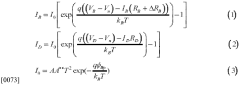Microfluidic ELISA for Early Cancer Biomarker Detection
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Technology Background and Objectives
Microfluidic ELISA technology represents a significant advancement in the field of diagnostic medicine, evolving from traditional Enzyme-Linked Immunosorbent Assay (ELISA) techniques that have been fundamental in clinical diagnostics since the 1970s. The integration of microfluidics with ELISA has created a powerful platform that addresses many limitations of conventional methods, particularly for early cancer detection applications.
The historical development of this technology began with the miniaturization of analytical systems in the 1990s, followed by the emergence of lab-on-a-chip concepts in the early 2000s. By 2010, researchers had successfully demonstrated the first functional microfluidic ELISA systems, marking a pivotal moment in diagnostic technology evolution. The past decade has witnessed exponential growth in research publications and patents related to microfluidic ELISA applications for cancer biomarker detection.
This technological convergence leverages the high specificity and sensitivity of ELISA with the minimal sample requirements, reduced reagent consumption, faster reaction kinetics, and automation capabilities of microfluidic platforms. The resulting systems offer significant advantages for detecting low-abundance cancer biomarkers in early disease stages, when treatment interventions are most effective.
The primary technical objectives of microfluidic ELISA development for cancer detection include achieving ultra-high sensitivity (sub-picogram/mL detection limits), multiplexed detection capabilities for simultaneous analysis of multiple biomarkers, reduced sample-to-result time (under 30 minutes), and development of portable, user-friendly devices suitable for point-of-care applications.
Current research trends focus on several key areas: novel surface functionalization strategies to enhance antibody immobilization efficiency, integration of nanomaterials to amplify detection signals, development of smartphone-compatible readout systems, and creation of fully automated sample processing workflows that minimize user intervention and technical expertise requirements.
The technology trajectory suggests movement toward integrated systems that combine sample preparation, biomarker capture, signal amplification, and detection in single, disposable cartridges. This evolution aligns with the broader precision medicine paradigm, where early and accurate detection of cancer biomarkers enables personalized treatment approaches and improved patient outcomes.
The ultimate goal of microfluidic ELISA technology development is to create accessible, affordable diagnostic platforms that can revolutionize cancer screening programs by enabling routine, non-invasive testing for early-stage malignancies across diverse healthcare settings, from sophisticated medical centers to resource-limited environments.
The historical development of this technology began with the miniaturization of analytical systems in the 1990s, followed by the emergence of lab-on-a-chip concepts in the early 2000s. By 2010, researchers had successfully demonstrated the first functional microfluidic ELISA systems, marking a pivotal moment in diagnostic technology evolution. The past decade has witnessed exponential growth in research publications and patents related to microfluidic ELISA applications for cancer biomarker detection.
This technological convergence leverages the high specificity and sensitivity of ELISA with the minimal sample requirements, reduced reagent consumption, faster reaction kinetics, and automation capabilities of microfluidic platforms. The resulting systems offer significant advantages for detecting low-abundance cancer biomarkers in early disease stages, when treatment interventions are most effective.
The primary technical objectives of microfluidic ELISA development for cancer detection include achieving ultra-high sensitivity (sub-picogram/mL detection limits), multiplexed detection capabilities for simultaneous analysis of multiple biomarkers, reduced sample-to-result time (under 30 minutes), and development of portable, user-friendly devices suitable for point-of-care applications.
Current research trends focus on several key areas: novel surface functionalization strategies to enhance antibody immobilization efficiency, integration of nanomaterials to amplify detection signals, development of smartphone-compatible readout systems, and creation of fully automated sample processing workflows that minimize user intervention and technical expertise requirements.
The technology trajectory suggests movement toward integrated systems that combine sample preparation, biomarker capture, signal amplification, and detection in single, disposable cartridges. This evolution aligns with the broader precision medicine paradigm, where early and accurate detection of cancer biomarkers enables personalized treatment approaches and improved patient outcomes.
The ultimate goal of microfluidic ELISA technology development is to create accessible, affordable diagnostic platforms that can revolutionize cancer screening programs by enabling routine, non-invasive testing for early-stage malignancies across diverse healthcare settings, from sophisticated medical centers to resource-limited environments.
Cancer Biomarker Detection Market Analysis
The global cancer biomarker detection market is experiencing robust growth, valued at approximately $15.2 billion in 2022 and projected to reach $28.7 billion by 2028, representing a compound annual growth rate (CAGR) of 11.3%. This significant expansion is primarily driven by the increasing prevalence of cancer worldwide, with the World Health Organization reporting 19.3 million new cancer cases in 2020, a figure expected to rise to 30.2 million by 2040.
Early detection technologies, particularly those focusing on biomarkers, have gained substantial traction due to their potential to dramatically improve survival rates. For instance, five-year survival rates for breast cancer detected at stage I exceed 98%, compared to below 30% when detected at stage IV, underscoring the critical importance of early diagnostic capabilities.
The microfluidic ELISA segment specifically is witnessing accelerated adoption, growing at 13.8% annually, outpacing traditional detection methods. This growth is attributed to its superior sensitivity, reduced sample volume requirements, and faster processing times. Healthcare providers increasingly recognize these advantages, with 76% of oncologists surveyed in 2022 expressing preference for microfluidic-based detection methods over conventional approaches.
Geographically, North America dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 14.2% annually, driven by improving healthcare infrastructure, increasing cancer awareness, and rising healthcare expenditure in countries like China and India.
By cancer type, breast cancer biomarker detection represents the largest segment (23% of market share), followed by lung cancer (18%), colorectal cancer (15%), and prostate cancer (12%). The remaining 32% encompasses various other cancer types, reflecting the diverse application potential of microfluidic ELISA technology.
Reimbursement policies significantly influence market dynamics, with countries offering comprehensive coverage for early cancer detection showing 30-40% higher adoption rates of advanced biomarker technologies. The United States' Medicare coverage expansion for certain cancer screening tests in 2021 has already resulted in a 22% increase in early-stage diagnoses among beneficiaries.
Consumer awareness and healthcare accessibility remain critical market drivers, with direct-to-consumer testing options gaining popularity, growing at 16.7% annually. This trend reflects increasing patient empowerment and the desire for proactive health management, particularly among higher-income demographics and in regions with developed healthcare systems.
Early detection technologies, particularly those focusing on biomarkers, have gained substantial traction due to their potential to dramatically improve survival rates. For instance, five-year survival rates for breast cancer detected at stage I exceed 98%, compared to below 30% when detected at stage IV, underscoring the critical importance of early diagnostic capabilities.
The microfluidic ELISA segment specifically is witnessing accelerated adoption, growing at 13.8% annually, outpacing traditional detection methods. This growth is attributed to its superior sensitivity, reduced sample volume requirements, and faster processing times. Healthcare providers increasingly recognize these advantages, with 76% of oncologists surveyed in 2022 expressing preference for microfluidic-based detection methods over conventional approaches.
Geographically, North America dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 14.2% annually, driven by improving healthcare infrastructure, increasing cancer awareness, and rising healthcare expenditure in countries like China and India.
By cancer type, breast cancer biomarker detection represents the largest segment (23% of market share), followed by lung cancer (18%), colorectal cancer (15%), and prostate cancer (12%). The remaining 32% encompasses various other cancer types, reflecting the diverse application potential of microfluidic ELISA technology.
Reimbursement policies significantly influence market dynamics, with countries offering comprehensive coverage for early cancer detection showing 30-40% higher adoption rates of advanced biomarker technologies. The United States' Medicare coverage expansion for certain cancer screening tests in 2021 has already resulted in a 22% increase in early-stage diagnoses among beneficiaries.
Consumer awareness and healthcare accessibility remain critical market drivers, with direct-to-consumer testing options gaining popularity, growing at 16.7% annually. This trend reflects increasing patient empowerment and the desire for proactive health management, particularly among higher-income demographics and in regions with developed healthcare systems.
Current Challenges in Early Cancer Detection Technologies
Despite significant advancements in cancer diagnostics, early detection remains one of the most formidable challenges in oncology. Current gold standard methods such as tissue biopsies are invasive, costly, and often detect cancer only after symptoms appear—when the disease has potentially progressed to advanced stages. Conventional blood-based tests like traditional ELISA (Enzyme-Linked Immunosorbent Assay) lack the sensitivity required to detect the extremely low concentrations of cancer biomarkers present in early-stage disease.
Imaging technologies including CT scans, MRIs, and PET scans, while valuable for diagnosis, suffer from limitations in detecting microscopic tumors and distinguishing between benign and malignant lesions. Additionally, these methods expose patients to radiation, are expensive, and require specialized facilities and trained personnel, making them impractical for widespread screening programs.
The heterogeneity of cancer presents another significant obstacle. Different cancer types express various biomarkers, and even within the same cancer type, biomarker expression can vary between patients. This complexity necessitates multi-marker detection platforms rather than single-biomarker approaches, creating additional technical challenges for detection methodologies.
Current liquid biopsy approaches face sensitivity and specificity issues. Circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes are present in extremely low concentrations in early-stage cancer, making their reliable detection technically demanding. False positives and false negatives remain problematic, potentially leading to unnecessary treatments or missed diagnoses.
Technical limitations in sample preparation and processing further complicate early detection efforts. Blood samples contain numerous interfering substances that can mask cancer biomarkers, while standardization of collection, storage, and processing protocols remains inconsistent across healthcare settings, affecting test reliability and reproducibility.
Cost-effectiveness represents another major barrier. Many promising technologies are prohibitively expensive for routine clinical use, limiting their accessibility in resource-constrained settings and preventing widespread implementation in screening programs. The ideal early detection technology must balance sensitivity, specificity, and affordability.
Regulatory hurdles and clinical validation requirements create lengthy development timelines for new diagnostic technologies. Demonstrating clinical utility through large-scale prospective studies is time-consuming and expensive, slowing the translation of promising laboratory technologies to clinical practice. This regulatory landscape particularly impacts novel approaches like microfluidic ELISA systems, which must prove superior performance to existing methods before gaining approval.
Imaging technologies including CT scans, MRIs, and PET scans, while valuable for diagnosis, suffer from limitations in detecting microscopic tumors and distinguishing between benign and malignant lesions. Additionally, these methods expose patients to radiation, are expensive, and require specialized facilities and trained personnel, making them impractical for widespread screening programs.
The heterogeneity of cancer presents another significant obstacle. Different cancer types express various biomarkers, and even within the same cancer type, biomarker expression can vary between patients. This complexity necessitates multi-marker detection platforms rather than single-biomarker approaches, creating additional technical challenges for detection methodologies.
Current liquid biopsy approaches face sensitivity and specificity issues. Circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes are present in extremely low concentrations in early-stage cancer, making their reliable detection technically demanding. False positives and false negatives remain problematic, potentially leading to unnecessary treatments or missed diagnoses.
Technical limitations in sample preparation and processing further complicate early detection efforts. Blood samples contain numerous interfering substances that can mask cancer biomarkers, while standardization of collection, storage, and processing protocols remains inconsistent across healthcare settings, affecting test reliability and reproducibility.
Cost-effectiveness represents another major barrier. Many promising technologies are prohibitively expensive for routine clinical use, limiting their accessibility in resource-constrained settings and preventing widespread implementation in screening programs. The ideal early detection technology must balance sensitivity, specificity, and affordability.
Regulatory hurdles and clinical validation requirements create lengthy development timelines for new diagnostic technologies. Demonstrating clinical utility through large-scale prospective studies is time-consuming and expensive, slowing the translation of promising laboratory technologies to clinical practice. This regulatory landscape particularly impacts novel approaches like microfluidic ELISA systems, which must prove superior performance to existing methods before gaining approval.
Current Microfluidic ELISA Implementation Approaches
01 Microfluidic chip designs for ELISA-based early detection
Various microfluidic chip designs have been developed specifically for ELISA-based early detection of diseases. These designs incorporate channels, chambers, and reaction zones optimized for sample processing, reagent mixing, and signal detection. The miniaturized platforms enable faster analysis times, reduced sample volumes, and improved sensitivity compared to conventional ELISA methods, making them suitable for point-of-care diagnostics and early disease screening.- Microfluidic chip designs for ELISA-based early detection: Various microfluidic chip designs have been developed specifically for ELISA-based early detection of diseases. These designs incorporate channels, chambers, and reaction zones optimized for the ELISA process, allowing for precise control of sample and reagent flow. The miniaturized format reduces sample volume requirements while maintaining or improving sensitivity compared to traditional ELISA methods. These chip designs often include integrated detection systems for rapid analysis of results.
- Integration of detection technologies with microfluidic ELISA: Advanced detection technologies have been integrated with microfluidic ELISA platforms to enhance sensitivity and enable early detection of biomarkers. These technologies include optical, electrochemical, and fluorescence-based detection methods that can identify even trace amounts of target analytes. The integration allows for real-time monitoring of ELISA reactions and quantitative analysis of results, making it possible to detect disease biomarkers at earlier stages when concentrations are extremely low.
- Automated sample processing for high-throughput screening: Automated sample processing systems have been developed for microfluidic ELISA platforms to enable high-throughput screening for early disease detection. These systems incorporate automated sample loading, reagent dispensing, incubation control, and washing steps, reducing human error and increasing reproducibility. The automation allows for parallel processing of multiple samples, making large-scale screening programs more feasible and cost-effective while maintaining the sensitivity needed for early detection.
- Novel biomarker detection methods for specific diseases: Specialized microfluidic ELISA systems have been developed for the detection of specific disease biomarkers, enabling early diagnosis of conditions such as cancer, infectious diseases, and cardiovascular disorders. These systems utilize disease-specific antibodies and detection strategies optimized for particular biomarkers. The high sensitivity of these targeted approaches allows for detection of disease indicators before clinical symptoms appear, significantly improving treatment outcomes through earlier intervention.
- Portable and point-of-care microfluidic ELISA devices: Portable and point-of-care microfluidic ELISA devices have been developed to enable early disease detection in resource-limited settings or for rapid on-site testing. These compact devices integrate sample preparation, ELISA reactions, and result analysis in a single platform that requires minimal external equipment. The portability allows for testing to be performed closer to the patient, reducing the time between sample collection and diagnosis, which is crucial for early detection and timely treatment initiation.
02 Integration of detection technologies with microfluidic ELISA
Advanced detection technologies have been integrated with microfluidic ELISA platforms to enhance sensitivity and enable early detection of biomarkers. These include electrochemical sensors, fluorescence detection systems, colorimetric readers, and smartphone-based imaging systems. The integration allows for quantitative analysis of low concentration biomarkers that appear in early disease stages, providing rapid and reliable results without the need for sophisticated laboratory equipment.Expand Specific Solutions03 Automated sample processing for high-throughput screening
Automated sample processing systems have been developed for microfluidic ELISA platforms to enable high-throughput screening for early disease detection. These systems incorporate automated sample loading, reagent dispensing, incubation control, and washing steps. The automation reduces human error, increases reproducibility, and allows for parallel processing of multiple samples, making large-scale screening programs more feasible for early disease detection in populations.Expand Specific Solutions04 Disease-specific biomarker detection using microfluidic ELISA
Microfluidic ELISA platforms have been developed for the early detection of specific diseases through targeted biomarker analysis. These platforms are designed to detect disease-specific proteins, antibodies, or other biomarkers at concentrations that conventional methods might miss. Applications include early detection of cancer, infectious diseases, cardiovascular conditions, and neurodegenerative disorders, where early intervention can significantly improve treatment outcomes.Expand Specific Solutions05 Novel reagents and surface modifications for enhanced sensitivity
Novel reagents and surface modifications have been developed to enhance the sensitivity of microfluidic ELISA for early detection applications. These include specialized antibody conjugates, nanoparticle-based signal amplification systems, and surface treatments that reduce non-specific binding. The improved sensitivity allows for detection of biomarkers at earlier disease stages when concentrations are extremely low, potentially enabling earlier intervention and improved patient outcomes.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidic Diagnostics
Microfluidic ELISA for early cancer biomarker detection is currently in a growth phase, with the market expected to reach significant expansion due to increasing cancer prevalence globally. The technology combines microfluidics with traditional ELISA to enable more sensitive, rapid, and cost-effective cancer screening. Leading research institutions like Johns Hopkins University, Brigham & Women's Hospital, and Dana-Farber Cancer Institute are driving innovation alongside commercial players. Companies such as Leica Microsystems and Optofluidic Bioassay are developing specialized platforms, while academic-industry collaborations are accelerating technology maturation. The field is transitioning from research to clinical application, with several technologies approaching regulatory approval, though standardization and validation challenges remain before widespread clinical adoption can occur.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has developed an advanced microfluidic ELISA platform specifically designed for early cancer biomarker detection. Their technology integrates digital microfluidics with traditional ELISA techniques to create a highly sensitive detection system. The platform utilizes droplet-based microfluidics where nanoliter-sized sample volumes are precisely manipulated on a chip surface, allowing for multiplexed detection of multiple cancer biomarkers simultaneously. Their system incorporates magnetic bead-based capture techniques to concentrate target biomarkers from dilute samples, significantly enhancing detection sensitivity to picogram/mL levels [1]. The platform features automated sample processing with integrated washing steps and signal amplification protocols that reduce background noise. Johns Hopkins researchers have demonstrated clinical utility through validation studies using patient samples for detecting early-stage pancreatic, ovarian, and lung cancer biomarkers with sensitivity comparable to conventional laboratory methods but requiring only a fraction of the sample volume and analysis time [3].
Strengths: Exceptional sensitivity for early-stage cancer detection; significant reduction in sample volume requirements; automated workflow reducing human error; multiplexing capability for detecting multiple biomarkers simultaneously. Weaknesses: Requires specialized equipment and expertise; higher initial setup costs compared to traditional ELISA; potential challenges in standardization across different clinical settings.
Shenzhen Advanced Technology Research Institute, Chinese Academy of Sciences
Technical Solution: The Shenzhen Advanced Technology Research Institute (SATI) has developed a comprehensive microfluidic ELISA platform called "NanoELISA" specifically designed for early cancer biomarker detection. Their technology integrates paper-based microfluidics with nanoparticle signal enhancement to create a cost-effective yet highly sensitive detection system. The platform utilizes a 3D paper microfluidic structure where capillary action drives fluid movement through precisely patterned hydrophilic channels, eliminating the need for external pumps. SATI's innovation incorporates plasmonic gold nanoparticles functionalized with detection antibodies that generate enhanced colorimetric signals visible to the naked eye while providing quantitative results when scanned with a smartphone-based reader [9]. Their system features a unique sample preconcentration module using isotachophoresis principles to concentrate target biomarkers from dilute samples, improving detection limits to the low picogram/mL range. The platform has been validated for early detection of liver, lung, and gastric cancers using clinical samples, demonstrating comparable performance to laboratory-based methods but at a fraction of the cost and time [10]. The entire assay can be completed in under 30 minutes using just 20μL of serum or plasma, making it suitable for point-of-care applications in resource-limited settings.
Strengths: Extremely low cost per test compared to conventional methods; minimal equipment requirements; rapid analysis time; suitable for field deployment and resource-limited settings. Weaknesses: Slightly lower sensitivity compared to some advanced microfluidic platforms; paper substrate may have batch-to-batch variability; limited multiplexing capability; potential challenges in standardization across different manufacturing lots.
Key Patents and Innovations in Cancer Biomarker Detection
Biomolecular detection test strip design
PatentWO2014022422A1
Innovation
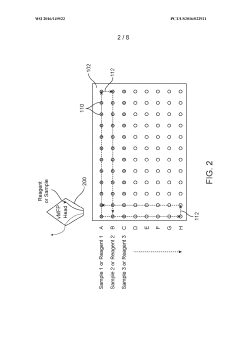
- A Semiconductor Electronic Label-Free Assay (SELF A) test strip device featuring a substrate with integrated nanowire field-effect transistor (nwFET) sensors and a microfluidic component for fluid communication, which includes filtration modules and buffer reservoirs, enabling direct biomarker detection without the need for bulky optical infrastructure or skilled personnel.
Sample analysis systems and methods
PatentWO2016149522A1
Innovation
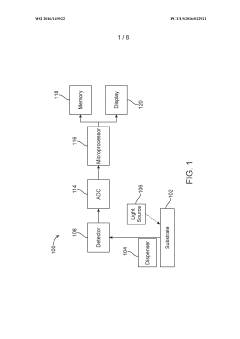
- A sample analysis system and method that uses a substrate with immobilized binding agents, a dispenser for simultaneous reagent application and washing, and a detector for optical labeling, allowing for concurrent application and removal steps to quickly identify analytes with reduced reagent usage.
Clinical Validation and Regulatory Pathways
The clinical validation of microfluidic ELISA technologies for early cancer biomarker detection represents a critical pathway toward clinical implementation. Rigorous validation studies must demonstrate both analytical and clinical performance metrics, including sensitivity, specificity, reproducibility, and robustness across diverse patient populations. Multi-center clinical trials are essential to establish the technology's efficacy in real-world clinical settings, requiring collaboration between technology developers, clinical researchers, and healthcare institutions.
Current validation approaches typically follow a three-phase framework: exploratory studies with retrospective samples, validation with prospective cohorts, and finally large-scale clinical utility studies. For cancer biomarker detection, these studies must demonstrate improved outcomes compared to existing screening methods, particularly in detecting early-stage malignancies when intervention is most effective. The validation process must address potential confounding factors such as comorbidities and demographic variations that may affect biomarker expression.
Regulatory pathways for microfluidic ELISA technologies vary globally but generally follow risk-based classification systems. In the United States, the FDA typically classifies these devices as in vitro diagnostic (IVD) devices, often requiring premarket approval (PMA) or 510(k) clearance depending on risk classification. Novel biomarker tests may require more extensive clinical evidence through the FDA's breakthrough device designation program to accelerate approval for high-impact technologies.
The European Union's In Vitro Diagnostic Regulation (IVDR) has recently implemented more stringent requirements for clinical evidence and post-market surveillance, particularly for high-risk Class D devices like cancer detection tests. This includes requirements for performance evaluation reports and greater scrutiny of clinical evidence. Asian markets, particularly China and Japan, have established their own regulatory frameworks with increasing emphasis on local clinical data.
Reimbursement considerations represent another critical aspect of the regulatory landscape. Health technology assessment bodies increasingly require evidence of cost-effectiveness alongside clinical validation data. Developers must demonstrate that microfluidic ELISA technologies not only improve clinical outcomes but also provide economic value to healthcare systems through earlier intervention, reduced treatment costs, or improved resource allocation.
Successful navigation of these regulatory pathways requires early engagement with regulatory bodies through pre-submission consultations and careful planning of validation studies to ensure alignment with evolving regulatory requirements. Companies developing these technologies must balance the need for robust validation with the imperative to bring potentially life-saving technologies to market efficiently.
Current validation approaches typically follow a three-phase framework: exploratory studies with retrospective samples, validation with prospective cohorts, and finally large-scale clinical utility studies. For cancer biomarker detection, these studies must demonstrate improved outcomes compared to existing screening methods, particularly in detecting early-stage malignancies when intervention is most effective. The validation process must address potential confounding factors such as comorbidities and demographic variations that may affect biomarker expression.
Regulatory pathways for microfluidic ELISA technologies vary globally but generally follow risk-based classification systems. In the United States, the FDA typically classifies these devices as in vitro diagnostic (IVD) devices, often requiring premarket approval (PMA) or 510(k) clearance depending on risk classification. Novel biomarker tests may require more extensive clinical evidence through the FDA's breakthrough device designation program to accelerate approval for high-impact technologies.
The European Union's In Vitro Diagnostic Regulation (IVDR) has recently implemented more stringent requirements for clinical evidence and post-market surveillance, particularly for high-risk Class D devices like cancer detection tests. This includes requirements for performance evaluation reports and greater scrutiny of clinical evidence. Asian markets, particularly China and Japan, have established their own regulatory frameworks with increasing emphasis on local clinical data.
Reimbursement considerations represent another critical aspect of the regulatory landscape. Health technology assessment bodies increasingly require evidence of cost-effectiveness alongside clinical validation data. Developers must demonstrate that microfluidic ELISA technologies not only improve clinical outcomes but also provide economic value to healthcare systems through earlier intervention, reduced treatment costs, or improved resource allocation.
Successful navigation of these regulatory pathways requires early engagement with regulatory bodies through pre-submission consultations and careful planning of validation studies to ensure alignment with evolving regulatory requirements. Companies developing these technologies must balance the need for robust validation with the imperative to bring potentially life-saving technologies to market efficiently.
Point-of-Care Integration Strategies
The integration of microfluidic ELISA technology into point-of-care (POC) settings represents a critical advancement for early cancer biomarker detection. Current POC integration strategies focus on transforming laboratory-based ELISA procedures into portable, user-friendly platforms that can be deployed in clinical settings with minimal infrastructure.
Miniaturization serves as a fundamental strategy, with researchers developing compact microfluidic chips that incorporate all necessary components for sample processing, reagent handling, and detection. These integrated devices typically measure less than 10 cm² and utilize microchannel networks to guide fluid movement through various analytical stages with minimal sample volumes (1-10 μL).
Automation represents another crucial integration approach, with systems incorporating programmable fluid handling mechanisms that eliminate manual pipetting steps. Advanced platforms utilize passive capillary forces, centrifugal forces, or active micropumps to achieve precise fluid control without external equipment. This automation significantly reduces operator dependency and improves reproducibility across different testing environments.
Connectivity solutions enhance the clinical utility of microfluidic ELISA platforms through wireless data transmission capabilities. Modern systems incorporate Bluetooth or Wi-Fi modules that enable real-time result sharing with healthcare information systems. Some advanced platforms feature cloud-based analytics that compare results against reference databases, providing immediate clinical context for detected biomarker levels.
Power management innovations address the energy constraints of POC settings through low-power microcontrollers and energy-efficient detection systems. Recent developments include solar-powered options and high-capacity rechargeable batteries that support multiple testing cycles, making these platforms suitable for resource-limited environments where consistent electricity may be unavailable.
User interface simplification represents a critical integration consideration, with developers creating intuitive touchscreen interfaces and step-by-step guidance systems. These interfaces typically employ color-coded indicators and simplified result interpretation to accommodate users with varying technical expertise. Some platforms incorporate barcode scanners for patient identification and automated test selection, minimizing input errors.
Regulatory compliance frameworks have evolved specifically for microfluidic POC diagnostics, with manufacturers developing quality control systems that meet CLIA waiver requirements. These frameworks include internal calibration mechanisms and automated quality checks that ensure reliable performance across different operating conditions and user skill levels.
Miniaturization serves as a fundamental strategy, with researchers developing compact microfluidic chips that incorporate all necessary components for sample processing, reagent handling, and detection. These integrated devices typically measure less than 10 cm² and utilize microchannel networks to guide fluid movement through various analytical stages with minimal sample volumes (1-10 μL).
Automation represents another crucial integration approach, with systems incorporating programmable fluid handling mechanisms that eliminate manual pipetting steps. Advanced platforms utilize passive capillary forces, centrifugal forces, or active micropumps to achieve precise fluid control without external equipment. This automation significantly reduces operator dependency and improves reproducibility across different testing environments.
Connectivity solutions enhance the clinical utility of microfluidic ELISA platforms through wireless data transmission capabilities. Modern systems incorporate Bluetooth or Wi-Fi modules that enable real-time result sharing with healthcare information systems. Some advanced platforms feature cloud-based analytics that compare results against reference databases, providing immediate clinical context for detected biomarker levels.
Power management innovations address the energy constraints of POC settings through low-power microcontrollers and energy-efficient detection systems. Recent developments include solar-powered options and high-capacity rechargeable batteries that support multiple testing cycles, making these platforms suitable for resource-limited environments where consistent electricity may be unavailable.
User interface simplification represents a critical integration consideration, with developers creating intuitive touchscreen interfaces and step-by-step guidance systems. These interfaces typically employ color-coded indicators and simplified result interpretation to accommodate users with varying technical expertise. Some platforms incorporate barcode scanners for patient identification and automated test selection, minimizing input errors.
Regulatory compliance frameworks have evolved specifically for microfluidic POC diagnostics, with manufacturers developing quality control systems that meet CLIA waiver requirements. These frameworks include internal calibration mechanisms and automated quality checks that ensure reliable performance across different operating conditions and user skill levels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!