection, while recent developments have expanded to include chemiluminescence, electrochemical, and fluorescence-based detection methods within microfluidic platforms. This diversification has significantly broadened the application scope and enhanced detection sensitivity.
The primary objective of nanozyme-based catalysis in microfluidic ELISA is to develop highly sensitive, stable, and cost-effective point-of-care diagnostic platforms. Specifically, researchers aim to achieve sub-picomolar detection limits for various biomarkers while maintaining rapid analysis times (under 30 minutes) and minimizing sample volume requirements (less than 10 μL). These improvements would represent a significant advancement over conventional ELISA techniques.
Additional technical goals include enhancing the specificity of nanozyme catalysis through surface modification strategies, developing multiplexed detection capabilities within single microfluidic chips, and creating smartphone-compatible readout systems for resource-limited settings. The field is also moving toward environmentally friendly "green" synthesis methods for nanozymes to reduce the environmental impact of manufacturing processes.
Looking forward, the convergence of nanozyme technology with artificial intelligence and machine learning presents opportunities for developing adaptive diagnostic systems capable of real-time optimization of detection parameters. The ultimate vision is to create fully integrated lab-on-a-chip devices that combine sample preparation, nanozyme-catalyzed detection, and result interpretation in a single platform accessible to non-specialists in diverse healthcare settings.
The integration of nanozymes into microfluidic ELISA (Enzyme-Linked Immunosorbent Assay) systems marks a significant advancement in biosensing technology. Traditional ELISA methods rely on natural enzymes like horseradish peroxidase (HRP) or alkaline phosphatase, which suffer from inherent limitations including poor stability, high production costs, and complex storage requirements. Nanozymes address these challenges by offering superior thermal stability, extended shelf life, and resistance to denaturation while maintaining comparable catalytic efficiency.
The technological evolution trajectory shows a clear progression from basic proof-of-concept demonstrations to increasingly sophisticated applications. Early nanozymes primarily focused on simple colorimetric detection, while recent developments have expanded to include chemiluminescence, electrochemical, and fluorescence-based detection methods within microfluidic platforms. This diversification has significantly broadened the application scope and enhanced detection sensitivity.
The primary objective of nanozyme-based catalysis in microfluidic ELISA is to develop highly sensitive, stable, and cost-effective point-of-care diagnostic platforms. Specifically, researchers aim to achieve sub-picomolar detection limits for various biomarkers while maintaining rapid analysis times (under 30 minutes) and minimizing sample volume requirements (less than 10 μL). These improvements would represent a significant advancement over conventional ELISA techniques.
Additional technical goals include enhancing the specificity of nanozyme catalysis through surface modification strategies, developing multiplexed detection capabilities within single microfluidic chips, and creating smartphone-compatible readout systems for resource-limited settings. The field is also moving toward environmentally friendly "green" synthesis methods for nanozymes to reduce the environmental impact of manufacturing processes.
Looking forward, the convergence of nanozyme technology with artificial intelligence and machine learning presents opportunities for developing adaptive diagnostic systems capable of real-time optimization of detection parameters. The ultimate vision is to create fully integrated lab-on-a-chip devices that combine sample preparation, nanozyme-catalyzed detection, and result interpretation in a single platform accessible to non-specialists in diverse healthcare settings.
Market Analysis for Microfluidic ELISA Technologies
The microfluidic ELISA market is experiencing robust growth, driven by increasing demand for point-of-care diagnostics and personalized medicine. The global market for microfluidic-based diagnostic devices was valued at approximately $15 billion in 2022 and is projected to reach $42 billion by 2028, representing a compound annual growth rate (CAGR) of 18.7%. Within this broader market, nanozyme-enhanced microfluidic ELISA technologies are emerging as a particularly promising segment.
Healthcare facilities represent the largest end-user segment, accounting for nearly 45% of the market share. This dominance stems from the critical need for rapid, accurate diagnostic tools in clinical settings. Research institutions constitute the second-largest segment at 30%, followed by pharmaceutical companies at 15%, and other end-users at 10%.
Geographically, North America leads the market with approximately 40% share, attributed to advanced healthcare infrastructure and substantial R&D investments. Europe follows at 30%, while the Asia-Pacific region, particularly China, Japan, and India, is witnessing the fastest growth rate of 22% annually, driven by expanding healthcare access and increasing research activities.
The integration of nanozymes into microfluidic ELISA platforms addresses several critical market needs. First, it significantly reduces detection time from hours to minutes, meeting the growing demand for rapid diagnostics. Second, it enhances sensitivity, enabling detection of biomarkers at previously undetectable concentrations. Third, it improves stability, allowing for longer shelf life and operation under varied environmental conditions.
Market analysis reveals several key trends driving adoption. The shift toward decentralized testing is accelerating demand for portable, user-friendly diagnostic platforms. The COVID-19 pandemic has further catalyzed this trend, highlighting the need for rapid, accessible testing solutions. Additionally, the growing prevalence of chronic diseases necessitates more frequent monitoring, creating sustained demand for efficient diagnostic tools.
Consumer preferences are evolving toward minimally invasive procedures requiring smaller sample volumes, which aligns perfectly with microfluidic ELISA capabilities. Furthermore, healthcare providers increasingly value multiplex testing capabilities that can detect multiple analytes simultaneously, a feature that nanozyme-enhanced microfluidic platforms can readily provide.
Market barriers include high initial development costs, regulatory hurdles, and competition from established traditional ELISA methods. However, the long-term cost-effectiveness, superior performance metrics, and expanding application scope of nanozyme-based microfluidic ELISA technologies position them favorably for continued market penetration and growth.
Healthcare facilities represent the largest end-user segment, accounting for nearly 45% of the market share. This dominance stems from the critical need for rapid, accurate diagnostic tools in clinical settings. Research institutions constitute the second-largest segment at 30%, followed by pharmaceutical companies at 15%, and other end-users at 10%.
Geographically, North America leads the market with approximately 40% share, attributed to advanced healthcare infrastructure and substantial R&D investments. Europe follows at 30%, while the Asia-Pacific region, particularly China, Japan, and India, is witnessing the fastest growth rate of 22% annually, driven by expanding healthcare access and increasing research activities.
The integration of nanozymes into microfluidic ELISA platforms addresses several critical market needs. First, it significantly reduces detection time from hours to minutes, meeting the growing demand for rapid diagnostics. Second, it enhances sensitivity, enabling detection of biomarkers at previously undetectable concentrations. Third, it improves stability, allowing for longer shelf life and operation under varied environmental conditions.
Market analysis reveals several key trends driving adoption. The shift toward decentralized testing is accelerating demand for portable, user-friendly diagnostic platforms. The COVID-19 pandemic has further catalyzed this trend, highlighting the need for rapid, accessible testing solutions. Additionally, the growing prevalence of chronic diseases necessitates more frequent monitoring, creating sustained demand for efficient diagnostic tools.
Consumer preferences are evolving toward minimally invasive procedures requiring smaller sample volumes, which aligns perfectly with microfluidic ELISA capabilities. Furthermore, healthcare providers increasingly value multiplex testing capabilities that can detect multiple analytes simultaneously, a feature that nanozyme-enhanced microfluidic platforms can readily provide.
Market barriers include high initial development costs, regulatory hurdles, and competition from established traditional ELISA methods. However, the long-term cost-effectiveness, superior performance metrics, and expanding application scope of nanozyme-based microfluidic ELISA technologies position them favorably for continued market penetration and growth.
Current Challenges in Nanozyme-Based Detection Systems
Despite the promising potential of nanozyme-based microfluidic ELISA systems, several significant challenges impede their widespread adoption and commercial viability. The integration of nanozymes into microfluidic platforms faces stability issues, as these artificial enzymes often exhibit decreased catalytic activity when immobilized on microfluidic channel surfaces. Environmental factors such as pH, temperature, and ionic strength significantly affect nanozyme performance, making standardization difficult across different detection scenarios.
Reproducibility remains a critical concern in nanozyme-based detection systems. Batch-to-batch variations during nanozyme synthesis lead to inconsistent catalytic properties, undermining the reliability essential for clinical diagnostics. Additionally, the complex surface chemistry of nanozymes complicates their uniform functionalization with recognition elements like antibodies, resulting in variable binding efficiencies and detection sensitivities.
Selectivity limitations present another substantial hurdle. Many nanozymes exhibit broad substrate specificity, potentially generating false positive results in complex biological samples containing multiple similar analytes. This cross-reactivity significantly restricts their application in real-world clinical settings where sample matrices are inherently complex and contain numerous potentially interfering substances.
The quantification accuracy of nanozyme-based detection systems requires improvement. Current systems often display non-linear responses across wide concentration ranges, complicating the establishment of reliable calibration curves. The signal amplification mechanisms, while powerful, can sometimes lead to signal saturation at higher analyte concentrations, limiting the dynamic range of detection.
Mass production scalability presents significant engineering challenges. The synthesis protocols for high-quality nanozymes with consistent properties remain difficult to scale up without compromising performance. Furthermore, the integration of nanozyme production with microfluidic device manufacturing lacks standardized protocols, creating bottlenecks in commercial development.
Regulatory hurdles compound these technical challenges. The novel nature of nanozymes raises safety concerns regarding their potential toxicity and environmental impact. Regulatory frameworks for nanozyme-based diagnostic devices remain underdeveloped, creating uncertainty for commercial development. The lack of standardized validation protocols specifically designed for nanozyme-based detection systems further complicates regulatory approval processes.
Addressing these multifaceted challenges requires interdisciplinary collaboration between materials scientists, microfluidic engineers, analytical chemists, and regulatory experts to develop robust, reliable, and commercially viable nanozyme-based microfluidic ELISA detection systems.
Reproducibility remains a critical concern in nanozyme-based detection systems. Batch-to-batch variations during nanozyme synthesis lead to inconsistent catalytic properties, undermining the reliability essential for clinical diagnostics. Additionally, the complex surface chemistry of nanozymes complicates their uniform functionalization with recognition elements like antibodies, resulting in variable binding efficiencies and detection sensitivities.
Selectivity limitations present another substantial hurdle. Many nanozymes exhibit broad substrate specificity, potentially generating false positive results in complex biological samples containing multiple similar analytes. This cross-reactivity significantly restricts their application in real-world clinical settings where sample matrices are inherently complex and contain numerous potentially interfering substances.
The quantification accuracy of nanozyme-based detection systems requires improvement. Current systems often display non-linear responses across wide concentration ranges, complicating the establishment of reliable calibration curves. The signal amplification mechanisms, while powerful, can sometimes lead to signal saturation at higher analyte concentrations, limiting the dynamic range of detection.
Mass production scalability presents significant engineering challenges. The synthesis protocols for high-quality nanozymes with consistent properties remain difficult to scale up without compromising performance. Furthermore, the integration of nanozyme production with microfluidic device manufacturing lacks standardized protocols, creating bottlenecks in commercial development.
Regulatory hurdles compound these technical challenges. The novel nature of nanozymes raises safety concerns regarding their potential toxicity and environmental impact. Regulatory frameworks for nanozyme-based diagnostic devices remain underdeveloped, creating uncertainty for commercial development. The lack of standardized validation protocols specifically designed for nanozyme-based detection systems further complicates regulatory approval processes.
Addressing these multifaceted challenges requires interdisciplinary collaboration between materials scientists, microfluidic engineers, analytical chemists, and regulatory experts to develop robust, reliable, and commercially viable nanozyme-based microfluidic ELISA detection systems.
Existing Nanozyme-Microfluidic Integration Approaches
01 Metal-based nanozymes for catalytic detection
Metal-based nanozymes, including gold, silver, and platinum nanoparticles, exhibit enzyme-like catalytic activities that can be utilized for detection purposes. These nanomaterials can mimic various natural enzymes such as peroxidase, oxidase, and catalase, enabling colorimetric or electrochemical detection of target analytes. The catalytic efficiency can be tuned by controlling the size, shape, and surface properties of the metal nanoparticles, making them versatile tools for sensing applications in biomedical diagnostics and environmental monitoring.- Metal-based nanozymes for catalytic detection: Metal-based nanozymes, including noble metals and metal oxides, exhibit enzyme-like catalytic activities that can be utilized for detection purposes. These nanomaterials mimic natural enzymes such as peroxidase, oxidase, and catalase, enabling colorimetric or electrochemical detection of various analytes. The catalytic efficiency can be tuned by controlling the size, shape, and composition of the metal nanozymes, making them versatile tools for sensing applications in biomedical diagnostics and environmental monitoring.
- Carbon-based nanozymes for biosensing applications: Carbon-based nanomaterials such as graphene, carbon nanotubes, and carbon dots can function as nanozymes with intrinsic enzyme-like activities. These materials offer advantages including large surface area, excellent conductivity, and the ability to be functionalized with various groups to enhance their catalytic performance. Carbon-based nanozymes are particularly useful in electrochemical biosensing platforms where they can catalyze reactions for the detection of biomolecules, pathogens, and environmental pollutants with high sensitivity and selectivity.
- Nanozyme-based colorimetric detection systems: Nanozymes can catalyze reactions that produce visible color changes, enabling simple colorimetric detection methods. These systems typically utilize the peroxidase-like activity of nanozymes to catalyze the oxidation of chromogenic substrates in the presence of hydrogen peroxide. The resulting color change can be observed visually or measured using spectrophotometric techniques, allowing for both qualitative and quantitative analysis. Colorimetric nanozyme-based detection offers advantages such as simplicity, rapid response, and compatibility with point-of-care testing applications.
- Nanozyme-based electrochemical sensing platforms: Electrochemical sensing platforms incorporating nanozymes leverage their catalytic properties to enhance electron transfer and signal amplification. These systems can detect various analytes through electrochemical techniques such as amperometry, voltammetry, and impedance spectroscopy. The nanozymes can be immobilized on electrode surfaces or integrated into composite materials to create sensitive and selective sensors. These platforms offer advantages including high sensitivity, wide linear range, and the potential for miniaturization and integration into portable devices.
- Nanozyme cascade systems for enhanced detection: Nanozyme cascade systems involve multiple nanozymes working in sequence to amplify detection signals. These systems mimic natural enzyme cascades by coupling the product of one nanozyme-catalyzed reaction as the substrate for another, resulting in signal amplification. By integrating different types of nanozymes with complementary catalytic activities, these cascade systems can achieve improved sensitivity and specificity for target analytes. This approach is particularly valuable for detecting low-abundance biomarkers in complex biological samples.
02 Carbon-based nanozymes for biosensing applications
Carbon-based nanomaterials such as graphene, carbon nanotubes, and carbon dots can function as nanozymes with intrinsic enzyme-like activities. These materials offer advantages including high surface area, excellent conductivity, and good biocompatibility. Their catalytic properties can be harnessed for the detection of various biomolecules, heavy metals, and environmental pollutants. The detection mechanisms typically involve colorimetric, fluorescent, or electrochemical changes that occur when the target analyte interacts with the carbon-based nanozyme, allowing for sensitive and selective sensing.Expand Specific Solutions03 Metal-organic framework (MOF) nanozymes for sensitive detection
Metal-organic frameworks (MOFs) represent a class of porous materials that can be engineered to exhibit nanozyme-like catalytic activities. These MOF-based nanozymes combine the advantages of homogeneous and heterogeneous catalysts, offering high surface area, tunable pore size, and diverse metal centers that can catalyze various reactions. They have been applied for the detection of glucose, hydrogen peroxide, phenolic compounds, and other substances with high sensitivity and selectivity. The modular nature of MOFs allows for rational design of catalytic sites to enhance detection performance.Expand Specific Solutions04 Nanozyme-based colorimetric and fluorescent detection systems
Nanozymes can catalyze reactions that produce colorimetric or fluorescent signals, enabling visual or instrument-based detection of target analytes. These detection systems typically involve the nanozyme-catalyzed oxidation of chromogenic or fluorogenic substrates in the presence of hydrogen peroxide or other oxidizing agents. The resulting color or fluorescence change can be correlated with the concentration of the target analyte. This approach offers advantages such as simplicity, rapid response, and compatibility with point-of-care testing. Various nanomaterials including metal nanoparticles, metal oxides, and composite structures have been developed for such detection systems.Expand Specific Solutions05 Nanozyme-based electrochemical sensing platforms
Nanozymes can be integrated into electrochemical sensing platforms to achieve sensitive and selective detection of various analytes. These systems utilize the catalytic properties of nanozymes to enhance electron transfer processes at electrode surfaces, resulting in amplified electrochemical signals. The nanozymes can be immobilized on electrode surfaces or incorporated into composite materials to create stable and reusable sensing platforms. This approach has been applied for the detection of glucose, hydrogen peroxide, heavy metals, and various biomarkers with high sensitivity and wide linear range. The combination of nanozymes with electrochemical techniques offers advantages such as low detection limits, minimal sample preparation, and potential for miniaturization.Expand Specific Solutions
Leading Organizations in Nanozyme and Microfluidic Research
Nanozyme-based catalysis in microfluidic ELISA detection is emerging as a transformative technology in the early-growth phase of its industry lifecycle. The market is projected to expand significantly, driven by increasing demand for rapid, sensitive diagnostic solutions. Currently, the technology demonstrates moderate maturity with key players advancing different approaches. Leading organizations like Ventana Medical Systems (Roche) and Singapore Health Services are developing commercial applications, while academic institutions including Xiamen University, National Center for Nanoscience & Technology, and Cornell University are pioneering fundamental research. Industrial players such as Nicoya Lifesciences and Life Technologies are bridging research-to-market gaps by developing supporting technologies and platforms. The competitive landscape shows a balanced distribution between established diagnostic companies and research institutions, indicating a collaborative ecosystem focused on translating nanozyme innovations into practical diagnostic solutions.
Agency for Science, Technology & Research
Technical Solution: The Agency for Science, Technology & Research (A*STAR) has developed a sophisticated nanozyme-microfluidic ELISA platform utilizing carbon-based nanozymes (graphene quantum dots) with enhanced peroxidase-like activity. Their system features a centrifugal microfluidic design that enables automated sample processing through precisely controlled rotational forces, eliminating the need for external pumps. A*STAR's technology incorporates nanozyme-embedded hydrogel microstructures within detection chambers that provide controlled release of catalytic nanoparticles, optimizing reaction kinetics and signal development. Their platform includes integrated filtration membranes for on-chip sample preparation, allowing direct processing of complex biological samples like whole blood. The system achieves multiplexed detection through spatially separated reaction chambers, enabling simultaneous analysis of multiple biomarkers from a single sample. Detection sensitivity reaches the picogram/mL level with a total assay time under 20 minutes, representing significant improvements over conventional ELISA methods.
Strengths: Fully automated sample-to-answer capability reducing operator dependency; excellent reproducibility through precise fluidic control; capability to process complex biological samples directly; multiplexed detection capability for comprehensive analysis. Weaknesses: More complex manufacturing process increasing production costs; requires specialized equipment for centrifugal operation; potential challenges in scaling up production while maintaining quality control.
Xiamen University
Technical Solution: Xiamen University has pioneered an innovative nanozyme-based microfluidic ELISA system utilizing cerium oxide nanoparticles (CeO2) with multi-enzyme mimetic properties. Their approach integrates nanozymes directly into 3D-printed microfluidic channels with specialized surface treatments that enhance catalytic activity while preventing biofouling. The university's technology features a unique sequential reaction chamber design that allows for automated sample processing, reagent mixing, and signal development without external intervention. Their system incorporates smartphone-based colorimetric analysis for quantitative readouts, making it suitable for resource-limited settings. Notably, their nanozymes demonstrate remarkable stability under ambient conditions for over six months, eliminating cold-chain requirements. The platform achieves detection limits in the sub-nanogram range for various disease biomarkers, with a total assay time under 30 minutes compared to several hours for conventional ELISA methods.
Strengths: Exceptional operational simplicity requiring minimal user training; remarkable reagent stability eliminating cold chain requirements; versatility in detecting multiple biomarker types; cost-effective manufacturing using 3D printing technology. Weaknesses: Lower sensitivity compared to some competing nanozyme systems; potential for cross-reactivity in multiplex detection scenarios; limited throughput compared to conventional plate-based systems.
Key Patents and Publications in Nanozyme Catalysis
Detecting an analyte
PatentActiveUS11867699B2
Innovation
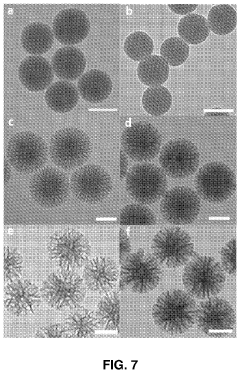
- The use of mesoporous silica nanoparticles with radial pore channels for enhanced enzyme loading and accessibility, and quantum dots immobilized within these nanoparticles to improve signal amplification and light efficiency in detection methods and displays.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Regulatory Considerations for Nanozyme Diagnostic Applications
The regulatory landscape for nanozyme-based diagnostic applications presents significant complexity due to the novel nature of these materials at the intersection of nanotechnology and enzyme mimetics. Currently, most regulatory frameworks worldwide lack specific guidelines for nanozymes, requiring manufacturers to navigate existing regulations for both in vitro diagnostics (IVDs) and nanomaterials simultaneously.
In the United States, the FDA regulates nanozyme-based diagnostics primarily through the Center for Devices and Radiological Health (CDRH) as medical devices. The classification depends on the intended use and risk profile, with most microfluidic ELISA systems falling under Class II, requiring 510(k) clearance. Manufacturers must demonstrate analytical validity, clinical validity, and safety profiles specific to nanozyme catalytic properties.
The European Union applies the In Vitro Diagnostic Regulation (IVDR 2017/746), which has stricter requirements than previous directives. Nanozyme-based systems require conformity assessment procedures with notified body involvement, particularly focusing on risk management and performance evaluation. The unique catalytic mechanisms of nanozymes necessitate specialized stability and reproducibility documentation.
Safety considerations represent a critical regulatory challenge. Nanozymes' potential for bioaccumulation and long-term toxicity remains poorly understood, requiring extensive biocompatibility testing. Regulatory bodies increasingly demand data on nanomaterial characterization, including size distribution, surface chemistry, and catalytic stability under various environmental conditions.
Quality control presents another significant hurdle. The batch-to-batch reproducibility of nanozyme catalytic activity must be rigorously demonstrated, with standardized protocols for activity measurement still under development. ISO standards 13485 (Quality Management Systems) and 10993 (Biocompatibility) provide general frameworks, but specific nanozyme testing protocols remain to be established.
Looking forward, regulatory harmonization efforts are emerging through international collaborations. The International Medical Device Regulators Forum (IMDRF) has begun discussions on nanomaterial-based diagnostics, potentially leading to standardized approaches. Companies developing nanozyme-based microfluidic ELISA platforms should engage early with regulatory authorities through pre-submission consultations to navigate this evolving landscape effectively.
In the United States, the FDA regulates nanozyme-based diagnostics primarily through the Center for Devices and Radiological Health (CDRH) as medical devices. The classification depends on the intended use and risk profile, with most microfluidic ELISA systems falling under Class II, requiring 510(k) clearance. Manufacturers must demonstrate analytical validity, clinical validity, and safety profiles specific to nanozyme catalytic properties.
The European Union applies the In Vitro Diagnostic Regulation (IVDR 2017/746), which has stricter requirements than previous directives. Nanozyme-based systems require conformity assessment procedures with notified body involvement, particularly focusing on risk management and performance evaluation. The unique catalytic mechanisms of nanozymes necessitate specialized stability and reproducibility documentation.
Safety considerations represent a critical regulatory challenge. Nanozymes' potential for bioaccumulation and long-term toxicity remains poorly understood, requiring extensive biocompatibility testing. Regulatory bodies increasingly demand data on nanomaterial characterization, including size distribution, surface chemistry, and catalytic stability under various environmental conditions.
Quality control presents another significant hurdle. The batch-to-batch reproducibility of nanozyme catalytic activity must be rigorously demonstrated, with standardized protocols for activity measurement still under development. ISO standards 13485 (Quality Management Systems) and 10993 (Biocompatibility) provide general frameworks, but specific nanozyme testing protocols remain to be established.
Looking forward, regulatory harmonization efforts are emerging through international collaborations. The International Medical Device Regulators Forum (IMDRF) has begun discussions on nanomaterial-based diagnostics, potentially leading to standardized approaches. Companies developing nanozyme-based microfluidic ELISA platforms should engage early with regulatory authorities through pre-submission consultations to navigate this evolving landscape effectively.
Commercialization Pathways for Nanozyme-Based ELISA Platforms
The commercialization of nanozyme-based ELISA platforms represents a significant opportunity for translating laboratory innovations into market-ready diagnostic solutions. Several viable pathways exist for bringing these technologies to market, each with distinct advantages and challenges.
Direct-to-market strategies involve developing complete diagnostic systems incorporating nanozyme-enhanced microfluidic ELISA technology. This approach requires substantial investment in manufacturing infrastructure, regulatory approval processes, and distribution networks, but offers maximum control over the final product and potentially higher profit margins. Companies pursuing this pathway must develop comprehensive quality management systems to ensure consistent performance across production batches.
Licensing arrangements present an alternative commercialization route, wherein the core nanozyme technology is licensed to established diagnostic companies. This approach leverages existing manufacturing capabilities and market access of industry incumbents while generating revenue through licensing fees and royalties. Successful licensing requires robust intellectual property protection and demonstration of clear advantages over conventional enzyme systems in terms of stability, cost, or performance.
Strategic partnerships with diagnostic equipment manufacturers or clinical laboratory service providers offer a hybrid approach. These collaborations can accelerate market entry by combining nanozyme innovation with established platforms and distribution channels. Joint development agreements typically involve shared investment and risk, with corresponding sharing of commercial returns.
Contract manufacturing represents another viable pathway, particularly for startups lacking production infrastructure. This model allows technology developers to focus on R&D while outsourcing manufacturing to specialized facilities with existing regulatory compliance and quality systems.
The regulatory landscape significantly influences commercialization strategy selection. Nanozyme-based diagnostics will require appropriate regulatory clearances, with pathways varying by jurisdiction and intended use. In the United States, FDA approval processes differ substantially between laboratory-developed tests and commercial diagnostic kits, affecting time-to-market and development costs.
Reimbursement considerations also play a crucial role in commercialization planning. Early engagement with payers to demonstrate clinical utility and cost-effectiveness can facilitate market adoption and ensure sustainable revenue streams. Health economic analyses comparing nanozyme-based platforms with conventional ELISA methods will be essential for justifying premium pricing or securing favorable reimbursement decisions.
Ultimately, successful commercialization will depend on selecting pathways aligned with available resources, competitive landscape, and target market segments. A phased approach may be optimal, beginning with niche applications where nanozyme advantages are most compelling before expanding to broader diagnostic markets.
Direct-to-market strategies involve developing complete diagnostic systems incorporating nanozyme-enhanced microfluidic ELISA technology. This approach requires substantial investment in manufacturing infrastructure, regulatory approval processes, and distribution networks, but offers maximum control over the final product and potentially higher profit margins. Companies pursuing this pathway must develop comprehensive quality management systems to ensure consistent performance across production batches.
Licensing arrangements present an alternative commercialization route, wherein the core nanozyme technology is licensed to established diagnostic companies. This approach leverages existing manufacturing capabilities and market access of industry incumbents while generating revenue through licensing fees and royalties. Successful licensing requires robust intellectual property protection and demonstration of clear advantages over conventional enzyme systems in terms of stability, cost, or performance.
Strategic partnerships with diagnostic equipment manufacturers or clinical laboratory service providers offer a hybrid approach. These collaborations can accelerate market entry by combining nanozyme innovation with established platforms and distribution channels. Joint development agreements typically involve shared investment and risk, with corresponding sharing of commercial returns.
Contract manufacturing represents another viable pathway, particularly for startups lacking production infrastructure. This model allows technology developers to focus on R&D while outsourcing manufacturing to specialized facilities with existing regulatory compliance and quality systems.
The regulatory landscape significantly influences commercialization strategy selection. Nanozyme-based diagnostics will require appropriate regulatory clearances, with pathways varying by jurisdiction and intended use. In the United States, FDA approval processes differ substantially between laboratory-developed tests and commercial diagnostic kits, affecting time-to-market and development costs.
Reimbursement considerations also play a crucial role in commercialization planning. Early engagement with payers to demonstrate clinical utility and cost-effectiveness can facilitate market adoption and ensure sustainable revenue streams. Health economic analyses comparing nanozyme-based platforms with conventional ELISA methods will be essential for justifying premium pricing or securing favorable reimbursement decisions.
Ultimately, successful commercialization will depend on selecting pathways aligned with available resources, competitive landscape, and target market segments. A phased approach may be optimal, beginning with niche applications where nanozyme advantages are most compelling before expanding to broader diagnostic markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!