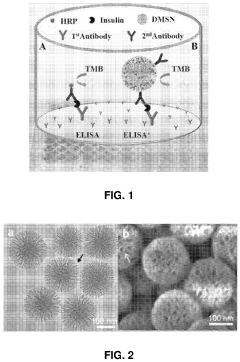
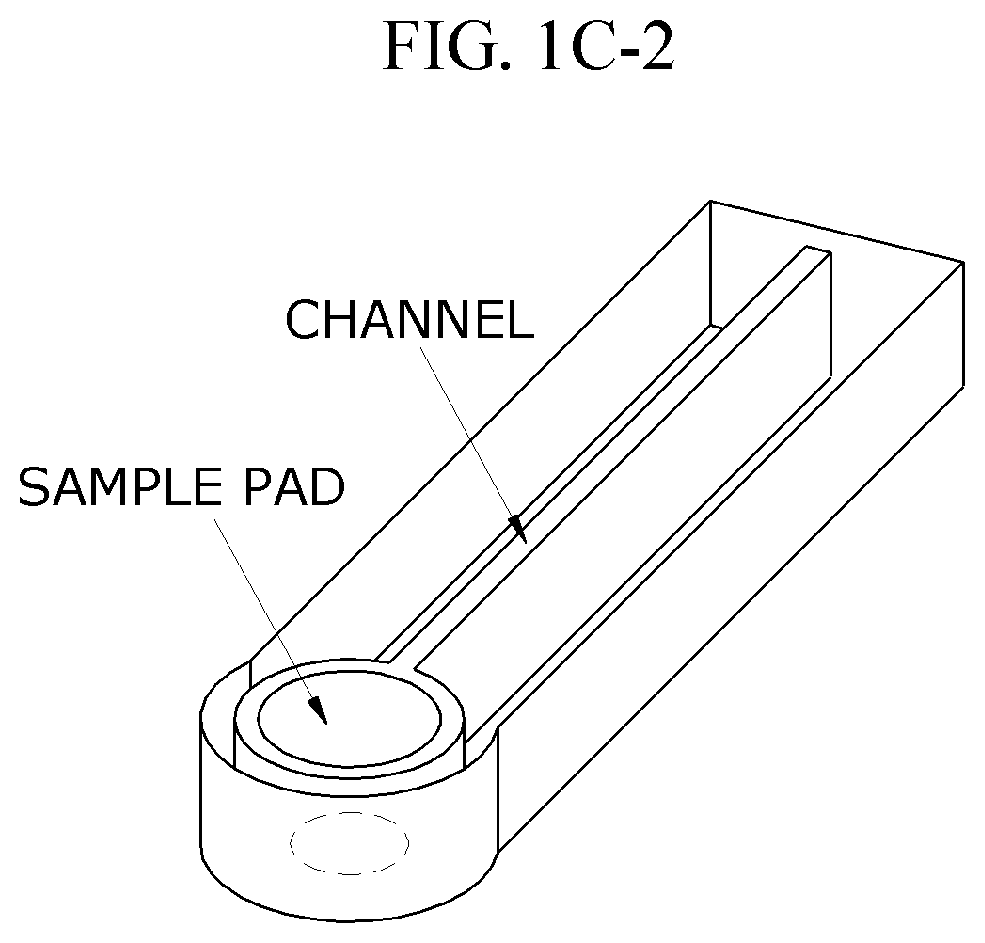
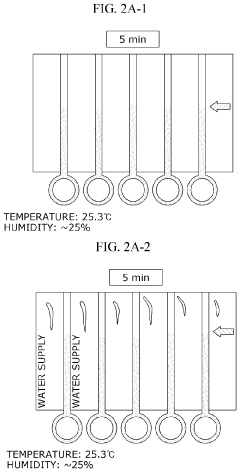
Comparative Study of Colorimetric and Electrochemical Microfluidic ELISA
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Background and Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has been a cornerstone of clinical diagnostics since its development in the 1970s, providing a reliable method for detecting and quantifying antigens or antibodies in biological samples. The evolution of ELISA technology has been marked by continuous improvements in sensitivity, specificity, and throughput. In recent years, the integration of microfluidic platforms with ELISA has emerged as a transformative approach, addressing limitations of conventional ELISA such as large sample volumes, lengthy assay times, and the need for specialized laboratory equipment.
Microfluidic ELISA represents a convergence of immunoassay principles with microscale fluid handling technologies, enabling the manipulation of fluids at the microliter to nanoliter scale. This miniaturization offers significant advantages including reduced reagent consumption, faster reaction kinetics due to shortened diffusion distances, and potential for automation and integration with other analytical processes.
Within the microfluidic ELISA domain, two detection methodologies have gained particular prominence: colorimetric and electrochemical detection. Colorimetric detection, the traditional approach, relies on enzyme-catalyzed reactions that produce colored products measurable through optical techniques. Electrochemical detection, meanwhile, measures electrical signals generated by redox reactions, offering potential advantages in sensitivity and compatibility with electronic readout systems.
The technological trajectory of microfluidic ELISA has been influenced by advances in materials science, microfabrication techniques, and detection technologies. Early systems utilized glass or silicon substrates, while more recent developments have embraced polymeric materials such as polydimethylsiloxane (PDMS) and thermoplastics, enabling more cost-effective and versatile device fabrication.
The primary objective of current research in microfluidic ELISA is to develop platforms that combine the analytical power of ELISA with the efficiency and accessibility of microfluidic systems. Specific goals include achieving detection limits comparable to or better than conventional ELISA, reducing assay times from hours to minutes, enabling multiplexed detection of multiple analytes, and creating systems suitable for point-of-care applications in resource-limited settings.
Looking forward, the field is moving toward fully integrated lab-on-a-chip devices that incorporate sample preparation, immunoreactions, and detection in a single platform. The comparative analysis of colorimetric versus electrochemical detection methodologies represents a critical aspect of this development, as the choice of detection strategy significantly impacts system performance, complexity, and applicability across different use scenarios.
The convergence of microfluidics and ELISA technology is positioned at the intersection of several technological trends, including miniaturization of analytical systems, development of point-of-care diagnostics, and the growing emphasis on personalized medicine. This technological evolution aims to democratize access to sophisticated diagnostic capabilities while maintaining or enhancing analytical performance.
Microfluidic ELISA represents a convergence of immunoassay principles with microscale fluid handling technologies, enabling the manipulation of fluids at the microliter to nanoliter scale. This miniaturization offers significant advantages including reduced reagent consumption, faster reaction kinetics due to shortened diffusion distances, and potential for automation and integration with other analytical processes.
Within the microfluidic ELISA domain, two detection methodologies have gained particular prominence: colorimetric and electrochemical detection. Colorimetric detection, the traditional approach, relies on enzyme-catalyzed reactions that produce colored products measurable through optical techniques. Electrochemical detection, meanwhile, measures electrical signals generated by redox reactions, offering potential advantages in sensitivity and compatibility with electronic readout systems.
The technological trajectory of microfluidic ELISA has been influenced by advances in materials science, microfabrication techniques, and detection technologies. Early systems utilized glass or silicon substrates, while more recent developments have embraced polymeric materials such as polydimethylsiloxane (PDMS) and thermoplastics, enabling more cost-effective and versatile device fabrication.
The primary objective of current research in microfluidic ELISA is to develop platforms that combine the analytical power of ELISA with the efficiency and accessibility of microfluidic systems. Specific goals include achieving detection limits comparable to or better than conventional ELISA, reducing assay times from hours to minutes, enabling multiplexed detection of multiple analytes, and creating systems suitable for point-of-care applications in resource-limited settings.
Looking forward, the field is moving toward fully integrated lab-on-a-chip devices that incorporate sample preparation, immunoreactions, and detection in a single platform. The comparative analysis of colorimetric versus electrochemical detection methodologies represents a critical aspect of this development, as the choice of detection strategy significantly impacts system performance, complexity, and applicability across different use scenarios.
The convergence of microfluidics and ELISA technology is positioned at the intersection of several technological trends, including miniaturization of analytical systems, development of point-of-care diagnostics, and the growing emphasis on personalized medicine. This technological evolution aims to democratize access to sophisticated diagnostic capabilities while maintaining or enhancing analytical performance.
Market Analysis for Microfluidic Immunoassay Applications
The global microfluidic immunoassay market has experienced substantial growth in recent years, driven by increasing demand for point-of-care testing and personalized medicine. The market value reached approximately $2.5 billion in 2022 and is projected to grow at a compound annual growth rate of 10.3% through 2028, according to recent industry analyses.
Microfluidic ELISA technologies, particularly colorimetric and electrochemical detection methods, represent a significant segment within this market. Colorimetric microfluidic ELISA currently dominates with about 65% market share due to its established protocols, visual readout capabilities, and compatibility with existing laboratory infrastructure. However, electrochemical detection methods are gaining traction rapidly, showing a growth rate of 15.7% annually, outpacing the overall market.
Healthcare facilities represent the largest end-user segment, accounting for 42% of the market, followed by research institutions (28%), pharmaceutical companies (18%), and others (12%). Geographically, North America leads with 38% market share, followed by Europe (29%), Asia-Pacific (24%), and rest of the world (9%). The Asia-Pacific region demonstrates the fastest growth trajectory at 13.2% annually, primarily driven by increasing healthcare expenditure in China and India.
The demand for microfluidic immunoassay applications is being fueled by several factors. Rising incidence of chronic and infectious diseases necessitates rapid diagnostic solutions, while the growing elderly population requires more frequent testing. The COVID-19 pandemic significantly accelerated market adoption, creating a 27% surge in demand for rapid immunoassay technologies between 2020-2022.
Cost considerations play a crucial role in market dynamics. Traditional ELISA methods cost approximately $15-25 per test, while microfluidic versions currently range from $8-15, with production scaling expected to further reduce costs to $5-10 per test by 2025. This cost advantage, coupled with reduced sample volume requirements (5-10 μL versus 50-100 μL for traditional methods), drives market expansion.
Industry surveys indicate that end-users prioritize sensitivity (ranked important by 87% of respondents), ease of use (82%), cost-effectiveness (78%), and integration capabilities (65%) when selecting microfluidic immunoassay platforms. Colorimetric methods score higher on ease of use and cost metrics, while electrochemical approaches demonstrate superior performance in sensitivity and integration potential.
The market shows increasing demand for multiplexed assay capabilities, with 73% of potential customers expressing interest in platforms that can detect multiple analytes simultaneously. This trend particularly favors electrochemical detection methods, which can more readily accommodate multiplexed sensing architectures through electrode array configurations.
Microfluidic ELISA technologies, particularly colorimetric and electrochemical detection methods, represent a significant segment within this market. Colorimetric microfluidic ELISA currently dominates with about 65% market share due to its established protocols, visual readout capabilities, and compatibility with existing laboratory infrastructure. However, electrochemical detection methods are gaining traction rapidly, showing a growth rate of 15.7% annually, outpacing the overall market.
Healthcare facilities represent the largest end-user segment, accounting for 42% of the market, followed by research institutions (28%), pharmaceutical companies (18%), and others (12%). Geographically, North America leads with 38% market share, followed by Europe (29%), Asia-Pacific (24%), and rest of the world (9%). The Asia-Pacific region demonstrates the fastest growth trajectory at 13.2% annually, primarily driven by increasing healthcare expenditure in China and India.
The demand for microfluidic immunoassay applications is being fueled by several factors. Rising incidence of chronic and infectious diseases necessitates rapid diagnostic solutions, while the growing elderly population requires more frequent testing. The COVID-19 pandemic significantly accelerated market adoption, creating a 27% surge in demand for rapid immunoassay technologies between 2020-2022.
Cost considerations play a crucial role in market dynamics. Traditional ELISA methods cost approximately $15-25 per test, while microfluidic versions currently range from $8-15, with production scaling expected to further reduce costs to $5-10 per test by 2025. This cost advantage, coupled with reduced sample volume requirements (5-10 μL versus 50-100 μL for traditional methods), drives market expansion.
Industry surveys indicate that end-users prioritize sensitivity (ranked important by 87% of respondents), ease of use (82%), cost-effectiveness (78%), and integration capabilities (65%) when selecting microfluidic immunoassay platforms. Colorimetric methods score higher on ease of use and cost metrics, while electrochemical approaches demonstrate superior performance in sensitivity and integration potential.
The market shows increasing demand for multiplexed assay capabilities, with 73% of potential customers expressing interest in platforms that can detect multiple analytes simultaneously. This trend particularly favors electrochemical detection methods, which can more readily accommodate multiplexed sensing architectures through electrode array configurations.
Current Challenges in Colorimetric vs Electrochemical Detection
Despite significant advancements in microfluidic ELISA technologies, both colorimetric and electrochemical detection methods face distinct challenges that limit their widespread adoption and optimal performance. Colorimetric detection, while offering simplicity and visual readout advantages, struggles with sensitivity limitations in low-concentration analyte scenarios. The optical path length constraints inherent to microfluidic channels significantly reduce detection sensitivity compared to traditional plate-based ELISA. Additionally, colorimetric methods often require relatively large sample volumes to achieve reliable signal intensity, contradicting the core miniaturization benefits of microfluidic platforms.
Environmental factors pose substantial challenges for colorimetric detection, with ambient lighting conditions and reader positioning introducing variability in results. The subjective interpretation of color changes, particularly near detection thresholds, creates reproducibility concerns across different operators and settings. Furthermore, colorimetric approaches typically offer limited dynamic range, making quantification across wide concentration spans problematic without multiple dilutions.
Electrochemical detection methods, while addressing many sensitivity limitations, introduce their own set of challenges. The integration of electrodes within microfluidic channels presents significant fabrication complexities, often requiring specialized equipment and multi-step manufacturing processes that increase production costs. Electrode fouling and surface passivation during repeated measurements compromise long-term stability and reproducibility, necessitating frequent recalibration or replacement.
Signal interference remains a persistent issue in electrochemical detection, with non-specific binding events and matrix effects from complex biological samples generating background noise that can mask true analyte signals. The miniaturization of reference electrodes while maintaining stable potential presents particular difficulties in three-electrode systems, affecting measurement accuracy across different sample conditions.
Both detection methodologies face common challenges in achieving multiplexing capabilities. Colorimetric approaches are constrained by the limited number of distinguishable color changes, while electrochemical methods require complex electrode array designs and sophisticated signal processing to differentiate multiple analytes simultaneously. Cross-reactivity between detection channels further complicates multiplexed analysis in both approaches.
From a practical implementation perspective, colorimetric systems generally require less sophisticated instrumentation but face standardization difficulties across different imaging devices. Conversely, electrochemical systems offer better quantitative performance but demand more specialized equipment and expertise for operation and data interpretation. This creates a significant barrier to adoption in resource-limited settings where technical expertise may be scarce.
The integration of these detection methods with automated sample processing remains challenging, particularly for point-of-care applications where user intervention should be minimized. Balancing detection performance with system complexity represents an ongoing challenge for both approaches in the development of fully integrated microfluidic ELISA platforms.
Environmental factors pose substantial challenges for colorimetric detection, with ambient lighting conditions and reader positioning introducing variability in results. The subjective interpretation of color changes, particularly near detection thresholds, creates reproducibility concerns across different operators and settings. Furthermore, colorimetric approaches typically offer limited dynamic range, making quantification across wide concentration spans problematic without multiple dilutions.
Electrochemical detection methods, while addressing many sensitivity limitations, introduce their own set of challenges. The integration of electrodes within microfluidic channels presents significant fabrication complexities, often requiring specialized equipment and multi-step manufacturing processes that increase production costs. Electrode fouling and surface passivation during repeated measurements compromise long-term stability and reproducibility, necessitating frequent recalibration or replacement.
Signal interference remains a persistent issue in electrochemical detection, with non-specific binding events and matrix effects from complex biological samples generating background noise that can mask true analyte signals. The miniaturization of reference electrodes while maintaining stable potential presents particular difficulties in three-electrode systems, affecting measurement accuracy across different sample conditions.
Both detection methodologies face common challenges in achieving multiplexing capabilities. Colorimetric approaches are constrained by the limited number of distinguishable color changes, while electrochemical methods require complex electrode array designs and sophisticated signal processing to differentiate multiple analytes simultaneously. Cross-reactivity between detection channels further complicates multiplexed analysis in both approaches.
From a practical implementation perspective, colorimetric systems generally require less sophisticated instrumentation but face standardization difficulties across different imaging devices. Conversely, electrochemical systems offer better quantitative performance but demand more specialized equipment and expertise for operation and data interpretation. This creates a significant barrier to adoption in resource-limited settings where technical expertise may be scarce.
The integration of these detection methods with automated sample processing remains challenging, particularly for point-of-care applications where user intervention should be minimized. Balancing detection performance with system complexity represents an ongoing challenge for both approaches in the development of fully integrated microfluidic ELISA platforms.
Comparative Analysis of Colorimetric and Electrochemical Approaches
01 Microfluidic chip designs for ELISA
Various microfluidic chip designs have been developed specifically for ELISA applications. These designs incorporate channels, chambers, and reaction zones optimized for the sequential steps of ELISA protocols. The microfluidic architecture enables precise control of sample and reagent flow, minimizing consumption of valuable reagents while maintaining or improving sensitivity. These designs often include features for sample preparation, antibody binding, washing steps, and detection within a single integrated platform.- Microfluidic chip designs for ELISA: Various microfluidic chip designs have been developed specifically for ELISA applications. These designs incorporate channels, chambers, and reaction zones optimized for the sequential steps of ELISA protocols. The miniaturized platforms enable precise control of fluid flow, reduced sample volumes, and enhanced sensitivity compared to conventional ELISA methods. These chip designs often include integrated detection systems and can be fabricated using materials such as PDMS, glass, or polymers.
- Automated microfluidic ELISA systems: Automated systems for microfluidic ELISA incorporate pumps, valves, and control mechanisms to automate the entire assay process. These systems enable precise control of reagent delivery, incubation times, washing steps, and detection, reducing human intervention and improving reproducibility. The automation allows for high-throughput screening and can be integrated with data analysis software for rapid result interpretation. These systems are particularly valuable for clinical diagnostics and research applications requiring consistent results.
- Detection methods in microfluidic ELISA: Various detection methods have been integrated into microfluidic ELISA platforms to enhance sensitivity and specificity. These include optical detection (fluorescence, colorimetric, chemiluminescence), electrochemical detection, and surface plasmon resonance. Miniaturized sensors and detectors can be incorporated directly into the microfluidic chips, allowing for real-time monitoring of the assay. Advanced signal amplification strategies are also employed to improve the detection limits, making these systems suitable for detecting low-abundance biomarkers.
- Sample preparation and handling in microfluidic ELISA: Microfluidic ELISA platforms incorporate innovative approaches for sample preparation and handling. These include on-chip sample filtration, concentration, and purification to isolate target analytes from complex biological samples. Techniques such as dielectrophoresis, magnetic separation, and acoustic focusing are utilized to manipulate particles and cells within the microfluidic channels. These integrated sample preparation steps enhance the overall performance of the assay by reducing interference and improving detection sensitivity.
- Multiplexed microfluidic ELISA: Multiplexed microfluidic ELISA platforms enable the simultaneous detection of multiple analytes from a single sample. These systems incorporate parallel reaction chambers, spatially separated detection zones, or bead-based assays to achieve multiplexing capability. Advanced microfluidic designs allow for the controlled delivery of different reagents to specific detection zones. This multiplexing capability significantly increases the throughput and efficiency of the assay, making it particularly valuable for applications requiring comprehensive biomarker profiling.
02 Detection and signal enhancement methods
Microfluidic ELISA systems employ various detection methods to enhance sensitivity and accuracy. These include optical detection systems using fluorescence, chemiluminescence, or colorimetric approaches adapted for microscale environments. Signal enhancement techniques such as nanoparticle amplification, enzymatic signal boosting, and digital detection methods are incorporated to improve the lower limits of detection. These detection systems are often integrated directly into the microfluidic platform for real-time analysis.Expand Specific Solutions03 Automation and flow control systems
Automation technologies for microfluidic ELISA include precise flow control mechanisms, automated sample handling, and integrated pumping systems. These systems utilize pressure-driven flow, electrokinetic transport, or centrifugal forces to move fluids through the microchannels. Automated valve systems enable sequential delivery of reagents and washing solutions, reducing manual intervention and improving reproducibility. Complete automation of the ELISA workflow in microfluidic formats significantly reduces analysis time and human error.Expand Specific Solutions04 Surface functionalization and immobilization strategies
Various surface modification techniques are employed in microfluidic ELISA to enhance antibody immobilization and reduce non-specific binding. These include chemical functionalization of channel surfaces, creation of hydrophilic or hydrophobic regions, and incorporation of specific binding sites. Novel immobilization strategies using beads, membranes, or 3D structures within microchannels increase the surface area for antibody attachment, thereby improving sensitivity. These functionalization approaches are critical for maintaining the biological activity of immobilized proteins and reducing background signals.Expand Specific Solutions05 Point-of-care and portable applications
Microfluidic ELISA technologies have been adapted for point-of-care and portable diagnostic applications. These systems integrate sample preparation, analysis, and detection into compact, user-friendly devices suitable for field use or resource-limited settings. Innovations include paper-based microfluidic ELISA, smartphone-based detection systems, and battery-operated portable analyzers. These point-of-care applications focus on rapid results, minimal user intervention, and robust performance under varying environmental conditions, making diagnostic testing more accessible in diverse settings.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidic ELISA
The microfluidic ELISA technology market is currently in a growth phase, characterized by increasing adoption of point-of-care diagnostic solutions. The global market for microfluidic-based diagnostics is expanding rapidly, projected to reach significant value as healthcare systems prioritize rapid, cost-effective testing methods. Technologically, colorimetric and electrochemical detection methods represent different maturity levels, with colorimetric approaches being more established while electrochemical methods offer higher sensitivity and quantification capabilities. Leading players include established healthcare organizations like The General Hospital Corp. and Genentech, alongside specialized companies such as AmberGen and Optofluidic Bioassay focusing on innovative detection technologies. Academic institutions including MIT, South China Normal University, and research organizations like CNRS are driving fundamental advances, while companies like Agilent Technologies and Leica Microsystems provide essential supporting instrumentation and reagents.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed sophisticated microfluidic ELISA platforms that integrate both colorimetric and electrochemical detection methods. Their approach utilizes polymer-based microfluidic chips with multiple detection chambers connected by microchannel networks. CNRS researchers have pioneered the use of conductive polymers as electrode materials, which offer improved biocompatibility and simpler fabrication processes compared to traditional metal electrodes. Their electrochemical detection system employs impedance spectroscopy and cyclic voltammetry techniques, achieving detection limits as low as 0.1 pg/mL for certain protein biomarkers. The colorimetric component incorporates specialized enzyme substrates that produce enhanced chromogenic signals, with integrated optical fibers for improved signal acquisition. CNRS has also developed innovative surface functionalization techniques using plasma treatment and layer-by-layer deposition of polyelectrolytes, which significantly reduces non-specific binding while maintaining high capture antibody activity. Their system includes automated sample handling with precise microvalve control, enabling fully integrated sample-to-answer capabilities.
Strengths: Exceptional sensitivity for both detection methods; innovative materials science approach reduces manufacturing complexity; highly automated operation reduces user error; excellent reproducibility with coefficient of variation <5%. Weaknesses: Relatively high initial equipment cost; specialized reagents may limit accessibility in some settings; system requires regular maintenance and calibration; limited field testing in resource-constrained environments.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced microfluidic ELISA platforms that integrate both colorimetric and electrochemical detection methods. Their technology utilizes paper-based microfluidic devices (μPADs) that enable multiplexed detection of biomarkers with minimal sample volumes. MIT's approach incorporates screen-printed electrodes directly onto paper substrates, creating low-cost diagnostic tools that maintain high sensitivity. Their electrochemical detection system employs amperometric techniques with modified carbon electrodes to achieve detection limits in the picomolar range. MIT researchers have also pioneered smartphone-based readout systems that can quantify both colorimetric changes and electrochemical signals, making their technology suitable for point-of-care applications in resource-limited settings. Recent innovations include the integration of nanomaterials such as gold nanoparticles and carbon nanotubes to enhance signal amplification and improve detection sensitivity by up to 100-fold compared to conventional ELISA methods.
Strengths: Superior integration of both detection methods on a single platform; exceptional sensitivity in the picomolar range; highly portable and field-deployable; significant cost reduction compared to traditional ELISA systems. Weaknesses: Paper-based platforms may have limitations in reproducibility; electrochemical sensors require periodic calibration; potential cross-reactivity issues in complex biological samples.
Key Patents and Scientific Breakthroughs in Detection Methods
Detecting an analyte
PatentActiveUS11867699B2
Innovation
- The use of mesoporous silica nanoparticles with radial pore channels for enhanced enzyme loading and accessibility, and quantum dots immobilized within these nanoparticles to improve signal amplification and light efficiency in detection methods and displays.
Lateral-flow microfluidic chip and flow velocity control method thereof
PatentInactiveUS20190374940A1
Innovation
- Increasing vapor pressure around specific channels in the microfluidic chip by using a separate vapor supply device or a liquid reservoir adjacent to the channel, which suppresses fluid evaporation and accelerates flow velocity, allowing for efficient sequential reactions without additional processes or equipment.
Miniaturization and Integration Strategies for Point-of-Care Testing
The miniaturization and integration of diagnostic platforms represent critical advancements in point-of-care testing (POCT) technologies, particularly for ELISA-based detection methods. Current trends focus on reducing the size of conventional laboratory equipment while maintaining or enhancing analytical performance, enabling testing in resource-limited settings.
Microfluidic technologies have emerged as the cornerstone of miniaturization efforts, allowing for significant reduction in sample and reagent volumes. Colorimetric microfluidic ELISA systems typically utilize channels with dimensions ranging from 10-500 μm, reducing reagent consumption by up to 90% compared to conventional plate-based methods. Electrochemical detection systems have achieved even greater miniaturization, with some devices incorporating electrode arrays smaller than 100 μm².
Integration strategies for colorimetric detection primarily involve incorporating optical components such as LEDs, photodiodes, and CMOS sensors directly into portable devices. Companies like Abbott and Roche have successfully commercialized systems that integrate sample preparation, reaction chambers, and detection components in single-use cartridges smaller than a credit card.
For electrochemical microfluidic ELISA, integration focuses on combining electrode fabrication with microfluidic channels. Advanced manufacturing techniques including photolithography, screen printing, and inkjet printing enable the production of electrodes directly within microfluidic channels. This approach has demonstrated superior integration density, with recent platforms incorporating up to 16 detection sites in devices smaller than 4 cm².
Smartphone integration represents another significant advancement, transforming consumer electronics into analytical instruments. Colorimetric systems utilize phone cameras as detectors, while electrochemical systems connect via data ports or wireless protocols. This strategy has reduced the cost of reader devices by 60-80% while improving accessibility.
Power management remains a critical challenge, particularly for electrochemical systems requiring stable voltage sources. Recent innovations include energy-harvesting technologies and low-power circuit designs that extend battery life from hours to days. Colorimetric systems generally maintain an advantage in power efficiency, though with lower sensitivity limits.
Data processing integration has evolved from external computation to on-chip analysis. Machine learning algorithms embedded in microcontrollers now enable real-time signal processing and interpretation, reducing the need for expert analysis. Cloud connectivity further enhances these capabilities by enabling remote monitoring and epidemiological data collection.
The convergence of these miniaturization and integration strategies is driving the development of truly portable, user-friendly diagnostic platforms capable of laboratory-quality results in field settings, fundamentally transforming healthcare delivery models worldwide.
Microfluidic technologies have emerged as the cornerstone of miniaturization efforts, allowing for significant reduction in sample and reagent volumes. Colorimetric microfluidic ELISA systems typically utilize channels with dimensions ranging from 10-500 μm, reducing reagent consumption by up to 90% compared to conventional plate-based methods. Electrochemical detection systems have achieved even greater miniaturization, with some devices incorporating electrode arrays smaller than 100 μm².
Integration strategies for colorimetric detection primarily involve incorporating optical components such as LEDs, photodiodes, and CMOS sensors directly into portable devices. Companies like Abbott and Roche have successfully commercialized systems that integrate sample preparation, reaction chambers, and detection components in single-use cartridges smaller than a credit card.
For electrochemical microfluidic ELISA, integration focuses on combining electrode fabrication with microfluidic channels. Advanced manufacturing techniques including photolithography, screen printing, and inkjet printing enable the production of electrodes directly within microfluidic channels. This approach has demonstrated superior integration density, with recent platforms incorporating up to 16 detection sites in devices smaller than 4 cm².
Smartphone integration represents another significant advancement, transforming consumer electronics into analytical instruments. Colorimetric systems utilize phone cameras as detectors, while electrochemical systems connect via data ports or wireless protocols. This strategy has reduced the cost of reader devices by 60-80% while improving accessibility.
Power management remains a critical challenge, particularly for electrochemical systems requiring stable voltage sources. Recent innovations include energy-harvesting technologies and low-power circuit designs that extend battery life from hours to days. Colorimetric systems generally maintain an advantage in power efficiency, though with lower sensitivity limits.
Data processing integration has evolved from external computation to on-chip analysis. Machine learning algorithms embedded in microcontrollers now enable real-time signal processing and interpretation, reducing the need for expert analysis. Cloud connectivity further enhances these capabilities by enabling remote monitoring and epidemiological data collection.
The convergence of these miniaturization and integration strategies is driving the development of truly portable, user-friendly diagnostic platforms capable of laboratory-quality results in field settings, fundamentally transforming healthcare delivery models worldwide.
Regulatory Considerations for Diagnostic Microfluidic Platforms
The regulatory landscape for microfluidic diagnostic platforms, particularly those employing ELISA techniques, presents a complex framework that manufacturers and researchers must navigate. Colorimetric and electrochemical microfluidic ELISA systems face distinct regulatory challenges due to their different detection mechanisms and performance characteristics.
In the United States, the FDA classifies most microfluidic diagnostic devices under the in vitro diagnostic (IVD) regulatory framework. Colorimetric ELISA platforms typically undergo evaluation for analytical sensitivity, specificity, and reproducibility, with particular attention to optical detection systems and potential interference from sample matrices. Electrochemical systems face additional scrutiny regarding electrode stability, signal drift, and electrical safety considerations.
European regulations under the In Vitro Diagnostic Regulation (IVDR) impose more stringent requirements than the previous IVDD, affecting both colorimetric and electrochemical platforms. The IVDR's risk-based classification system places most ELISA-based diagnostic tools in higher risk categories, necessitating notified body involvement and comprehensive technical documentation. Electrochemical systems often require additional electromagnetic compatibility testing not typically needed for purely colorimetric approaches.
Quality system requirements represent another critical regulatory consideration. ISO 13485 certification is generally expected for manufacturers of both types of microfluidic ELISA platforms. However, electrochemical systems may face additional quality control challenges related to electrode manufacturing consistency and electrical component reliability that colorimetric systems do not encounter.
Clinical validation requirements differ substantially between regions and applications. Point-of-care applications of microfluidic ELISA face particularly rigorous usability testing requirements. Electrochemical systems often demonstrate advantages in quantitative precision but may face more complex validation protocols due to their electronic components and potential for electromagnetic interference.
Emerging regulatory frameworks for novel technologies present both challenges and opportunities. The FDA's Digital Health Software Precertification Program may offer streamlined pathways for electrochemical platforms with significant software components. Similarly, the European IVDR introduces new provisions for companion diagnostics that may affect how certain microfluidic ELISA platforms are regulated when used to guide therapeutic decisions.
Global harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually reducing regulatory disparities across major markets. However, significant differences remain in how colorimetric versus electrochemical microfluidic platforms are evaluated, particularly regarding performance standards, calibration requirements, and stability testing protocols.
In the United States, the FDA classifies most microfluidic diagnostic devices under the in vitro diagnostic (IVD) regulatory framework. Colorimetric ELISA platforms typically undergo evaluation for analytical sensitivity, specificity, and reproducibility, with particular attention to optical detection systems and potential interference from sample matrices. Electrochemical systems face additional scrutiny regarding electrode stability, signal drift, and electrical safety considerations.
European regulations under the In Vitro Diagnostic Regulation (IVDR) impose more stringent requirements than the previous IVDD, affecting both colorimetric and electrochemical platforms. The IVDR's risk-based classification system places most ELISA-based diagnostic tools in higher risk categories, necessitating notified body involvement and comprehensive technical documentation. Electrochemical systems often require additional electromagnetic compatibility testing not typically needed for purely colorimetric approaches.
Quality system requirements represent another critical regulatory consideration. ISO 13485 certification is generally expected for manufacturers of both types of microfluidic ELISA platforms. However, electrochemical systems may face additional quality control challenges related to electrode manufacturing consistency and electrical component reliability that colorimetric systems do not encounter.
Clinical validation requirements differ substantially between regions and applications. Point-of-care applications of microfluidic ELISA face particularly rigorous usability testing requirements. Electrochemical systems often demonstrate advantages in quantitative precision but may face more complex validation protocols due to their electronic components and potential for electromagnetic interference.
Emerging regulatory frameworks for novel technologies present both challenges and opportunities. The FDA's Digital Health Software Precertification Program may offer streamlined pathways for electrochemical platforms with significant software components. Similarly, the European IVDR introduces new provisions for companion diagnostics that may affect how certain microfluidic ELISA platforms are regulated when used to guide therapeutic decisions.
Global harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are gradually reducing regulatory disparities across major markets. However, significant differences remain in how colorimetric versus electrochemical microfluidic platforms are evaluated, particularly regarding performance standards, calibration requirements, and stability testing protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!